Abstract
Background/Aims
Evidence suggests that patients with dementia with Lewy bodies (DLB) may have more nocturnal sleep disturbance than patients with Alzheimer's disease (AD). We sought to confirm such observations using a large, prospectively collected, standardized, multicenter-derived database, i.e. the National Alzheimer's Coordinating Center Uniform Data Set.
Methods
Nocturnal sleep disturbance (NSD) data, as characterized by the Neuropsychiatric Inventory Questionnaire (NPI-Q), were derived from 4,531 patients collected between September 2005 and November 2008 from 32 National Institute on Aging participating AD centers. Patient and informant characteristics were compared between those with and without NSD by dementia diagnosis (DLB and probable AD). Finally, a logistic regression model was created to quantify the association between NSD status and diagnosis while adjusting for these patient/informant characteristics, as well as center.
Results
NSD was more frequent in clinically diagnosed DLB relative to clinically diagnosed AD (odds ratio = 2.93, 95% confidence interval = 2.22–3.86). These results were independent from the gender of the patient or informant, whether the informant lived with the patient, and other patient characteristics, such as dementia severity, depressive symptoms, and NPI-Q-derived measures of hallucinations, delusions, agitation and apathy. In AD, but not DLB, patients, NSD was associated with more advanced disease. Comorbidity of NSD with hallucinations, agitation and apathy was higher in DLB than in AD. There was also evidence that the percentage of DLB cases with NSD showed wide variation across centers.
Conclusion
As defined by the NPI-Q, endorsement of the nocturnal behavior item by informants is more likely in patients with DLB when compared to AD, even after the adjustment of key patient/informant characteristics.
Key Words: Dementia with Lewy bodies, Alzheimer's disease, Sleep, Neuropsychiatric Inventory Questionnaire
Introduction
Sleep disturbance occurs in many forms of dementia; however, it is possible that certain forms of dementia may be relatively more likely to be associated with disturbed sleep. For example, the Third International Consensus Conference definition of dementia with Lewy bodies (DLB) [1] indicates that rapid eye movement (REM) sleep behavior disorder (RBD) may be important in defining this condition. Although patients with Alzheimer's disease (AD) have also been known to have disrupted sleep [2] and may experience changes in circadian rhythms [2,3], few studies have compared the presence of disturbed sleep between these two neurodegenerative conditions. In this study, we report on a comparison of informant-reported nighttime behaviors in secondary data analyses of a large national database of well-characterized patients with AD and DLB using the National Institute on Aging (NIA) Uniform Data Set (UDS).
Methods
Data in this study were derived from the UDS covering the period of September 2005 to November 2008 maintained by the National Alzheimer's Coordinating Center (NACC) located at the University of Washington under the auspices of the NIA. Development of the UDS has been described elsewhere [4,5] but, in brief, consists of a deidentified, web-based, relational database, which serves as a repository for data collected by the 32 NIA-funded AD centers. Access to the NACC UDS database is available to all interested researchers associated with an NIA-sponsored AD research center via formal request to the NACC Steering Committee through the NACC website (https://www.alz.washington.edu; see Beekly et al. [4] and Lau et al. [6] for further information).
All patients or their proxies provided informed consent through an Institutional Review Board-approved protocol at each site. All patients entered in the UDS received a physician-based clinical diagnosis. Autopsy verification is pursued in many centers as a part of the NACC data collection but was not incorporated into the analyses presented here. As is customary of patients encountered in clinics focusing on dementias, a broad range of diagnoses are considered. In this study, analyses were limited to cases with a diagnosis of DLB or probable AD. Clinical diagnosis for each of these conditions followed consensus-based definitions and criteria [1,7,8]. Additional data employed in the study were patient and informant demographics (e.g. gender, age, and education), the 15-item form of the Geriatric Depression Scale (GDS) [9] and the Mini-Mental State Exam [10]. The Clinical Dementia Rating (CDR) [11] was used to rate the severity of each patient's dementia, calculated as the sum of boxes (SOB). Finally, we relied upon an informant-derived measure dealing with behavioral syndromes common in dementia patients, i.e. the Neuropsychiatric Inventory Questionnaire (NPI-Q) [12,13], which has been validated in AD patients, though not necessarily DLB patients [13]. The NPI-Q requires that the informant report on the patient's behaviors during the preceding month, which also entails severity, and is included as a part of the UDS. As described on the NACC website, one of the aims of the UDS is to ensure high-quality assurance for all data collected, and to that end, the NPI-Q is completed by a trained health professional via informant interview. In some cases, adult children of the patients served as informants. We focused on several specific items on the NPI-Q, including the presence or absence of agitation/aggression, hallucinations, delusions, nighttime behaviors, apathy, and depressed mood. The nighttime behavior item was: ‘Does the patient awaken you at night, rise too early in the morning, or take excessive naps during the day?’, which could be answered with ‘yes’ or ‘no’ (see Cummings et al. [12] for specific wording of other NPI-Q items). Informant responses to this item were categorized as positive or negative for nighttime sleep disturbance (NSD). Although clinical diagnoses in the UDS are based on expert consensus at each participating center, the possibility exists that information related to sleep gathered on the NPI-Q may also have been used diagnostically at some centers.
