Abstract
Background
The lateral (LA) and central (CE), but not basal (B), amygdala nuclei are necessary for reactive Pavlovian fear responses such as freezing. The amygdala also plays a key role in the acquisition and expression of active instrumental defensive behaviors, but little is known about the specific roles of amygdala nuclei. Using a Sidman active avoidance (AA) task, we examined the necessity of LA, B, and CE for learning and performance. Pavlovian freezing was simultaneously assessed to examine the contributions of amygdala nuclei to the transition from reactive to active defensive responding.
Methods
Rats received electrolytic lesions of LA, CE or B before AA training, or following overtraining. Rats that expressed low levels of AA performance during training received bilateral electrolytic lesions to CE to eliminate competing freezing reactions and rescue AA. AA performance and freezing were assessed.
Results
Damage to LA and B, but not CE, impaired the acquisition of AA. Performance of AA became amygdala-independent following overtraining. CE lesions abolished Pavlovian freezing and rescued instrumental AA performance in rats that expressed low levels of avoidance responses and high levels of freezing during training.
Conclusions
While the acquisition of Pavlovian fear depends on LA and CE, but not B, acquisition of instrumental AA is dependent on LA and B, but not CE. CE-dependent Pavlovian processes that control freezing can constrain avoidance behavior. Performance of well-trained AA becomes independent of all three amygdala nuclei. Thus, it appears that different output pathways of LA mediate reactive and active conditioned defensive responding.
Keywords: Amygdala, Avoidance, Freezing, Sidman, Lesion
Introduction
When faced with a threat, an animal's ability to regulate defensive behaviors is crucial for survival. Reactive defensive behaviors, such as freezing, increase the chance of survival by decreasing the odds of being detected by a predator (1,2,3). Nevertheless, when possible, active instrumental responses, such as running or hiding to avoid an aversive event, can be more effective defensive behaviors (4,5). Exerting control over environmental threats by engaging in active instrumental responding also prevents the arousal of fear responses (6,7). Reactive coping responses, while reducing the likelihood of being harmed, do not allow for control over exposure to threatening stimuli and do not eliminate fear arousal.
Excessive or prolonged fear is damaging to emotional well-being and physiological processes (8–10), and can be thought of as the primary problem for those suffering from anxiety disorders. Transitioning to active instrumental behaviors may be a more effective coping strategy, because this strategy prevents fear arousal. However, not all active coping strategies are beneficial. Avoidance responses that interfere with daily living are another defining characteristic of pathological anxiety (11,12). Thus, a major goal of anxiety therapy is to reduce unpleasant and harmful fear reactions, often by instilling an active coping strategy that is not itself, disruptive (13–16). Understanding how the brain mediates the transition from reactive to active defensive responding may therefore facilitate the development of innovative treatment options for anxiety disorders.
The amygdala is known to be crucially involved in aversive conditioning, in which reactive and active defensive behaviors are expressed as conditioned responses (17). However, research on the intra-amygdala circuits that mediate conditioning has focused on reactive defensive behaviors and the intra-amygdala circuits that mediate active defensive behaviors are poorly understood.
The principal behavioral approach to studying intra-amygdala circuits involved in reactive defensive behaviors is Pavlovian fear conditioning (18–21). Fear conditioning research suggests that the amygdala is composed of multiple functionally heterogeneous nuclei (22). The lateral (LA) and central (CE) nuclei of the amygdala mediate the acquisition and expression of reactive defensive behaviors (18,19,23–26; but see 27). LA is the main gateway for sensory inputs into the amygdala and appears to learn and store conditioned stimulus-unconditioned stimulus (CS-US) associations whereas CE is the principal output region and mainly participates by generating Pavlovian responses (28–30). On the other hand, the basal nucleus (B) is not required for fear conditioning but may play a key role in fear expression and extinction (31–33).
The amygdala also appears to contribute to the acquisition and performance of aversive instrumental behaviors such as active avoidance (AA) and punishment (17,34–41). However, most studies have not directly examined the roles of different amygdala nuclei. For example, some studies have made lesions or recorded from cells in the amygdala without distinguishing between LA and B. Given that Pavlovian conditioning is the first phase of AA, it is perhaps not surprising that the amygdala is involved (15). However, it is unlikely that the amygdala only contributes to avoidance by mediating Pavlovian conditioning since other studies have found that LA and B, but not CE, contribute to instrumental escape and punishment (31,37). Thus, one goal of the present study was to examine the roles of the LA, B and CE separately in AA.
It has often been noted that a substantial number of animals (~20%) fail to acquire AA responses (e.g. 42–44). Bolles and Popp (44) observed that “animals that flinch, freeze or develop stereotyped running reactions to the shock generally do not learn the avoidance response; those that explore, rear and attempt to climb the walls do learn”. Similarly, we have observed that animals that perform poorly in AA tasks tend to express persistent freezing responses even though freezing fails to avoid the aversive US (45). This suggests that the freezing reaction is a competing defensive behavior that can interfere with the performance of AA responses (46–51). Animals have also been bred and behaviorally characterized on the basis of good and poor performance in AA tasks, suggesting that the ability to acquire and perform AA responses is a heritable, trait-like characteristic (52–56). Because CE is critical for conditioned freezing, it is possible that dysregulation of this nucleus is responsible for the persistent freezing observed in the subset of animals that fail to acquire AA. If so, it might also be the case that CE dysregulation accounts for failure to acquire successful active coping skills in anxiety disorders.
