Abstract
Background
Microalbuminuria is a common diagnosis in the clinical care of patients with type 1 diabetes. Long-term outcomes after the development of microalbuminuria are variable.
Methods
We quantified the incidence of and risk factors for long-term renal outcomes after the development of microalbuminuria in the DCCT/EDIC Study. The DCCT randomly assigned 1441 persons with type 1 diabetes to intensive or conventional diabetes therapy, and participants were subsequently followed during the observational EDIC Study. During DCCT/EDIC, 325 participants developed incident persistent microalbuminuria (albumin excretion rate [AER] ≥ 30 mg/24hr on two consecutive study visits). We assessed their subsequent renal outcomes, including progression to macroalbuminuria (AER ≥ 300 mg/24hr x2), impaired glomerular filtration rate (estimated GFR < 60 mL/min/1.73m2 x2), and end stage renal disease (ESRD), and regression to normoalbuminuria (AER < 30 mg/24hr x2).
Results
Median follow-up after persistent microalbuminuria diagnosis was 13 years (maximum 23 years). 10-year cumulative incidences of progression to macroalbuminuria, impaired GFR, and ESRD and regression to normoalbuminuria were 28%, 15%, 3%, and 40%, respectively. Albuminuria outcomes were more favorable with intensive diabetes therapy, lower hemoglobin A1c, lack of retinopathy, female gender, lower blood pressure, and lower concentrations of LDL cholesterol and triglyceride. Lower hemoglobin A1c, lack of retinopathy, and lower blood pressure were also associated with decreased risk of impaired GFR.
Conclusions
After the development of persistent microalbuminuria, progression and regression of kidney disease each occur commonly. Intensive glycemic control, lower blood pressure, and a more favorable lipid profile are associated with improved outcomes.
Introduction
Microalbuminuria (urine albumin excretion rate 30 – 300 mg/24hr) is a common diagnosis in the clinical care of patients with type 1 diabetes, with a life-time cumulative incidence of 20–40%.1–4 Clinical guidelines recommend regular screening for microalbuminuria because it is common, is known to be associated with adverse renal and cardiovascular outcomes, and can be treated with antagonists of the renin-angiotensin-aldosterone system.4
The Diabetes Control and Complications Trial (DCCT) demonstrated clearly that hyperglycemia is a risk factor for developing microalbuminuria and that intensive diabetes therapy can prevent or delay the development of microalbuminuria.5, 6 Additional risk factors for microalbuminuria have been well-described and include older age, male gender, long duration of diabetes, smoking, obesity, elevated blood pressure, and genetic predisposition.7–13
Less is known about the long-term renal outcomes of individuals with type 1 diabetes after microalbuminuria develops, in part because few studies are able to accurately ascertain the time at which microalbuminuria occurs and follow participants in detail for long subsequent periods of time. Accurate characterization of long-term renal outcomes is critically important to provide prognosis for type 1 diabetes patients and to identify opportunities for therapeutic intervention.
The DCCT and its observational extension, the Epidemiology of Diabetes Interventions and Complications (EDIC) Study, offer a unique opportunity to assess long-term clinical renal outcomes after the development of microalbuminuria because the time of albuminuria onset can be precisely defined using extensive longitudinal measurements and participants have been followed up for more than two decades with standardized methods.5, 6 In this study, we describe the long-term renal outcomes of persistent microalbuminuria in the DCCT/EDIC cohort and assess risk factors for progression and regression of kidney disease. We focus on the potential impact of glycemia, because the DCCT was a randomized trial of intensive diabetes therapy and detailed longitudinal hemoglobin A1c data were collected during DCCT/EDIC.
Methods
The DCCT/EDIC Study
The DCCT was a multicenter clinical trial in type 1 diabetes mellitus examining the effects of intensive diabetes therapy, aimed at lowering glycemia as close to the non-diabetic range as safely possible, compared with conventional therapy.5, 6 The trial included two cohorts, a primary prevention cohort (1 to 5 years duration of diabetes, albumin excretion rate [AER] < 40mg/24hr, no retinopathy by fundus photography) and a secondary intervention cohort (1 to 15 years duration, AER ≤ 200mg/24hr, and at least one microaneurysm in either eye, but no more than moderate nonproliferative retinopathy). From 1983–89, 1441 participants between the ages of 13 and 39 years were enrolled and randomly assigned to intensive or conventional diabetes therapy. Intensive therapy included three or more insulin injections daily or use of an insulin pump with the aim of achieving HbA1c levels < 6.05%. The goal of conventional therapy was prevention of symptoms of hyperglycemia and hypoglycemia using one or two daily injections of insulin. Subjects were followed for a mean of 6.5 years until DCCT closeout in 1993.
At the end of the DCCT, all former conventional treatment participants were offered instruction in intensive therapy, and all participants returned to their own health care providers for ongoing diabetes care. All DCCT participants were invited to join the EDIC study, an observational extension of the DCCT, and 1375 (96% of the surviving cohort) agreed to participate. During the EDIC study, mean hemoglobin A1c levels, which had been separated by approximately 2% between conventional and intensive therapy groups during the DCCT, converged between the former treatment groups.6 DCCT/EDIC Study procedures were approved by the institutional review boards of participating centers, and all participants provided written informed consent. The study described herein includes data from DCCT baseline through EDIC Study year 14 (2006–2008).