Statistical Analyses
Descriptive statistics were calculated for patient and informant demographics and neurobehavioral instruments (e.g. Mini-Mental State Exam, NPI-Q, GDS) by diagnosis and NSD status. Continuous variables were summarized by means ± 1 standard deviation and categorical variables by percentages. Comparisons within diagnosis groups (probable AD and DLB) by NSD status were performed via the Wilcoxon rank sum or Pearson χ2 tests for continuous and categorical variables, respectively. The unadjusted p values from these comparisons were calculated, but the Bonferroni adjustment to the type I error (0.05/34 = 0.0015) may be applied to account for multiple comparisons.
To quantify the association between diagnosis and NSD, a multivariate logistic regression model was developed using data from all 32 centers. This model also accounted for patient characteristics (age, gender, race, marital status), informant gender, NPI-Q items (depression, hallucinations, delusions, agitation, apathy), and CDR SOB. Because it was of interest to investigate center differences, center ID was included as an adjustment variable and not accounted for as a random effect. All covariates, except CDR SOB, were categorical and modeled as a series of binary variables. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to summarize the model. The robustness of these estimates was assessed by refitting the model on data derived from patients that were living with others. All analyses were performed using R version 2.11 [14].
Results
As of November 1, 2008, 4,444 patients with a primary diagnosis of probable AD and 354 patients with a primary diagnosis of DLB were available for analysis. Of these patients, 4,531 (94%) had complete NPI-Q data and served as the basis for the current work. DLB (4.2%) and AD (5.7%) patients did not differ in the proportions of missing NPI-Q data (χ2 = 1.29, p = 0.26, d.f. = 1). However, those with missing NPI-Q data were significantly older (78.8 ± 9.7 vs. 76.1 ± 9.3 years, t = 4.60, p < 0.0001, d.f. = 4,796) and more likely to be women (62.6 vs. 37.5%, χ2 = 6.30, p = 0.01, d.f. = 1). Among those with complete NPI-Q data, there were 4,192 AD (1,810 men and 2,382 women) and 339 DLB (244 men and 95 women) patients, respectively. DLB patients were more likely to be men (72.0% of DLB patients were male, whereas only 43.2% of AD patients were male, χ2 = 104.96, p < 0.0001, d.f. = 1). AD patients were significantly older than DLB patients (76.2 ± 9.4 vs. 74.0 ± 7.8 years, t = 4.20, p < 0.0001, d.f. = 4,529). The presence of NSD was significantly higher among DLB patients relative to probable AD patients (63.1 vs. 26.6%, χ2 = 201.9, d.f. = 1, p < 0.0001).
Table 1 compares patient and informant characteristics for patients with and without NSD by diagnosis. Results suggest that among probable AD patients, those with NSD were more likely to be older and black, whereas among DLB patients, those with NSD were more likely to be male and married. Few other patient or informant characteristics appeared to distinguish diagnostic groups having or not having NSD.
Table 1.