In this set of experiments, we used a two-way Sidman AA shuttling task for two reasons. Sidman AA is behaviorally well-characterized and has been used for more than 50 years (57,58). Furthermore, because there is no explicit signal to predict the aversive US, this AA task yields a higher percentage of poor performers than signaled AA tasks. This allowed us to obtain a sufficient number of animals to study poor AA performance. We first tested the effect of damage to specific amygdala nuclei (LA, B or CE) on instrumental AA learning. Then, we examined the role of these nuclei in the performance of well-trained AA responses. Finally, we examined the role that CE-dependent freezing may play in constraining AA in poor performers.
Methods and Materials
Subjects
Male Sprague Dawley rats (Hilltop Labs, Scottdale, PA) initially weighing 250–300g were individually housed in plastic Nalgene cages on a 12h light-dark cycle in a temperature controlled room (22°C). Ad libitum food and water were available throughout the experiment. All procedures were performed in accordance with NIH's guidelines and were approved by the New York University Animal Care and Use Committee.
Surgery
Rats were anesthetized with ketamine and xylazine (i.p., 100 mg/kg; 6.0 mg/kg, Phoenix Pharmaceutical, Burlingame, CA), and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Small burr holes were drilled above the targeted brain area. A stainless steel monopolar electrode covered with epoxy (exposed tip of 500μm for CE and 250μm for LA and B lesions; model NE-300X and SNEX-300X, David Kopf Instruments) was lowered through an incision in the dura into the target site. Brain coordinates were measured from bregma (59). Lesions were created by passing current (+0.5mA) of different durations (Table 1). Sham animals underwent the same procedure, but no current was passed through the electrode. Animals recovered for 14 days following surgeries. Although neurotoxic lesions spare fibers of passage, they typically lack spatial specificity and often damage multiple nuclei. We used electrolytic lesions in this study because these are the most effective way to produce damage restricted from adjacent amygdala nuclei (15). Importantly, a recent study from our laboratory showed that combined damage to LA and B using excitotoxic lesions impairs signaled AA, suggesting that cells in LA and B, and not fibers passing through, mediate AA (45).
Table 1.
Lesion Parameters. B, basal amygdala; CE, central amygdala; LA, lateral amygdala.
| Brain Site | Anterior-Posterior (mm) | Medial-Lateral (mm) | Dorsal-Ventral (mm) | Current Duration (sec) |
|---|---|---|---|---|
| LA | −2.3 | ±5.2 | 7.8 | 8 |
| −2.8 | ±5.5 | 7.8 | 10 | |
| −3.3 | ±5.3 | 8.0 | 10 | |
| −3.8 | ±5.5 | 8.2 | 10 | |
| CE | −1.8 | ±3.8 | 8.4 | 12 |
| −2.3 | ±4.0 | 8.4 | 12 | |
| −2.8 | ±4.4 | 8.4 | 12 | |
| B | −2.1 | ±4.9 | 9.1 | 12 |
| −2.8 | ±4.9 | 9.3 | 15 | |
| −3.3 | ±5.3 | 9.2 | 15 | |
| −4.2 | ±5.3 | 9.3 | 15 |
Apparatus
Behavioral assessments occurred in two-way avoidance shuttle boxes (model H10-11RSC, Courlbourn Instruments, Whitehall, PA) within sound-attenuating chambers (model H10-24A). Shock was distributed through a metal grid floor. A houselight in each compartment illuminated the shuttle-boxes. Video-cameras located on the ceiling of each compartment were used to record sessions 1, 5, 10 & 15, as well as all test sessions. Shuttling was automatically registered by infrared beams and recorded throughout the sessions (Graphic State software, Coulbourn Instruments).
Sidman Avoidance
Rats received daily 25-minute sessions, usually 5 per week, where shuttling delayed the delivery of a scrambled footshock US (1mA; 0.5s) by 30s (“R-S” or response-shock interval). In the absence of shuttling, the US was delivered every 5s (“S-S” or shock-shock interval). Avoidance responses were defined as shuttles during the R-S interval only. All shuttles were marked by a brief feedback stimulus (houselights blink off for 0.3s). Freezing was defined as the absence of movement except that required for breathing (60) and was measured during the first 120s of the AA session (or 120s following the first footshock for session 1). For Experiment 1, rats received eight AA sessions following recovery from surgery. Pilot work determined that rats approach asymptotic performance in eight sessions with our protocol. For Experiment 2, rats received 15 AA sessions followed by surgery. After recovery, rats received another assessment of AA performance with 2 AA sessions. Note that only `good performers' (see Materials and Methods) were used to assess the role of amygdala lesions on post-overtraining performance. The other `poor performing' rats were used for the final rescue experiment. Good performers were defined as rats that had at least two consecutive sessions of 20 or more AA responses by session 10; all rats not meeting this criterion were considered poor performers. These rats were given CE or sham lesions following the 10th AA training session. After recovery, rats received two AA sessions to assess performance. Note that all lesion effects on AA responding were confirmed by analyses of shocks delivered during testing (supplementary Figure 1).
Histology
Animals were given an overdose of pentobarbital (100 mg/kg, i.p.) and perfused transcardially with 10% phosphate buffered formalin. Brains were stored in 10% phosphate-buffered formalin and 30% sucrose for at least 3 days and were then cut in 50 μm sections using a freezing microtome (every other section was collected). Nissl stains were then performed and tissue images were collected (Nikon Microphot-FXA, Melville, NY) and imported to ImageJ. Brain regions and damage were traced using a rat brain atlas (59) to quantify percent damage to regions of interest (ROIs) and adjacent areas.