Persistent microalbuminuria cohort
This study focuses on 325 DCCT/EDIC participants who developed incident persistent microalbuminuria (PMA) during the course of DCCT/EDIC Study observation. For this and other epidemiologic studies of the DCCT/EDIC cohort,13–15 PMA is defined as albumin excretion rate (AER) ≥ 30 mg/24hr on two consecutive study visits. This definition of PMA differs from the original DCCT definition of microalbuminuria used for assessment of treatment effect (single AER ≥ 40 mg/24hr) in order to reflect current American Diabetes Association and National Kidney Foundation recommendations, which include a threshold of 30 mg/24hr and persistence, and to reduce the impact of measurement error on the diagnosis of microalbuminuria.4, 16 AER was measured yearly during the DCCT and every 2 years during the EDIC Study. Urine was collected for 4 hours during a water diuresis, and albumin was measured by fluoroimmunoassay (coefficient of variation 9.4%).5 A diagnosis of PMA was based on two consecutive DCCT AER measurements, the last DCCT and first EDIC study AER measurement, or two consecutive EDIC Study measurements. The cohort of 325 participants with incident PMA did not include 68 separate participants with AER ≥ 30 mg/24hr at their first two DCCT visits (prevalent microalbuminuria).
Definitions of long-term renal outcomes
Among the PMA cohort, we assessed progression to macroalbuminuria, development of impaired GFR, development of ESRD, and regression to normoalbuminuria. Progression to macroalbuminuria was defined as AER ≥ 300 mg/24hr on two consecutive study visits. Impaired GFR was defined as estimated GFR < 60 mL/min/1.73m2 on two consecutive study visits.4, 16 Regression to normoalbuminuria was defined as AER < 30 mg/24hr on two consecutive study visits. Serum creatinine was measured yearly during the DCCT and EDIC Study using the modified Jaffe reaction, and estimated GFR was calculated using the Modification of Diet in Renal Disease formula.17 ESRD was defined as the initiation of maintenance dialysis (n=13) or kidney transplantation (n=8), assessed yearly by questionnaire. Renal outcomes were not mutually exclusive.
Covariates
Demographic data and smoking were assessed by self-report. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Blood pressure was measured by trained personnel.18 All laboratory measurements were completed at the DCCT/EDIC Study Central Biochemistry Laboratory. Hemoglobin A1c was measured using high-performance ion-exchange liquid chromatography (coefficient of variation <4%).19 Plasma lipids were measured using conventional enzymatic methods, with LDL cholesterol calculated using the Friedewald formula. Prevalence of any retinopathy (at least one microaneurysm in either eye) was assessed with 7-field stereo fundus photography.
Medication use was assessed yearly by self-report only during the EDIC study. Use of angiotensin converting enzyme (ACE) inhibitors was discouraged during the DCCT, and only 6% reported ACE inhibitor use on their first EDIC study visit. Angiotensin receptor blockers were available for clinical use only during the EDIC Study. Renin-angiotensin-aldosterone system (RAAS) inhibitors (ACE inhibitors and angiotensin receptor blockers) were combined and analyzed as a class. HMGCoA reductase inhibitors became available during the end of the DCCT (beginning in 1989), and use of a lipid-lowering agent was reported by only 2% of participants at their first EDIC study visit.
Statistical analysis
To describe the development of PMA over the clinical course of type 1 diabetes in our population, we first graphed the cumulative incidence of PMA by duration of diabetes in the full DCCT/EDIC cohort. We used a Turnbull estimator for interval censored observations to allow for both left censoring of participants with prevalent PMA and right censoring of participants who did not develop PMA during DCCT/EDIC observation.20
Among the 325 participants who developed incident PMA, long-term renal outcomes were evaluated separately. Time at risk began at PMA diagnosis and extended to diagnosis of the renal outcome being analyzed or to each participant’s penultimate AER measurement (for progression and regression of microalbuminuria), penultimate estimated GFR (for impaired GFR), or last study visit (for ESRD). Incidence rates were expressed in person-years. In prevalence analyses only, normoalbuminuria, microalbuminuria, and macroalbuminuria were assessed in 2-year intervals and mean AER was used if two AER measurements were available.
Risk factors for long-term renal outcomes after the diagnosis of PMA were assessed using cumulative incidence plots and discrete Cox proportional hazards models.21 We repeated all analyses using a Weibull model for interval censoring, with no substantial differences in results. Cox models assessed as exposures both characteristics at the time of PMA diagnosis and characteristics assessed at the time of follow-up exam (time-updated variables) and adjusted for age, gender, and diabetes duration at PMA diagnosis. Analyses were performed using SAS version 9.1 (Cary, NC).