Patient and informant characteristics of the NACC UDS database by diagnosis and NPI-Q-assessed NSD
| DLB (n = 339) |
Probable AD (n = 4,192) |
|||||||
|---|---|---|---|---|---|---|---|---|
| without NSD (n = 214) | with NSD (n = 125) | test statistic | p value | without NSD (n = 3,077) | with NSD (n = 1,115) | test statistic | p value | |
| Patient characteristics | ||||||||
| Age group | χ26 = 9.8 | 0.133 | χ26 = 13.4 | 0.038 | ||||
| <60 | 4 | 5 | 6 | 6 | ||||
| 60–64 | 10 | 7 | 6 | 5 | ||||
| 65–69 | 15 | 13 | 9 | 8 | ||||
| 70–74 | 15 | 29 | 16 | 14 | ||||
| 75–79 | 28 | 23 | 23 | 24 | ||||
| 80–84 | 17 | 15 | 23 | 22 | ||||
| >84 | 10 | 8 | 17 | 21 | ||||
| Gender | χ21 = 5.1 | 0.025 | χ21 = 3.5 | 0.061 | ||||
| Male | 65 | 76 | 42 | 46 | ||||
| Female | 35 | 24 | 58 | 54 | ||||
| Education | χ23 = 4.6 | 0.205 | χ23 = 4.1 | 0.253 | ||||
| <High school | 7 | 8 | 10 | 12 | ||||
| High school | 37 | 26 | 31 | 32 | ||||
| College | 30 | 37 | 38 | 35 | ||||
| Graduate | 26 | 29 | 21 | 21 | ||||
| Race/ethnicity | χ23 = 2.4 | 0.493 | χ23 = 59 | <0.001 | ||||
| Non-Hispanic White | 88 | 90 | 78 | 69 | ||||
| Non-Hispanic Black | 4 | 6 | 11 | 20 | ||||
| Non-Hispanic other | 2 | 1 | 2 | 3 | ||||
| Hispanic | 6 | 3 | 8 | 8 | ||||
| Living situation | χ21 = 10.5 | 0.001 | χ21 = 5.1 | 0.024 | ||||
| Alone | 10 | 2 | 15 | 13 | ||||
| With others | 90 | 98 | 85 | 87 | ||||
| Marital status | χ22 = 5.8 | 0.056 | χ22 = 10 | 0.007 | ||||
| Married/living with partner | 76 | 86 | 66 | 61 | ||||
| Not currently married | 22 | 13 | 31 | 36 | ||||
| Never married/other | 2 | 1 | 3 | 2 | ||||
| Informant characteristics | ||||||||
| Relationship with patient | χ21 = 3.9 | 0.047 | χ21 = 5.3 | 0.021 | ||||
| Spouse/partner | 69 | 79 | 58 | 54 | ||||
| Non-spouse/partner | 31 | 21 | 42 | 46 | ||||
| Living with patient | χ21 = 6.3 | 0.012 | χ21 = 3.5 | 0.060 | ||||
| No | 26 | 15 | 32 | 29 | ||||
| Yes | 74 | 85 | 68 | 71 | ||||
| Gender | χ21 = 4.3 | 0.039 | χ21 = H | 0.001 | ||||
| Male | 23 | 14 | 34 | 29 | ||||
| Female | 77 | 86 | 66 | 71 | ||||
| Education | χ23 = 1.8 | 0.608 | χ23 = 1.0 | 0.796 | ||||
| <High school | 3 | 2 | 2 | 2 | ||||
| High school | 23 | 30 | 24 | 24 | ||||
| College | 50 | 45 | 46 | 48 | ||||
| Graduate | 24 | 23 | 28 | 26 | ||||
| Reliability | χ21 = 0.3 | 0.571 | χ21 = 0.4 | 0.508 | ||||
| No | 98 | 99 | 97 | 97 | ||||
| Yes | 2 | 1 | 3 | 3 | ||||
Variables are summarized as percentages and comparisons within diagnosis by NSD were computed using Pearson's test. A 'yes' response on 'reliability' refers to cases for whom credibility of the informant maybe questionable.