Statistical Analysis
Data represent group means (± SEM) and were analyzed using GraphPad Prism (v5.0, GraphPad Software Inc., La Jolla, CA). Effects of amygdala lesions on AA training, AA performance after overtraining, and freezing comparisons between good and poor performers across AA training, were analyzed using two-way analyses of variance (ANOVAs) with repeated measures. Planned post-hoc comparisons of group means at different time points were analyzed using Bonferroni tests. Effects of amygdala lesions on AA performance following overtraining were analyzed using two-tailed paired t-tests. Finally, two-tailed unpaired t-tests were used to compare freezing and AA in CE-lesioned and Sham poor performers. Differences were considered significant if p values were less than 0.05.
Results
Histology
The percentage of combined bilateral damage for each amygdala nuclei in the experiments are shown in Table 2. A total of 111 rats were used in this study and 35 of these were part of the sham groups. The remaining 78 rats received bilateral electrolytic lesions aimed at the LA, B, or CE. Of these animals, 33 were excluded due to minimal damage to the target structure or excessive damage to adjacent amygdala nuclei. Animals were excluded from the study if they did not have at least 40% combined bilateral damage to the region of interest (ROI) or if they had more than 15% damage to the adjacent amygdala nuclei that were not targeted in the experiment. Given that damage to LA had no effect on AA performance following overtraining, we did not exclude animals from the Basal lesion group in the post-overtraining lesion experiment due to damage to LA. For the pre-training lesion experiments we used 20 sham animals, 10 for the overtraining and 5 for the poor performers experiment.
Table 2.
Mean ± SD percentage combined bilateral damage of targeted amygdala nuclei (bold) and adjacent amygdala nuclei. The minimum and maximum damage of targeted brain regions (i.e. region of interest; ROI) are shown in the Min ROI and Max ROI boxes; n refers to the number of lesioned animals included in the experiment. B, basal amygdala; CE, central amygdala; LA, lateral amygdala.
| Experiment | LA | B | CE | Min ROI | Max ROI | n | |||
|---|---|---|---|---|---|---|---|---|---|
| % | SD | % | SD | % | SD | ||||
| Pre-training LA | 65% | 16.26 | 3% | 3.27 | 2% | 2.39 | 43% | 86% | 10 |
| Pre-training B | 8% | 5.53 | 63% | 12.08 | 0% | 0.89 | 49% | 83% | 6 |
| Pre-training CE | 2% | 1.33 | 3% | 1.32 | 72% | 10.81 | 61% | 83% | 5 |
| Overtraining LA | 61% | 7.35 | 1% | 0.88 | 0% | 0.84 | 55% | 72% | 4 |
| Overtraining B | 52% | 17.62 | 72% | 16.34 | 3% | 2.29 | 47% | 93% | 8 |
| Overtraining CE | 2% | 1.72 | 2% | 1.44 | 64% | 16.74 | 44% | 83% | 5 |
| Poor Performers | 4% | 3.46 | 2% | 1.56 | 51% | 5.12 | 44% | 58% | 5 |
Pre-training LA or B lesions impair AA acquisition
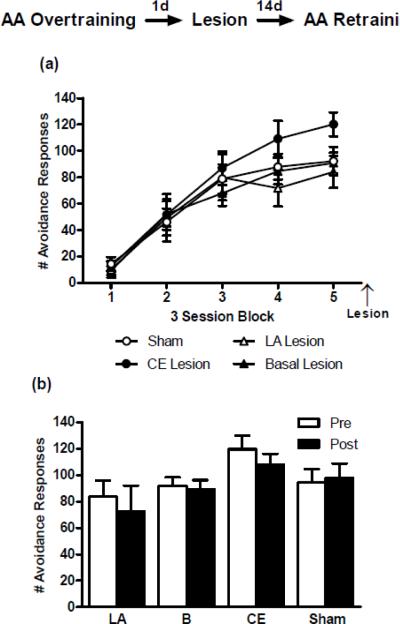
The mean number of AA responses per training block (2 sessions/block) for each lesion group is shown in Figure 1. A repeated measures ANOVA comparing AA responses throughout training showed significant effects of Group (F(3,117) = 12.39; p < 0.01), Block (F(3,117) = 14.10; p <0.01), and a the interaction (F(9,117) = 5.13; p <0.01). Bonferonni post-tests revealed that the LA- (blocks 2–4) and B-lesion (blocks 3–4) groups performed less AA responses than Shams (p values < 0.05). The CE lesion and Sham groups responded similarly throughout training, with the CE group responding slightly, but non-significantly, higher during Block 1 (p > 0.05). Together, these results show that pre-training LA or B lesions impaired AA responses, but CE lesions produced no impairment, and if anything, a slight facilitation of AA learning.
Figure 1.
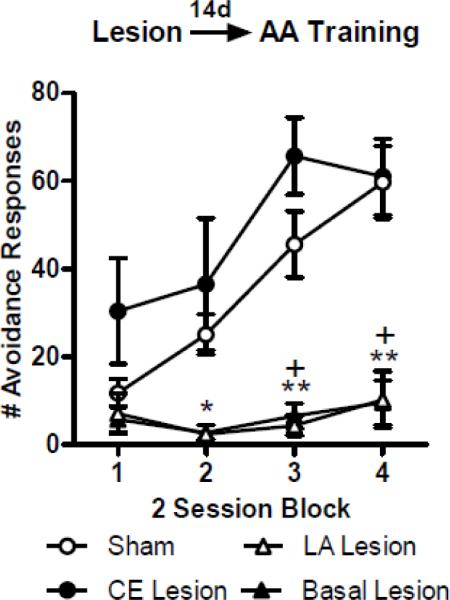
Mean ± SE number of active avoidance (AA) responses across training in rats that recieved pre-training sham surgeries (n = 20) or bilateral electrolytic lesion aimed at lateral amygdala (LA; n = 10), basal amygdala (B; n = 6) or central amygdala (CE; n = 5). Pre-training damage to the LA or B severely impaired acquisition of AA responses. Damage to the CE showed a trend towards facilitation of AA learning during block 1. (*) LA vs Sham = p< 0.05; (+) B vs Sham = p < 0.01.