Results
Incidence of persistent microalbuminuria
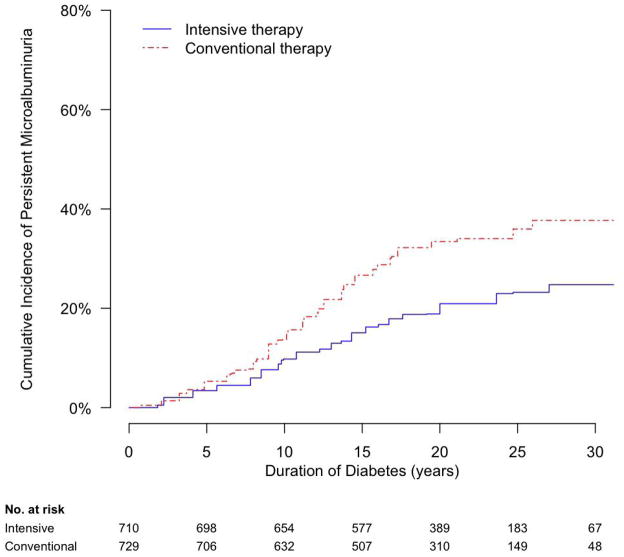
Among participants randomly assigned to DCCT conventional therapy, PMA developed most frequently during the second decade after diagnosis of diabetes (Figure 1). Cumulative incidences of PMA were 14%, 33%, and 38% at 10, 20, and 30 years duration of diabetes, respectively, and cumulative incidence appeared to plateau below 40%. Among participants assigned to intensive therapy, cumulative incidence of PMA was reduced, and development of PMA during the second decade after diagnosis of diabetes was particularly blunted (Figure 1). Cumulative incidences of PMA were 10%, 21%, and 25% at 10, 20, and 30 years duration of diabetes, respectively, and cumulative incidence appeared to plateau near 25%.
Figure 1.
Cumulative incidence of persistent microalbuminuria in the DCCT/EDIC Study, by duration of type 1 diabetes and DCCT treatment assignment.
Clinical characteristics
Of the 325 participants who developed incident PMA, 41% were women and 35% had been assigned to intensive diabetes therapy (Table 1). At the time of PMA diagnosis, mean age was 33 years, mean duration of diabetes was 14 years, 87% of participants had retinopathy, and 48% were in the EDIC phase of the study. Median AER was 48 mg/24hr, and mean estimated GFR was 114 mL/min/1.73m2. Compared with participants assigned to DCCT conventional therapy who developed PMA, participants assigned to intensive therapy who developed PMA did so at a slightly later duration of diabetes (15 versus 13 years), were slightly heavier (mean BMI 27 versus 26 kg/m2), had lower hemoglobin A1c levels (mean 8.9% versus 9.6%), and were more likely to report use of RAAS inhibitors (10% versus 6%) and lipid-lowering agents (8% versus 3%); other characteristics did not differ.
Table 1.
Characteristics of 325 participants in the DCCT/EDIC Study at the time incident persistent microalbuminuria was diagnosed
| Demographic data and medical history | |
| Age (years) | 33 (10) |
| Female gender | 132 (41%) |
| Caucasian race | 304 (94%) |
| Duration of diabetes (years) | 14 (6) |
| DCCT cohort | |
| Primary prevention | 140 (43%) |
| Secondary prevention | 185 (57%) |
| DCCT treatment assignment | |
| Intensive therapy | 115 (35%) |
| Conventional therapy | 210 (65%) |
| Time of diagnosis of persistent microalbuminuria | |
| During DCCT | 170 (52%) |
| During EDIC Study | 155 (48%) |
| Retinopathy | 231 (87%) |
| Active smoking | 99 (32%) |
| RAAS inhibitor use | 24 (7%) |
| Lipid-lowering medication use | 16 (5%) |
| Physical examination | |
| Body mass index (kg/m2) | 26.0 (4.1) |
| Systolic blood pressure (mmHg) | 122 (14) |
| Diastolic blood pressure (mmHg) | 78 (9) |
| Laboratory data | |
| Hemoglobin A1c (%) | 9.4 (1.8) |
| Albumin excretion rate (mg/24hr) | 48 (37, 76) |
| Estimated GFR (mL/min/1.73 m2) | 114 (30) |
| Total cholesterol (mg/dL) | 190 (38) |
| HDL cholesterol (mg/dL) | 51 (13) |
| Triglycerides (mg/dL) | 108 (89) |
| LDL cholesterol (mg/dL) | 119 (32) |
Data are mean (standard deviation) or n (%), except for albumin excretion ratio (median, inter-quartile range). DCCT = Diabetes Control and Complications Trial; EDIC = Epidemiology of Diabetes Interventions and Complications; RAAS = renin-angiotensin-aldosterone system; GFR = glomerular filtration rate.