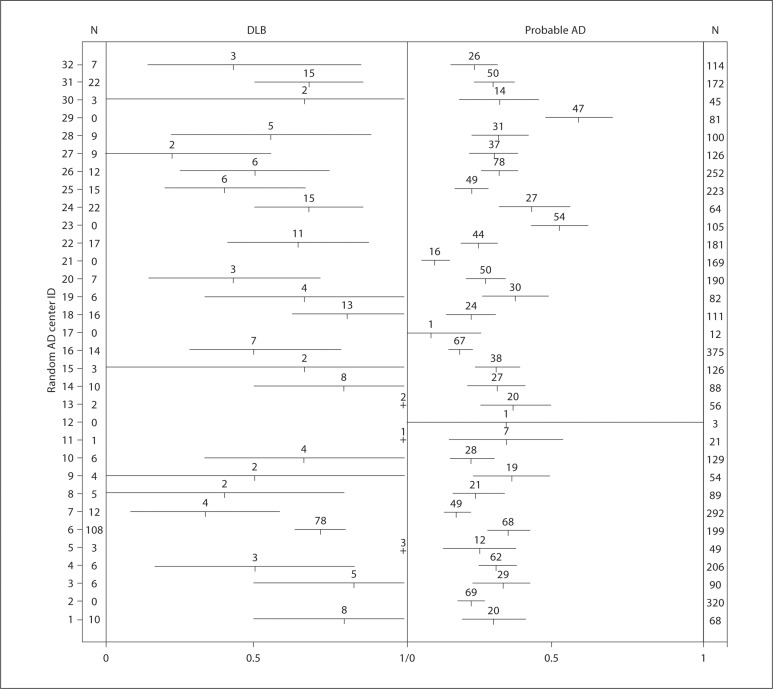
Differences among centers were apparent. Of the 13 centers reporting 9 or more DLB cases, the presence of NSD ranged from 22.2 to 80.0%. The frequencies of NSD in all centers and their 95% bootstrapped CI are presented in figure 1 and suggest that, along with wide CI among some centers contributing only a small number of cases, there was considerable variation in the presence of NSD in DLB by center, relative to the presence of NSD in AD.
Fig. 1.
Proportion of subjects with NPI-Q-assessed NSD by diagnosis and by center. Intervals represent 95% bootstrapped CI and values above each interval correspond to the number of subjects with NSD at the specific center. The total number of subjects with DLB (or probable AD) for each center are denoted in column N, respectively.
Table 2 examines the extent to which dementia severity and depressive symptoms may have differentiated patients with informant-reported NSD from those without NSD. The CDR SOB was significantly higher in AD patients with informant-reported NSD, but this relationship was not seen in DLB, implying that in AD patients NSD may be a late-stage, rather than early-stage, disease correlate. Higher depression scores were also associated with NSD in AD, but not DLB, patients.
Table 2.
Disease severity and depressive symptoms in relation to NPI-Q-assessed NSD in DLB and AD patients
| DLB (n = 339) |
Probable AD (n = 4,192) |
|||||||
|---|---|---|---|---|---|---|---|---|
| without NSD (n = 125) | with NSD (n = 214) | test statistic | p value | without NSD (n = 3,077) | with NSD (n = 1,115) | test statistic | p value | |
| MMSE score (mean ± 1 SD) | 23.0 ± 16.9 | 24.0 ± 16.5 | F1, 337 = 0.29 | 0.59 | 21.6 ± 14.4 | 21.7 ± 16.7 | F1, 4,190 = 4.3 | 0.037 |
| CDRSOB | 6.9 ± 5.1 | 7.2 ± 4.5 | F1,337 = 2.0 | 0.15 | 6.7 ± 4.3 | 8.0 ± 4.7 | F1, 4,190 = 70 | <0.001 |
| NPI-Q depression item, % | 45 | 52 | χ21 = 1.8 | 0.18 | 33 | 48 | χ21 = 74 | <0.001 |
| GDS | 4.3 ± 3.4 | 4.5 ± 3.3 | Fl,283 = 0.63 | 0.43 | 2.3 ± 2.5 | 2.7 ± 2.7 | F1, 3,653 = 18 | <0.001 |
MMSE = Mini-Mental State Exam. Continuous variables are summarized with mean ± 1 standard deviation and categorical variables by percentages, p values represent comparisons using the Wilcoxon (for continuous variables) or Pearson's test (for categorical variables). For GDS: 3,655 (87%) and 285 (84%) responses noted for probable AD and DLB, respectively.