Lesions of LA, B or CE have no effect on overtrained AA performance
To test the effects of amygdala damage on performance of well-trained AA responses, we overtrained rats for 15 sessions. After overtraining, the rats were assigned to one of four lesion groups (LA, B, CE or Sham) matched on their level of AA performance. Poor performing rats were excluded from this study (see Materials and Methods). Mean number of AA responses per block (3 sessions/block) shown in Figure 2a. A two-way ANOVA revealed no main effect for Group (F(3,92) = 0.57; p = 0.64) and no significant interaction (F(12,92) = 1.12; p = 0.35), but there was an effect for Session (F(4,92) = 80.55; p< 0.01) given that animals were selected for having acquired the task across training. Although the groups were perfectly matched pre-lesion, exclusion of rats following lesion verification resulted in slight, but non-significant, differences in final pre-lesion AA performance levels. Therefore, we compared AA performance post-lesion (2 AA sessions) to the same group's pre-lesion performance (final 2 training sessions) with paired two-tailed t-tests (Figure 2b). None of the lesions had an effect on AA performance, B-lesion (t(7) = 0.26; p = 0.80); LA (t(3) = 0.55; p = 0.62); CE-lesion (t(4) = 1.99; p = 0.12); Sham (t(9) = 0.41; p = 0.69). Therefore, if the AA response is overtrained, damage to LA, CE or B has no effect on AA performance.
Figure 2.
(a) Mean ± SE number of active avoidance (AA) responses across overtraining in rats that received post-overtraining sham surgeries (n = 10) or bilateral electrolytic lesions aimed at lateral amygdala (LA; n = 4), basal amygdala (B; n = 8) or central amygdala (CE; n = 5). Each block is composed of three training sessions for a total of 15 training sessions. (b) Mean ± SE number of AA responses in the last 2 overtraining sessions compared to the first 2 post-lesion retraining sessions. Post-overtraining lesions had no effect on the number of AA responses during retraining.
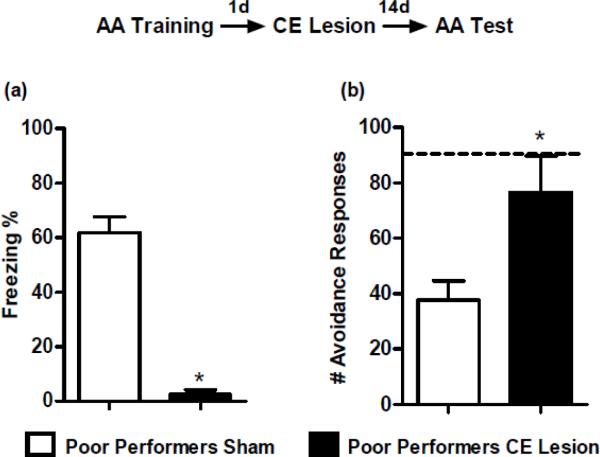
CE lesion rescues AA in poor performing rats
A comparison of the mean number of AA responses between poor and good performers is illustrated in Figure 3a. To examine the possible interfering effect of CE-dependent freezing on AA poor performers, we first compared the levels of freezing between poor and good performers during sessions 1, 5, and 10 (Figure 3b). A two-way ANOVA showed that poor performers froze more than good performers (F(1,56) = 12.93; p < 0.01). Furthermore, a significant interaction (F(2,56) = 3.34; p < 0.05) revealed that as training progresses poor performers freeze more; whereas good performers freeze less (session 10: p < 0.01).
Figure 3.
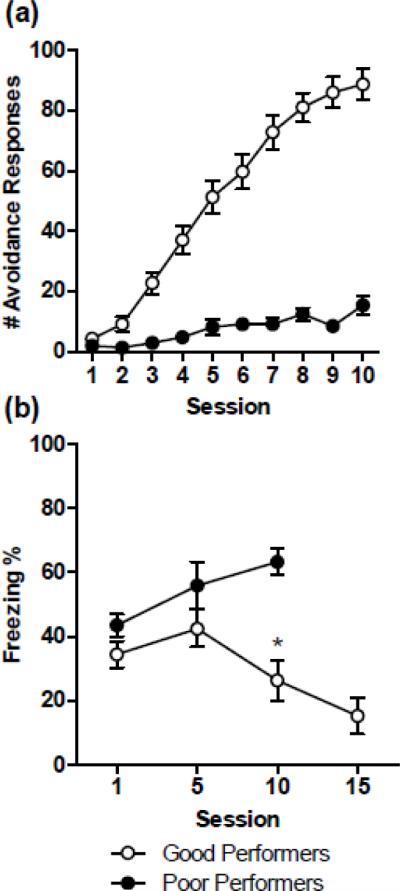
(a) Mean ± SE number of active avoidance (AA) responses in good (n = 38) and poor (n = 19) performers across training. Poor performers were animals from the overtraining experiment that did not reach the learning criteria of at least two consecutive sessions of 20 or more AA responses. (b) Mean ± SE percentage freezing at the start of sessions 1, 5, 10 and 15. Freezing levels of good performers decrease as AA training progresses, while freezing remains high in poor performers. (*) session 10: p < 0.01.