Long-term renal outcomes
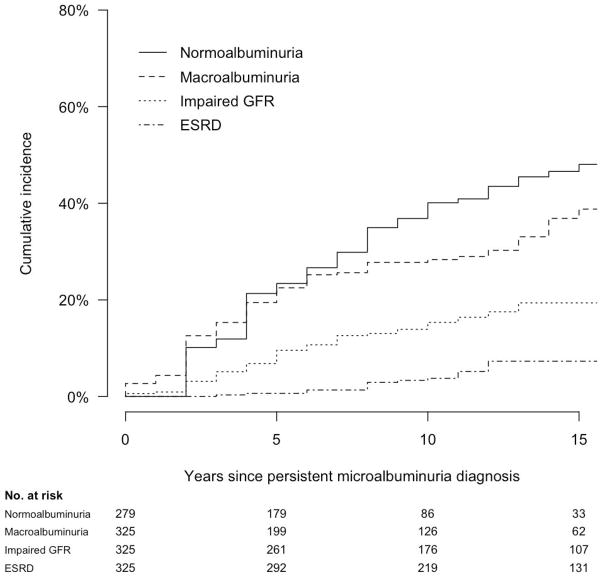
Mean and median follow-up periods after the diagnosis of PMA were each 13 years, with a maximum follow-up of 23 years (Table 2). Follow-up data for AER and estimated GFR were complete for 95% and 93% of study visits, respectively. 26 of the 325 participants with incident PMA died during follow-up (8%). Ninety-eight participants progressed to macroalbuminuria, 60 developed impaired GFR, 21 developed ESRD, and 117 regressed to normoalbuminuria. 26, 4, 0, and 44 of these events occurred during the DCCT itself, respectively, with the remainder occurring during EDIC follow-up. Cumulative 10-year incidences of progression to macroalbuminuria, impaired GFR, ESRD, and regression to normoalbuminuria were 28%, 15%, 4%, and 40%, respectively (Table 2 and Figure 2). At the time of progression or regression, RAAS inhibitor use was reported by 37% of participants who progressed to macroalbuminuria, 71% of participants who developed impaired GFR, and 25% of participants who regressed to normoalbuminuria.
Table 2.
Incidence of long-term renal outcomes after the diagnosis of persistent microalbuminuria among 325 participants in the DCCT/EDIC Study
| Follow-up | Events | |||||||
|---|---|---|---|---|---|---|---|---|
| Median (years) | Range (years) | Person-years | N | Incidence rate* | Cumulative incidence | |||
| 5 years | 10 years | 15 years | ||||||
| Overall follow-up | 13 | 1 – 23 | 4096 | - | - | - | - | - |
| Normoalbuminuria | 8 | 2 – 21 | 1302 | 117 | 9.1 | 23% | 40% | 48% |
| Macroalbuminuria | 8 | 0 – 21 | 2578 | 98 | 3.8 | 22% | 28% | 39% |
| Impaired GFR | 11 | 0 – 22 | 3416 | 60 | 1.8 | 10% | 15% | 19% |
| ESRD | 12 | 1 – 23 | 4026 | 21 | 0.5 | 1% | 4% | 7% |
per 100 person-years. GFR = glomerular filtration rate; ESRD = end-stage renal disease.
Figure 2.
Cumulative incidence of long-term renal outcomes after the development of persistent microalbuminuria (time 0) among 325 participants in the DCCT/EDIC Study.
Seventeen participants regressed to normoalbuminuria more than 10 years after the initial PMA diagnosis. At the time of regression to normoalbuminuria, mean HbA1c was 7.7% and mean BP 121/77 mmHg. Prevalences of RAAS inhibitor and lipid-lowering medication use were 47% and 12%, respectively. Three of these late regressors developed sustained macroalbuminuria prior to regressing to normoalbuminuria, but none developed sustained impaired GFR prior to regressing.
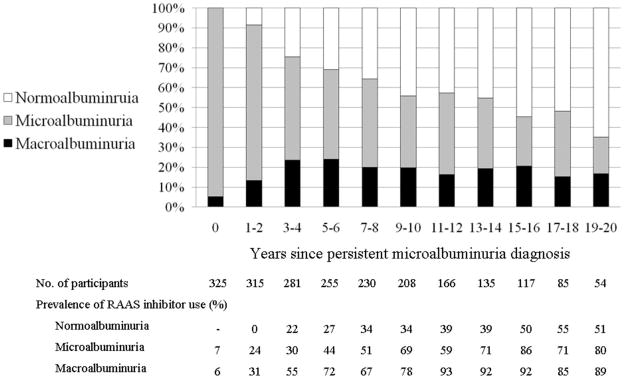
Macroalbuminuria prevalence increased over the first 4 years following PMA diagnosis, then stabilized near 20% (Figure 3). Further increases in macroalbuminuria may have been obscured by an increasing prevalence of RAAS inhibitor use among participants with AER measured in the microalbuminuria range (exceeding 50% 7–8 years after PMA diagnosis). Normoalbuminuria became increasingly prevalent over the full course of follow-up, exceeding 50% after 13–14 years. Fewer than 50% of participants who regressed to prevalent normoalbuminuria during the first 14 years after PMA diagnosis were using RAAS inhibitors (Figure 3).
Figure 3.
Prevalence of normoalbuminuria, microalbuminuria, and macroalbuminuria by time following the diagnosis of incident persistent microalbuminuria (time 0) among 325 participants in the DCCT/EDIC Study.