Informant-reported NSD was also associated with several other key aspects of behavioral disturbance on the NPI-Q, including delusions, hallucinations, agitation and apathy (table 3). These informant-reported phenomena were associated with informant-reported NSD in both DLB and AD. It is noteworthy that the comorbidities between these phenomena were higher in DLB, relative to AD patients, implying that such behavioral manifestations are more likely to represent an overlapping, common phenotype in the former, relative to the latter. The nonmotor symptoms of DLB may thus constitute a more recognizable constellation of behaviors than they do in AD, where such phenomena may be more likely to occur in a somewhat more isolated fashion.
Table 3.
Comorbidity of NPI-Q-assessed NSD with hallucinations, delusions, agitation and apathy in DLB and probable AD
| DLB (n = 339) | Probable AD (n = 4,192) | Test statistic | P value | |
|---|---|---|---|---|
| Hallucinations | 31.0 | 4.9 | χ2 1 = 333.2 | <0.001 |
| Delusions | 19.2 | 7.5 | χ2 1 = 55.5 | <0.001 |
| Agitation | 28.0 | 13.4 | χ2 1 = 54.5 | <0.001 |
| Apathy | 47.5 | 15.2 | χ2 1 = 226.0 | <0.001 |
Informants may have endorsed one or more NPI-Q items. Proportions represent the percentages of patients for each diagnostic group (DLB or AD) with NSD and a particular comorbidity (hallucinations, delusions, agitation, and apathy). Pearson χ2 and p values represent the comparison between DLB and AD.
The regression summary (OR and 95% CI) is presented in table 4. Because there were fewer cases missing NPI-Q items relative to missing data with the GDS, we included the NPI-Q depressed mood item in this model. These data suggested that although older age, male gender, black race, CDR SOB, and NPI-Q-derived assessments of depressive symptoms, hallucinations, delusions, agitation and apathy were associated with NSD, the presence of DLB diagnosis was by far the strongest predictor (OR = 2.93, 95% CI = 2.22–3.86). Significant differences were also noted among a few of the centers. Data were virtually identical when cases living alone were excluded (OR for diagnosis = 3.18, 95% CI = 2.39–4.23), suggesting that inclusion of those cases did not influence this association.
Table 4.
Multivariate logistic regression model of predictors of NPI-Q-assessed NSD
| OR | 95% CI | p value | |||
|---|---|---|---|---|---|
| Age group | <60 | 1.00 | – | – | |
| 60–64 | 1.01 | 0.66–1.54 | 0.972 | ||
| 65–69 | 1.13 | 0.76–1.66 | 0.550 | ||
| 70–74 | 1.27 | 0.89–1.80 | 0.191 | ||
| 75–79 | 1.39 | 0.99–1.95 | 0.061 | ||
| 80–84 | 1.40 | 0.99–1.98 | 0.055 | ||
| >84 | 1.64 | 1.14–2.35 | 0.007 | ||
| Patient gender | Male | 1.00 | – | – | |
| Female | 0.66 | 0.54–0.80 | <0.0001 | ||
| Informant gender | Male | 1.00 | – | – | |
| Female | 0.93 | 0.76–1.13 | 0.451 | ||
| Race | Non-Hispanic White | 1.00 | – | – | |
| Non-Hispanic Black | 1.39 | 1.10–1.74 | 0.005 | ||
| Non-Hispanic other | 1.05 | 0.65–1.71 | 0.835 | ||
| Hispanic | 0.98 | 0.73–1.32 | 0.902 | ||
| NPI-Q depression | no | 1.00 | – | – | |
| yes | 1.49 | 1.28–1.74 | <0.0001 | ||
| Diagnosis | Probable AD | 1.00 | – | – | |
| DLB | 2.93 | 2.22–3.86 | <0.0001 | ||
| CDR SOB | 1.03 | 1.01–1.05 | 0.003 | ||