Given that poor performers showed higher levels of freezing but lower levels of AA responses, we tested if damage to CE would decrease freezing and allow for the acquisition of AA responses across retraining. An unpaired t-test comparing poor performers with CE or Sham lesions during the first retraining session revealed that CE damage abolished freezing (t(8)= 9.51; p < 0.01; Figure 4a). CE-lesioned animals also showed significantly increased AA performance compared to Sham animals (t(8)= 2.72; p< 0.05; Figure 4b). Unexpectedly, the CE-lesioned group showed AA responding comparable to the asymptotic performance of good perfomers (dashed line in figure) immediately during the first retraining session, and maintained this performance during the second session. Together, these results suggest that damage to the CE abolishes freezing defensive behavior in this task and facilitates AA responses in poor performers.
Figure 4.
(a) Mean ± SE percentage freezing during the first retraining session of poor performers that received sham surgeries (n = 5) or bilateral electrolytic central amygdala (CE) lesions (n = 5). CE damage in poor performers abolished freezing reactions compared to sham. (b) Mean ± SE number of active avoidance (AA) responses for poor performers during the first two retraining sessions. CE-lesioned poor performers showed significantly higher levels of AA responses compared to the sham. In fact, the CE lesion increased AA responses to asymptotic levels of performance by the first retraining session. Dashed line represents the mean for good performers at training day 11, which corresponds to the first retraining session in poor performers.
Discussion
The present experiments expand our understanding of amygdala function by identifying roles for specific amygdala nuclei in different aspects of instrumental AA. The main conclusions that we draw are: 1) LA and B are critical for the acquisition of instrumental AA, but CE is not. 2) Well-trained AA responses become LA- and B-independent, and continue to be CE-independent. 3) The CE can constrain AA, possibly by inducing Pavlovian responses such as freezing that compete with AA performance. In the following, we will discuss each of these points in more detail.
LA and B are necessary for AA acquisition
Early studies employing varying degrees of amygdala damage produced inconclusive results; some studies reported impaired AA with amygdala lesions, some reported no effect, and still others reported facilitation (61–63; reviewed in: 34,35). Other studies though, found that the amygdala is critical for AA learning (17,36,38,39,41,45). Some of the discrepancy may be related to the differential involvement of various amygdala nuclei. Indeed, our results highlight the importance of studying the LA, B and CE as functionally heterogeneous nuclei. Thus, we found that damage to LA and B, but not CE, disrupted AA acquisition (Figure 1). This agrees with our previous finding on instrumental escape behavior and contrasts with Pavlovian fear conditioning findings (24,31,32). Selective damage to the CE, rather than disrupting learning, actually tended to facilitate acquisition of AA responses in the early stages of training when freezing normally dominates. Similar facilitation has been observed with damage to the septum or hippocampus, and has been hypothesized to occur due to a decrease in competing freezing reactions (61,64).
Our findings are consistent with the hypothesis that different intra-amygdala circuits that originate in the LA underlie emotional reactions and actions (15,31,45). According to this hypothesis, LA is critical for learning and storing Pavlovian CS-US associations and different outputs of LA (CE and B) use this association to generate defensive reactions and actions. Specifically, connections from LA to CE are required for Pavlovian reactions like freezing, while connections from LA to B mediate fear-motivated instrumental actions (e.g. avoidance, conditioned punishment, and escape from fear; 13,15,31,65; but see 37,66).
Amygdala involvement in AA is temporary if training is continued
Although the amygdala is critical for the learning (Figure 1) and early expression of AA responses (45), its involvement is transient when training continues beyond the point of asymptotic performance. Specifically, AA responses become independent of the LA and B following overtraining (Figure 2). These results are consistent with a study showing that muscimol inactivation aimed at B has no effect on AA performance following overtraining (39). Furthermore, training-induced neural activity decreases during overtraining, suggesting a disengagement of B as responses become habitual (36). One limitation of the inactivation study is that it is not possible to determine to what degree muscimol may have spread to the LA; thus, because we selectively damaged the LA following overtraining, our study conclusively shows that AA performance following overtraining also becomes independent of the LA. This disengagement of LA and B during overtraining represents another marked difference in how the amygdala processes information about Pavlovian versus instrumental tasks: combined damage to LA and B impairs the expression of fear conditioned responses even after overtraining (25,67)*. This is consistent with the notion that aversive Pavlovian CS-US associations are normally stored in LA, whereas aversive instrumental responses initially use this information for learning (conditioned negative reinforcement) but the memory is ultimately stored outside the amygdala.
CE-mediated Pavlovian processes constrain AA
Bilateral damage to CE significantly facilitated AA performance in poor performers. Following overtraining, CE lesions did not facilitate AA performance in good performers, although it showed this trend in the early stages of the pre-training experiment. This raises the interesting possibility that CE damage only facilitates AA performance when freezing would otherwise interfere with performance. Note that good performing rats showed minimal levels of freezing at the end of overtraining (Figure 3). An alternative interpretation is that with overtraining, AA performance is maximal and CE damage can produce no further facilitation. However, the rescue effect on AA responses, along with the dramatic decrease in freezing levels observed in CE-lesioned poor performers, provides strong support for the hypothesis that CE constrains AA performance when freezing and avoidance compete as defensive behaviors. Finally, the lack of a role for CE in instrumental AA at any stage marks an interesting contrast with the role of CE in Pavlovian fear reactions, where it is required both for acquisition and expression of freezing following overtraining (26,67).