Intensive diabetes therapy, glycemia, and long-term renal outcomes
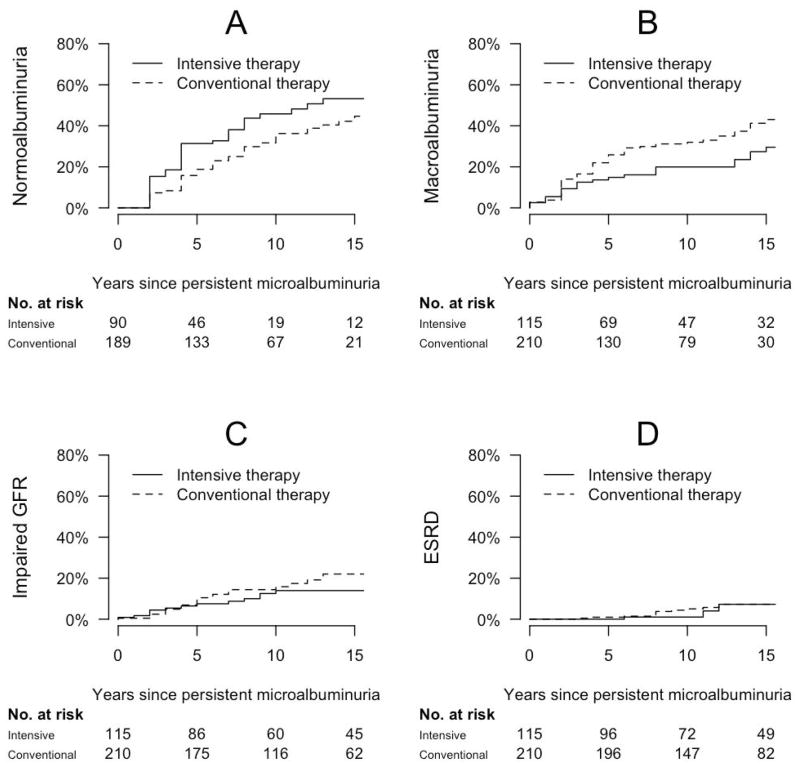
Participants who were assigned to intensive diabetes therapy during the DCCT and developed PMA experienced more favorable long-term renal outcomes than those who were assigned to conventional diabetes therapy and developed PMA (Figure 4). Adjusting for age, gender, and duration of diabetes, hazard ratios associated with intensive diabetes therapy were 0.64 for progression to macroalbuminuria (95% confidence interval 0.40, 1.02), 0.65 for impaired GFR (0.36, 1.16), and 1.92 for regression to normoalbuminuria (1.28, 2.86), p<0.05 for regression only (Table 3). Further adjusting for BMI and use of RAAS inhibitors and lipid-lowering medications, hazard ratios associated with intensive diabetes therapy were 0.61 for progression to macroalbuminuria (0.38, 0.98), 0.64 for impaired GFR (0.35, 1.17), and 2.16 for regression to normoalbuminuria (1.43, 3.26). Associations of intensive diabetes therapy with renal outcomes were stronger for participants diagnosed with PMA during the DCCT compared with participants diagnosed with PMA during EDIC follow-up, though these interactions was not statistically significant (data not shown). Lower hemoglobin A1c levels measured at PMA diagnosis or after PMA diagnosis were also associated with reduced risks of progression to macroalbuminuria and impaired GFR and increased probability of regression to normoalbuminuria (each p<0.05, Table 3).
Figure 4.
Cumulative incidence of long-term renal outcomes after the development of persistent microalbuminuria (time 0) among 325 participants in the DCCT/EDIC Study, by DCCT treatment assignment: (A) progression to macroalbuminuria; (B) impaired GFR; (C) end stage renal disease; (D) regression to normoalbuminuria.
Table 3.
Risk factors for long-term renal outcomes after the diagnosis of persistent microalbuminuria among 325 participants in the DCCT/EDIC Study
| Renal outcomes | ||||||
|---|---|---|---|---|---|---|
| Macroalbuminuria | Impaired GFR | Normoalbuminuria | ||||
| Risk factor | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Assessed at PMA diagnosis | ||||||
| Age (per year) | 1.00 (0.98–1.02) | 0.94 | 1.04 (1.01–1.07) | 0.01 | 1.00 (0.98–1.02) | 0.84 |
| Female gender | 0.52 (0.33–0.82) | 0.005 | 1.10 (0.65–1.87) | 0.73 | 2.53 (1.70–3.76) | <0.001 |
| Duration of diabetes (years) | ||||||
| < 10 years | 0.62 (0.38–1.02) | 0.06 | 0.54 (0.28–1.06) | 0.07 | 1.60 (1.04–2.45) | 0.03 |
| 10 –19 years | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) | |||
| ≥ 20 years | 0.27 (0.06–1.11) | 0.07 | 0.89 (0.31–2.59) | 0.84 | 1.38 (0.62–3.09) | 0.43 |
| DCCT intensive diabetes therapy | 0.64 (0.4–1.02) | 0.06 | 0.65 (0.36–1.16) | 0.14 | 1.92 (1.28–2.86) | 0.002 |
| Retinopathy | 3.15 (1.09–9.11) | 0.03 | 2.83 (0.64–12.5) | 0.17 | 0.46 (0.24–0.87) | 0.02 |
| Active smoking | 1.13 (0.73–1.75) | 0.57 | 1.04 (0.60–1.81) | 0.90 | 1.33 (0.87–2.03) | 0.19 |
| RAAS inhibitor use | 0.57 (0.17–1.94) | 0.37 | 1.17 (0.38–3.54) | 0.79 | 0.59 (0.14–2.57) | 0.49 |
| Lipid-lowering medication use | 2.16 (0.8–5.83) | 0.13 | 2.52 (0.84–7.55) | 0.10 | 1.04 (0.29–3.76) | 0.95 |
| Body mass index (per kg/m2) | 1.01 (0.96–1.07) | 0.64 | 0.95 (0.88–1.02) | 0.18 | 0.96 (0.91–1.01) | 0.13 |
| Systolic BP (per 10 mmHg) | 1.30 (1.10–1.54) | 0.003 | 1.06 (0.86–1.30) | 0.60 | 0.85 (0.72–0.99) | 0.04 |
| Diastolic BP (per 10 mmHg) | 1.38 (1.08–1.77) | 0.01 | 1.21 (0.89–1.65) | 0.23 | 0.71 (0.56–0.90) | 0.004 |
| Hemoglobin A1c (per %) | 1.25 (1.11–1.40) | <0.001 | 1.33 (1.15–1.53) | <0.001 | 0.76 (0.67–0.85) | <0.001 |
| Total cholesterol (per 10 mg/dL) | 1.14 (1.05–1.23) | 0.001 | 1.07 (0.97–1.18) | 0.16 | 0.88 (0.82–0.94) | <0.001 |