| NPI-Q: hallucinations | no | 1.00 | – | – | |
| yes | 2.35 | 1.88–2.94 | <0.0001 | ||
| NPI-Q: delusions | no | 1.00 | – | – | |
| yes | 1.25 | 1.04–1.51 | 0.020 | ||
| NPI-Q: agitation | no | 1.00 | – | – | |
| yes | 1.59 | 1.36–1.86 | <0.0001 | ||
| NPI-Q: apathy | no | 1.00 | – | – | |
| yes | 1.58 | 1.36–1.84 | <0.0001 | ||
| Marital status | |||||
| Married | 1.00 | – | – | ||
| Not currently married | 1.21 | 1.00–1.48 | 0.053 | ||
| Never married/other | 1.13 | 0.69–1.86 | 0.619 | ||
| NACC center number | 6 | 1.00 | – | – | |
| 1 | 0.76 | 0.42–1.37 | 0.365 | ||
| 2 | 0.44 | 0.30–0.65 | <0.0001 | ||
| 3 | 0.73 | 0.43–1.23 | 0.238 | ||
| 4 | 0.74 | 0.49–1.13 | 0.166 | ||
| 5 | 0.84 | 0.43–1.67 | 0.628 | ||
| 7 | 0.40 | 0.26–0.61 | <0.0001 | ||
| 8 | 0.45 | 0.25–0.81 | 0.008 | ||
| 9 | 0.94 | 0.50–1.77 | 0.857 | ||
| 10 | 0.63 | 0.38–1.03 | 0.067 | ||
| 11 | 0.88 | 0.34–2.31 | 0.796 | ||
| 12 | 1.12 | 0.08–15.27 | 0.934 | ||
| 13 | 0.90 | 0.48–1.69 | 0.736 | ||
| 14 | 0.94 | 0.56–1.59 | 0.826 | ||
| 15 | 0.80 | 0.49–1.30 | 0.364 | ||
| 16 | 0.49 | 0.34–0.72 | 0.001 | ||
| 17 | 0.16 | 0.02–1.33 | 0.090 | ||
| 18 | 0.80 | 0.49–1.30 | 0.368 | ||
| 19 | 1.09 | 0.64–1.86 | 0.739 | ||
| 20 | 0.62 | 0.40–0.95 | 0.027 | ||
| 21 | 0.24 | 0.13–0.44 | <0.0001 | ||
| 22 | 0.63 | 0.41–0.97 | 0.038 | ||
| 23 | 1.34 | 0.80–2.24 | 0.264 | ||
| 24 | 1.24 | 0.72–2.12 | 0.437 | ||
| 25 | 0.66 | 0.44–1.01 | 0.055 | ||
| 26 | 0.92 | 0.63–1.35 | 0.666 | ||
| 27 | 0.52 | 0.32–0.85 | 0.009 | ||
| 28 | 1.05 | 0.63–1.73 | 0.864 | ||
| 29 | 2.56 | 1.46–4.49 | 0.001 | ||
| 30 | 0.79 | 0.19–3.21 | 0.741 | ||
| 31 | 0.87 | 0.57–1.32 | 0.500 | ||
| 32 | 0.57 | 0.32–1.01 | 0.053 | ||
CDR SOB represents each unit increase in SOB. Center 6 was used as reference group because it contained the largest number of DLB patients.
Discussion
These data indicate that substantial proportions of DLB and AD patients experience some aspect of disturbed sleep, with the former showing an apparent presence well over twice that of the latter. The data thus confirm what preliminary analyses in much smaller samples of such patients from particular sites [15,16,17,18,19] and of patients with more generally defined parkinsonism [20] have demonstrated previously. Patients with DLB are more likely to suffer from NSD than patients with AD. The neurobiological substrates for the preferential association of sleep disturbance with synucleinopathies versus amyloidopathies remain speculative, but are thought to involve the primacy of brainstem degeneration in parkinsonian-like conditions [17,21,22]. Such deterioration may impact upon nuclei such as the pedunculopontine nucleus, the sublaterodorsal nucleus, the magnocellular reticular formation, or other structures and their afferent/efferent projections, which ultimately lead to dysregulation of normal REM sleep atonia [21,23]. Among the AD patients, but not the DLB patients, NSD was more likely to be associated with more advanced disease (i.e., CDR). This is consistent with the observation that sleep disturbance may be one of the earliest signs of a synucleinopathy [24] and one that remains prominent throughout disease course or may even decrease [25], whereas in AD it may be a sign of late-stage disease [26,27].