At first glance, the deficit of AA in poor performers may seem like a learning impairment (Figure 3a), but our data are more consistent with a performance deficit. First, if these animals had not acquired the AA response during training, then their performance should have progressively improved during retraining, similar to the CE pre-training lesion group (Figure 1). However, damage to CE enhanced AA performance to asymptotic levels on the first day of retraining, and severely impaired Pavlovian freezing. This suggests that these animals had acquired the AA response but were unable to perform, possibly due to interference from freezing. Importantly, although poor performers showed drastically reduced responding across the 10 training sessions compared to good performers, they did successfully avoid approximately 10 times per session for the last 5 sessions. So these animals apparently had enough learning trials (where they performed the AA response) to acquire the instrumental association, even though they failed to show much memory expression before the CE lesions. Second, researchers that have bred lines of rats for their poor performance in AA tasks have shown that these animals are not impaired in learning other aversive conditioning tasks. In fact, these animals generally show enhanced acquisition of inhibitory avoidance (43), which can also be thought of as a reactive coping response. Therefore, poor performers may instead be biased towards reactive defensive behaviors which interfere with the development of AA responses. Evidence for such interference comes from a number of studies. For example, Blanchard and Blanchard (47) showed that immobility is negatively correlated with the level of AA responses in a circular runway AA task. Thus, freezing interferes with the development of AA responses and manipulations that impair freezing are expected to facilitate AA performance (46–48,69; but see 70).
Outstanding issues
The bed nucleus of the stria terminalis (BNST) and the periaqueductal gray (PAG) contribute to freezing expression and may play a role in the CE-lesion rescue effect (71–76). BNST receives projections from B that are likely damaged with electrolytic CE lesions. Future studies will evaluate excitotoxic CE lesions in poor performers to determine if neurons in CE mediate the constraining influence on AA performance or whether fibers passing through CE to other brain regions mediate this effect. It will be also be important to examine the effects of direct damage to BNST or PAG on AA in poor performers. Nevertheless, inactivation of CE using small infusions of muscimol, which should have no effect on amygdala projections to BNST or PAG, inhibits the expression of fear-conditioned freezing (26). This suggests that damage to CE is sufficient to decrease freezing, and possibly allow for the AA rescue effect.. Finally, CE lesions have been reported to induce hyperactivity (77,78). Thus, one could argue that our CE lesions did not rescue true avoidance behavior, but rather caused the animals to run around more resulting in apparent avoidance responses. However,. if hyperactivity alone were enough to result in apparent avoidance, then our pre-training CE lesions should have produced much higher responding during the first training session (Figure 1). Also, CE lesions in the poor performers resulted in AA responding nearly identical in magnitude to the asymptotic performance of good performers, which may be unlikely if hyperactivity alone produced the effect. Nevertheless, hyperactivity could be a contributing factor to the improved performance observed with CE lesions.
Summary
LA and B are required for the initial acquisition of instrumental avoidance responses but play a transient role in the performance of AA responses. In contrast, CE is not required but can constrain AA, most likely because of its role in mediating competing Pavlovian responses such as freezing. These findings are in agreement with the hypothesis that the LA and B mediate fear motivated actions while the LA and CE mediate fear reactions (31).
Pathological anxiety affects a large portion of the population and exacts significant emotional and financial costs (12,79). Effective cures for pathological anxiety have been elusive, with many cognitive-behavioral and pharmacological interventions providing only temporary recovery or suppression of symptoms (80). Although avoidance is a hallmark of many anxiety disorders, it is likely to be so prevalent precisely because it is an effective means of coping with intense fear. Acquisition of instrumental escape and avoidance prevents Pavlovian fear reactions associated with the subjective state of fear and gives the subject control in dangerous environments (6,7,81). Importantly, this effective suppression of fear is long lasting. Thus, we and others have suggested that training of active instrumental coping responses that do not interfere with daily living, under the guidance of a therapist, may be an ideal strategy for treating pathological anxiety (13–16,82). This training may be enhanced pharmacologically, provided that we understand the underlying brain mechanisms and appropriately target them. The present studies identify two small amygdala nuclei, LA and B, as critical components of AA. They also implicate CE as a constraint on the transition from reactive to active responding to fear-eliciting cues. Future studies will attempt to facilitate LA and B processes important for AA acquisition while also blocking constraining CE processes in the hopes of identifying novel strategies for treating pathological anxiety.
Supplementary Material
Acknowledgements
This research was supported by the National Institute of Mental Health (NIMH) Grant F31MH086294 to Gabriel Lázaro-Muñoz; NIMH Grant RO1MH038774 to Joseph E. LeDoux; and NIMH Grant F32MH077458 to Christopher K. Cain. We are grateful to June-Seek Choi, Karim Nader, Bruce Overmier, and Rick Servatius for helpful comments and to Claudia Farb for assistance with histology and lesion quantification.
Footnotes
Financial Disclosures none
Rats with combined damage to LA and B prior to overtraining Pavlovian fear conditioning eventually acquire fear responses, suggesting that other brain regions such as CE are capable of encoding Pavlovian fear conditioning (27,67). However, Pavlovian fear memories encoded in animals with combined damage to LA and B are acquired more slowly and are more susceptible to the deteriorating effects of time on fear memory (68).