| HDL cholesterol (per 10 mg/dL) | 1.10 (0.85–1.42) | 0.48 | 1.21 (0.87–1.67) | 0.26 | 0.96 (0.78–1.18) | 0.69 |
| Triglycerides (per 10 mg/dL) | 1.06 (1.01–1.10) | 0.01 | 1.04 (0.98–1.10) | 0.15 | 0.93 (0.87–0.98) | 0.007 |
| LDL cholesterol (per 10 mg/dL) | 1.12 (1.03–1.22) | 0.01 | 1.04 (0.93–1.16) | 0.52 | 0.88 (0.82–0.95) | 0.001 |
| AER | ||||||
| 30–49 | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) | |||
| 50–99 | 1.98 (1.23–3.18) | 0.005 | 1.42 (0.75–2.69) | 0.28 | 0.85 (0.54–1.36) | 0.50 |
| ≥ 100 | 3.33 (1.95–5.69) | <0.001 | 3.17 (1.69–5.93) | <0.001 | 0.70 (0.40–1.25) | 0.23 |
| Time-updated risk factors | ||||||
| Systolic BP (per 10 mmHg) | 1.71 (1.49–1.97) | <0.001 | 1.91 (1.65–2.21) | <0.001 | 0.82 (0.70–0.95) | 0.008 |
| Diastolic BP (per 10 mmHg) | 2.03 (1.63–2.53) | <0.001 | 2.17 (1.67–2.82) | <0.001 | 0.68 (0.55–0.84) | <0.001 |
| Hemoglobin A1c (per %) | 1.25 (1.1–1.42) | <0.001 | 1.13 (0.95–1.33) | 0.16 | 0.79 (0.69–0.90) | <0.001 |
HR = hazard ratio, adjusted for age, gender, and duration of diabetes at the time of persistent microalbuminuria diagnosis; CI = confidence interval; PMA = persistent microalbuminuria; RAAS = renin-angiotensin-aldosterone system.
Other risk factors
Albuminuria outcomes were also more favorable (decreased risk of progression to macroalbuminuria and increased probability of regression to normoalbuminuria) with female gender, absence of retinopathy, lower blood pressure (at diagnosis or at follow-up), and lower concentrations of LDL cholesterol and triglyceride (Table 3). Younger age, absence of retinopathy, and lower blood pressure (the latter during follow-up) were also associated with decreased risk of impaired GFR. RAAS inhibitor use was not associated with improved albuminuria or GFR outcomes, without (Table 3) or with adjustment for blood pressure. Level of AER at PMA diagnosis was strongly associated with progression to macroalbuminuria and impaired GFR but only weakly associated with regression to normoalbuminuria.
Discussion
This study characterizes long-term renal outcomes from the time of development of PMA in type 1 diabetes. Progression to macroalbuminuria, impaired GFR, and ESRD occurred with clinically important frequencies (cumulative incidences 28%, 15%, and 3% at 10 years, respectively). However, regression to normoalbuminuria was the most common renal outcome, with a 10-year cumulative incidence of 40%. Thus, the development of persistent microalbuminuria does not necessarily represent the start of an inexorable downhill course for diabetic nephropathy. Among persons who develop microalbuminuria, lower levels of glycemia and intensive insulin therapy remain associated with more favorable long-term renal outcomes, providing provocative evidence that glycemic control may help prevent the progression of existing kidney disease in addition to the development of microalbuminuria. Additional modifiable risk factors associated with long-term renal outcomes included blood pressure (for albuminuria and GFR outcomes) and lipid levels (for albuminuria outcomes only).
Progression of PMA to macroalbuminuria and impaired GFR was common in our study. This progression carries important implications, since it portends substantially increased risk of cardiovascular events and mortality.7, 22 While progression to ESRD has been uncommon in the DCCT/EDIC cohort,6 this study reinforces the adverse prognostic renal implications of microalbuminuria by noting substantial cumulative incidences of ESRD of 4% and 7% 10 and 15 years after the development of PMA, respectively. Furthermore, ESRD incidence rates will likely increase with further EDIC follow-up.
Microalbuminuria was once viewed as the first step in a committed course of progressive diabetic kidney disease in type 1 diabetes, but it is now widely recognized that microalbuminuria may commonly regress to normoalbuminuria.23 While RAAS inhibitors clearly reduce urine albumin excretion and can induce regression, the majority of regression to normoalbuminuria in our study was spontaneous, as previously observed in other studies.23, 24 Our results also document regression to normoalbuminuria after more than a decade of PMA, mostly without RAAS inhibitor use and generally in the setting of excellent control of glycemia and blood pressure.
We observed an incidence of regression to normoalbuminuria higher than in some previous studies11, 12, 23 and comparable to others.25–27 Our relatively high incidence rate is likely due to our unique study design. Participants were longitudinally screened for the onset of PMA, as currently recommended for clinical care.4 This approach yields a representative mix of persons with incident PMA, which is less likely to be selected for persistent and severe disease compared to populations with prevalent microalbuminuria. Rates of regression are also likely to vary with frequency of AER ascertainment. More frequent AER sampling is likely to detect microalbuminuria of shorter duration which is more susceptible to regression, and more frequent AER sampling after microalbuminuria diagnosis is likely to increase the observed rate of regression, each contributing to higher observed rates of disease “regression.” Our sampling interval (every 1–2 years) is consistent with current screening recommendations and actual clinical care.
The DCCT and United Kingdom Prospective Diabetes Study clearly demonstrated an important role for glycemic control in the prevention of early diabetic kidney disease.5, 6,28 However, the role of glycemic control in preventing progression of established kidney disease remains controversial.12 In particular, it has been suggested that glycemic control may improve metabolic and endothelial abnormalities leading to microalbuminuria, but not necessarily fibrotic processes leading to macroalbuminuria, impaired GFR, and ESRD. In the DCCT, intensive diabetes therapy significantly reduced the incidence of macroalbuminuria, but it has been difficult to determine whether this was due to the large effect of intensive diabetes therapy on preventing the development of microalbuminuria, or to effects on progression of microalbuminuria to macroalbuminuria per se.5 The current study identifies hyperglycemia as an important risk factor for progression of established microalbuminuria to macroalbuminuria and impaired GFR. Furthermore, our results suggest that intensive diabetes therapy may help prevent this progression, whether applied before, at, or after the time of PMA diagnosis. The latter results are not definitive, because participants were not randomized to diabetes therapy at the time of PMA diagnosis. The direction and magnitude of resulting bias cannot be determined, but it is reasonable to speculate that benefits of intensive diabetes therapy were actually underestimated in our study, because outcomes of participants who developed PMA with conventional therapy were compared to outcomes of participants who developed PMA despite intensive therapy. Thus, our results suggest that glycemic control remains important for preventing renal progression in type 1 diabetes even after microalbuminuria has developed.
Our results highlight the known importance of BP control in the prevention of diabetic kidney disease and again note associations of lipid levels with renal outcomes.11, 27, 29, 30 We again note that men with type 1 diabetes have less favorable albuminuria outcomes.7, 11, 13 Gender was not associated with the development of impaired GFR in our analyses, though this relationship may be biased by inclusion of gender in the formula used to estimate GFR.17 Our data cannot be validly used to assess the impact of RAAS inhibitors or lipid-lowering medications on disease progression, as the use of these medications in this observational study is highly subject to confounding by indication.31 As a result, and given rigorous randomized clinical trials demonstrating that RAAS inhibitors prevent progression of kidney disease in types 1 and 2 diabetes,32–35 our data should not discourage use of RAAS inhibitors in the setting of microalbuminuria.
Although our study population is not an inception cohort, our observed PMA cumulative incidence (25–40% at 30 years diabetes duration) and timing (largely during the second decade of diabetes duration) are similar to those in prior studies.7, 11 This concordance reinforces the prior observation that complication rates in DCCT participants assigned to conventional diabetes therapy are similar to historical rates in a community-based cohort.36
The main strengths of this study derive from the careful longitudinal characterization of DCCT/EDIC participants over a long duration of follow-up, allowing identification of PMA at or near its time of onset and accurate diagnosis of subsequent outcomes. By requiring persistence of microalbuminuria at diagnosis and persistence of normoalbuminuria, macroalbuminuria, and impaired GFR during follow-up, we are able to report changes in disease status with confidence. As a result, our results offer relevant guidance to clinicians and patients who are newly diagnosed with PMA.
This study also has limitations. We evaluated GFR only as a dichotomous outcome, while early renal function decline is also likely to have important clinical implications.37 A relatively small number of ESRD cases were observed. The incidence and outcomes of PMA may be changing over time with changes in clinical care, including increased RAAS inhibitor use. We did not assess cardiovascular and mortality outcomes, though because death has been uncommon in the DCCT/EDIC Study relative to renal outcomes,38 it is unlikely that the incidence of renal outcomes was substantially biased due to informative censoring.