References
- 1.Bolles RC, Collier AC. The effect of predictive cues on freezing in rats. Animal Learning and Behavior. 1976;4(1):6–8. [Google Scholar]
- 2.Hirsh SM, Bolles RC. On the ability of prey to recognize predators. Zeitschrift fur Tierpsychologie. 1980;54:71–84. [Google Scholar]
- 3.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Bleecher MD, editors. Evolution and learning. Erlbaum; Hillsdale, NJ: 1988. pp. 185–211. [Google Scholar]
- 4.Bolles RC. Species-specific defense reactions and avoidance learning. Psychol. Rev. 1970;77:32–48. [Google Scholar]
- 5.Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behaviors of laboratory and wild Rattus norvegicus. J Comp Psychol. 1986;100(2):101–7. [PubMed] [Google Scholar]
- 6.Solomon RL, Wynne LC. Traumatic avoidance learning: The principles of anxiety conservation and partial irreversibility. Psychol Rev. 1954;61:353–85. doi: 10.1037/h0054540. [DOI] [PubMed] [Google Scholar]
- 7.Cain CK, LeDoux JE. Escape from fear: A detailed behavioral analysis of two atypical responses reinforced by CS termination. J Exp Psych: Animal Behav Process. 2007;33:451–63. doi: 10.1037/0097-7403.33.4.451. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues SM, Ledoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 9.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 10.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 12.NIMH . Anxiety Disorders. National Institute of Mental Health, National Institutes of Health, US Department of Health and Human Services; Bethesda, MD: 2007. [Google Scholar]
- 13.LeDoux JE, Gorman JM. A call to action: Overcoming anxiety through active coping. Amer J Psychiatry. 2001;158:1953–55. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- 14.van der Kolk BA. Clinical implications of neuroscience research in PTSD. Annals New York Acad Sciences. 2006;1071:277–293. doi: 10.1196/annals.1364.022. [DOI] [PubMed] [Google Scholar]
- 15.Cain CK, LeDoux JE. Brain mechanisms of Pavlovian and instrumental aversive conditioning. In: Nutt DJ, Blanchard RJ, Blanchard DC, Griebel G, editors. Handbook of Anxiety and Fear. Volume 17. Elsevier Academic Press; Amsterdam: 2008a. pp. 103–125. [Google Scholar]
- 16.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25(7–8):669–78. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard RJ, Blanchard DC. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81(2):281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 18.Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala. Oxford University Press; Oxford, UK: 2000. pp. 213–288. [Google Scholar]
- 19.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 20.Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–34. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 21.Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- 22.LeDoux JE. The amygdala. Current Biology. 2007;17(20):868–79. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 24.Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. Neuroscience. 2004;24:3810–5. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neuroscience. 2006;26:12387–96. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neuroscience. 1999;19:8696–703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neuroscience. 1990;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neuroscience. 1995;15(3):2301–11. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paré D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92(1):1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 31.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 32.Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neuroscience. 2005;25(42):9680–5. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–6. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 34.Goddard GV. Functions of the amygdala. Psychol Rev. 1964;62:89–109. doi: 10.1037/h0044853. [DOI] [PubMed] [Google Scholar]
- 35.Sarter MF, Markowitsch HJ. Involvement of the amygdala in learning and memory: a critical review, with emphasis on anatomical relations. Behav Neurosci. 1985;99(2):342–80. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- 36.Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–6. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- 37.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 38.Poremba A, Gabriel M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. J Neuroscience. 1997;17:5237–44. doi: 10.1523/JNEUROSCI.17-13-05237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poremba A, Gabriel M. Amygdala neurons mediate acquisition but not maintenance of instrumental avoidance behavior in rabbits. J Neuroscience. 1999;19:9635–41. doi: 10.1523/JNEUROSCI.19-21-09635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holahan MR, White NM. Amygdala inactivation blocks expression of conditioned memory modulation and the promotion of avoidance and freezing. Behav Neurosci. 2004;118(1):24–35. doi: 10.1037/0735-7044.118.1.24. [DOI] [PubMed] [Google Scholar]
- 41.Rorick-Kehn LM, Steinmetz JE. Amygdalar unit activity during three learning tasks: eyeblink classical conditioning, Pavlovian fear conditioning, and signaled avoidance conditioning. Behav Neurosci. 2005;119(5):1254–73. doi: 10.1037/0735-7044.119.5.1254. [DOI] [PubMed] [Google Scholar]
- 42.Brush FR. On the difference between animals that learn and do not learn to avoid electric shock. Psychonomic Science. 1966;5:123–124. [Google Scholar]
- 43.Brush FR. Selection for differences in avoidance learning: The Syracuse strains differ in anxiety, not learning ability. Behav Genetics. 2003;33:677–96. doi: 10.1023/a:1026135231594. [DOI] [PubMed] [Google Scholar]
- 44.Bolles RC, Popp RJ., Jr. Parameters affecting the acquisition of Sidman avoidance. J Exp Anal Behav. 1964;7:315–21. doi: 10.1901/jeab.1964.7-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi J, LeDoux JE. 2003 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2003. Lesions of the lateral/basal but not the central nucleus of the amygdala impair post-training performance of fear-induced 2-way active avoidance signaled by a conditioned stimulus. Program No. 623.5. Online. [Google Scholar]
- 46.Schwartzbaum JS, Green RH, Beatty WW, Thompson JB. Acquisition of avoidance behavior following septal lesions in the rats. J Comp Physiol Psychol. 1967;63(1):95–104. doi: 10.1037/h0024145. [DOI] [PubMed] [Google Scholar]