In conclusion, progression and regression of kidney disease each occur commonly after the development of PMA in type 1 diabetes, suggesting that the development of PMA offers a valuable occasion to assess and target renal interventions. In particular, intensive diabetes therapy appears to improve renal outcomes after the development of PMA, in addition to preventing the initial development of microalbuminuria.
Acknowledgments
A complete list of participants in the DCCT/EDIC research group can be found in Archives of Ophthalmology, 2008;126(12):1713.
Funding/Support: The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translational Science Awards Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. Additional funding for this analysis was provided by National Center for Research Resources grant UL1 RR 025014.
Footnotes
Contributors of free or discounted supplies and/or equipment: Lifescan, Roche, Aventis, Eli Lilly, OmniPod, Can-Am, B-D, Animas, Medtronic, Medtronic Minimed, Bayer (donation one time in 2008), Omron.
Contributor Information
Ian H. de Boer, University of Washington, Seattle, WA.
Tessa C. Rue, University of Washington, Seattle, WA.
Patricia A. Cleary, The George Washington University, Rockville, MD.
John M. Lachin, The George Washington University, Rockville, MD.
Mark E. Molitch, Northwestern University, Chicago, IL.
Michael W. Steffes, University of Minnesota, Minneapolis, MN.
Wanjie Sun, The George Washington University, Rockville, MD.
Bernard Zinman, University of Toronto, Toronto, Ontario.
John D. Brunzell, University of Washington, Seattle, WA.
References
- 1.Parving HH, Oxenboll B, Svendsen PA, Christiansen JS, Andersen AR. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 1982 Sep;100(4):550–555. doi: 10.1530/acta.0.1000550. [DOI] [PubMed] [Google Scholar]
- 2.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982 Jul 26;1(8287):1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984 Feb 9;310(6):356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 4.Standards of medical care in diabetes--2009. Diabetes Care. 2009 Jan;32( Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int. 1995 Jun;47(6):1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 6.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Jama. 2003 Nov 22;290(16):2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983 Dec;25(6):496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- 8.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989 May 4;320(18):1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- 9.Coonrod BA, Ellis D, Becker DJ, et al. Predictors of microalbuminuria in individuals with IDDM. Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 1993 Oct;16(10):1376–1383. doi: 10.2337/diacare.16.10.1376. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int. 2001 Jul;60(1):219–227. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 11.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. Bmj. 2004 May 8;328(7448):1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossing P, Hougaard P, Parving HH. Progression of microalbuminuria in type 1 diabetes: ten-year prospective observational study. Kidney Int. 2005 Oct;68(4):1446–1450. doi: 10.1111/j.1523-1755.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- 13.de Boer IH, Sibley SD, Kestenbaum B, et al. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol. 2007 Jan;18(1):235–243. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boright AP, Paterson AD, Mirea L, et al. Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes. 2005 Apr;54(4):1238–1244. doi: 10.2337/diabetes.54.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Kateb H, Boright AP, Mirea L, et al. Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes. 2008 Jan;57(1):218–228. doi: 10.2337/db07-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 17.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 18.de Boer IH, Kestenbaum B, Rue TC, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008 Sep 22;168(17):1867–1873. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005 Apr;51(4):753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. Journal of the Royal Statistical Society, Series B. 1976;38:290–295. [Google Scholar]
- 21.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981 Jun;2(2):93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 22.Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987 Jun 27;294(6588):1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003 Jun 5;348(23):2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 24.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The Early Natural History of Nephropathy in Type 1 Diabetes: III. Predictors of 5-Year Urinary Albumin Excretion Rate Patterns in Initially Normoalbuminuric Patients. Diabetes. 2005 Jul;54(7):2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 25.Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant. 2001 Aug;16(7):1382–1386. doi: 10.1093/ndt/16.7.1382. [DOI] [PubMed] [Google Scholar]
- 26.Bojestig M, Arnqvist HJ, Karlberg BE, Ludvigsson J. Glycemic control and prognosis in type I diabetic patients with microalbuminuria. Diabetes Care. 1996 Apr;19(4):313–317. doi: 10.2337/diacare.19.4.313. [DOI] [PubMed] [Google Scholar]
- 27.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia. 2004 Jun;47(6):1020–1028. doi: 10.1007/s00125-004-1413-8. [DOI] [PubMed] [Google Scholar]
- 28.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003 Jan;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002 Sep;62(3):963–970. doi: 10.1046/j.1523-1755.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 30.Molitch ME, Rupp D, Carnethon M. Higher levels of HDL cholesterol are associated with a decreased likelihood of albuminuria in patients with longstanding type 1 diabetes. Diabetes Care. 2006 Jan;29(1):78–82. doi: 10.2337/diacare.29.01.06.dc05-1583. [DOI] [PubMed] [Google Scholar]
- 31.Avorn J. In defense of pharmacoepidemiology--embracing the yin and yang of drug research. N Engl J Med. 2007 Nov 29;357(22):2219–2221. doi: 10.1056/NEJMp0706892. [DOI] [PubMed] [Google Scholar]
- 32.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993 Nov 11;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 33.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001 Sep 20;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 34.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001 Sep 20;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 35.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001 Sep 20;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 36.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005) Arch Intern Med. 2009 Jul 27;169(14):1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007 Apr;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 38.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]