- 47.Blanchard RJ, Blanchard DC. Reactive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969a;68:129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 48.Blanchard RJ, Blanchard DC, Fial RA. Hippocampal lesions in rats and their effect on activity, avoidance, and aggression. J Comp Physiol Psych. 1970;71(1):92–102. doi: 10.1037/h0028958. [DOI] [PubMed] [Google Scholar]
- 49.Anisman H, Waller TG. Facilitative and disruptive effects of prior exposure to shock on subsequent avoidance performance. J Comp Physiol Psychol. 1972;78(1):113–22. doi: 10.1037/h0032299. [DOI] [PubMed] [Google Scholar]
- 50.Overmier JB, Seligman ME. Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 1967;63(1):28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- 51.Overmier JB. Interference with avoidance behavior: failure to escape traumatic shock. J Exp Psychol. 1968;78:340–43. doi: 10.1037/h0026365. [DOI] [PubMed] [Google Scholar]
- 52.Bignami G. Selection for high rates and low rates of avoidance conditioning in the rat. Anim Behav. 1965;13:221–7. doi: 10.1016/0003-3472(65)90038-2. [DOI] [PubMed] [Google Scholar]
- 53.Brush FR, Froehlich JC, Sakellaris PC. Genetic selection for avoidance behavior in the rat. Behav Genet. 1979;9:309–16. doi: 10.1007/BF01068209. [DOI] [PubMed] [Google Scholar]
- 54.Ryzhova LIu, Kulagin DA, Lopatina NG. Correlated variability in motor activity and emotionality in selecting rats for high and low values of active avoidance conditioned reflexes. Genetika. 1983;19:121–5. [PubMed] [Google Scholar]
- 55.Blizard DA, Adams N. The Maudsley reactive and nonreactive strains: a new perspective. Behav Genet. 2002;32(5):277–99. doi: 10.1023/a:1020206120248. [DOI] [PubMed] [Google Scholar]
- 56.Servatius RJ, Jiao X, Beck KD, Pang KC, Minor TR. Rapid avoidance acquistion in Wistar-Kyoto rats. Behav Brain Res. 2008;192(2):191–7. doi: 10.1016/j.bbr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Sidman M. Avoidance conditioning with brief shock and no exteroceptive warning signal. Science. 1953a;118:157–8. doi: 10.1126/science.118.3058.157. [DOI] [PubMed] [Google Scholar]
- 58.Sidman M. Two temporal parameters of the maintenance of avoidance behavior by the white rat. J Comp Physiol Pshycol. 1953b;46:253–61. doi: 10.1037/h0060730. [DOI] [PubMed] [Google Scholar]
- 59.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th edition Elsevier Academic Press; 2005. [Google Scholar]
- 60.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969b;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 61.King FA. Effects of septal and amygdaloid lesions on emotional behavior and conditioned avoidance responses in the rat. J Nerv Ment Dis. 1958;126(1):57–63. doi: 10.1097/00005053-195801000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Grossman SP, Grossman L, Walsh L. Functional organization of the rat amygdala with respect to avoidance behavior. J Comp Physiol Psychol. 1975;88(2):829–850. doi: 10.1037/h0076396. [DOI] [PubMed] [Google Scholar]
- 63.Roozendaal B, Koolhaas JM, Bohus B. The central amygala is involved in conditioning but not retention of active and reactive shock avoidance in male rats. Behav Neural Biology. 1993;59:143–49. doi: 10.1016/0163-1047(93)90873-g. [DOI] [PubMed] [Google Scholar]
- 64.Isaacson RL, Douglas RJ, Moore RY. The effect of radical hippocampal ablation on acquisition of avoidance response. J Comp Physiol Psychol. 1961;54(6):625–28. [Google Scholar]
- 65.Cain CK, LeDoux JE. Emotional Processing and Motivation: In Search of Brain Mechanisms. In: Elliot AJ, editor. Handbook of Approach and Avoidance Motivation. Psychology Press, Taylor and Francis Group, LLC; New York: 2008b. pp. 17–34. [Google Scholar]
- 66.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in Neuroscience. 2006;39(5):272–79. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Memory. 2007;14:634–44. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poulus AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistence of fear memory across time requires the basolateral amygdala complex. PNAS. 2009;106(28):11737–41. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krieckhaus EE, Simmons HJ, Thomas GJ, Kenyon J. Septal lesions enhance shock avoidance behavior in the rat. Exp Neurol. 1964;9:107–13. doi: 10.1016/0014-4886(64)90010-x. [DOI] [PubMed] [Google Scholar]
- 70.de Oca BM, Minor TR, Fanselow MS. Brief flight to a familiar enclosure in response to a conditional stimulus in rats. J Gen Psychol. 2007;134(2):153–72. doi: 10.3200/GENP.134.2.153-172. [DOI] [PubMed] [Google Scholar]
- 71.Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 72.Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Oxford University Press; Oxford, UK: 2000. pp. 31–116. [Google Scholar]
- 73.Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, LeDoux JE. Lesions of the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128(1):7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 74.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neuroscience. 1988;8(7):2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neuroscience. 1993;107(6):1093–8. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 76.Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn Memory. 1999;6(5):491–9. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jallestad FK, Markowska A, Bakke HK. Behavioral effects after ibotenic acid, 6-OHDA and electrolytic lesions in the central amygdala nucleus of the rat. Physiol Behav. 1986;37:855–62. [PubMed] [Google Scholar]
- 78.Werka T, Zielinski K. CS modality transfer of two-way avoidance in rats with central and basolateral amygdala lesions. Behav Brain Res. 1998;93:11–24. doi: 10.1016/s0166-4328(97)00131-9. [DOI] [PubMed] [Google Scholar]
- 79.Kessler RC, Heeringa S, Lakoma MD, Petukhova M, Rupp AE, Schoenbaum M, et al. The individual-level and societal-level effects of mental disorders on earnings in the United States: Results from the National Comorbidity Survey Replication. Amer J Psychiatry. 2008;165(6):703–11. doi: 10.1176/appi.ajp.2008.08010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cloitre M. Effective psychotherapies for posttraumatic stress disorder: a review and critique. CNS Spectrums. 2009;14(1):32–43. [PubMed] [Google Scholar]
- 81.Kamin LJ, Brimer CJ, Black AH. Conditioned suppression as a monitor of fear of the CS during the course of avoidance training. J Comp Physiol Psychol. 1962;56:497–501. doi: 10.1037/h0047966. [DOI] [PubMed] [Google Scholar]
- 82.Stovall-McClough KC, Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. J Consult Clin Psychol. 2006;74(2):219–28. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.