Hypersomnolence as captured by a positive response to the phrase on the NPI-Q ‘take excessive naps during the day’ could reflect a circadian dysrhythmia, coexistent obstructive and/or central sleep apnea syndrome, restless legs syndrome/periodic limb movement disorder, a narcolepsy-like disorder, another primary sleep disorder, or an effect of medications. It may also reflect apathy, which may overlap with sleepiness to some degree. The frequencies of these various sleep disorders among DLB and AD patients are not well characterized, but these warrant further study, as they could be differentially expressed in DLB versus AD and provide diagnostic clues. Furthermore, as reflected by endorsement of frequent napping, hypersomnolence could contribute to the fluctuations in cognition and/or arousal which are considered a core feature of DLB [17].
Weaknesses of the current analyses include the fact that the UDS database essentially represents a convenience sample of patients presenting at academic AD centers, lack of autopsy verification of clinical diagnosis, lack of control over medication usage and daytime activity level, as well as the limitation of the NPI-Q as the sole source of information regarding nocturnal behavior. There are many disadvantages of the NPI-Q nighttime behavior item. The NPI-Q was selected for inclusion in the UDS because of its demonstrated utility and validity in gathering data on a wide variety of behavioral syndromes associated with dementia [12], but its use was not driven specifically by an interest in sleep or sleep behavior. We could locate only one very preliminary study reporting convergent validity of the NPI-Q sleep item with other sleep questions, which themselves were summarized globally into a single score [28]. The fundamental limitation of the item is that it consists of three separate, undifferentiated components (nocturnal awakenings, arising too early in the morning, or excessive daytime napping), which may or may not occur concurrently in dementia patients [2,29,30,31]. (The final component, daytime sleepiness, arguably does not even constitute a ‘nighttime’ behavior, though one might infer that it could represent an outcome of poor sleep at night [32,33]. Our finding that informant-endorsed apathy on the NPI-Q also overlapped with the nighttime behavior item further suggests possible overlap with daytime sleepiness). Furthermore, none of these three components implicit within the single question specifically target dream enactment behaviors during REM (so-called ‘active sleep’) that constitute one of the suggestive criteria (i.e., the behavioral manifestation of RBD) for DLB diagnosis [1]. Such an omission could represent a ‘false negative’.
Of consequence in the current work is that the more likely presence of NSD in DLB relative to AD was a robust finding. It occurred independently from the patient's gender or the informant's gender. It also occurred regardless of whether the informant lived with or was married to the patient. Various centers also differed in the presence of NSD at their respective sites, particularly for DLB cases. Among the 13 centers encountering 9 or more DLB cases, informant-endorsed NSD ranged from as low as 22% (center 27) to as high as 81% (center 18) (fig. 1), whereas the range for NSD in AD cases among those same centers was about half of that (as low as 17% for center 7 to as high as 42% for center 24). Informant endorsement variability can be interpreted in several different ways. It is possible that DLB patients at certain centers had more disturbed sleep and that the informant reports reflected that fact. The UDS did not collect data on household sleeping arrangements, which makes it impossible to know whether some informants might not be missing sleep disturbance, for example, by not sleeping in the same bedroom as the patients. Data such as these might enhance the understanding of these apparent site differences. However, this type of influence would not be expected to operate systematically at different sites and vary systematically between DLB and AD cases. On the other hand, the possibility exists that different centers might implicitly weight the informant's report of NSD differently when establishing a diagnosis of DLB. Although RBD is now acknowledged to represent a strongly suggestive feature of DLB [1], it is unclear how various centers may view these data as crucial or not crucial for establishing this differential diagnosis relative to AD. If the latter situation even represents the most remote possibility, it certainly draws attention to the importance of an independent and more detailed assessment of sleep/wake function in the evaluation of all dementia patients, perhaps even physiologically. For example, we have recently noted that phasic muscle activity in limbs [34], recorded from a single REM sleep period, is a useful objective physiologic marker to differentiate individuals with a history of RBD from controls, even when dream enactment behaviors were not detected when sleeping in the laboratory for a single night [35]. Such electromyographic activity, labeled the phasic electromyographic metric (PEM), may provide objective verification of the phenomena that informants are providing here via the NPI-Q. Such measures may also be particularly informative by providing insights into the underlying neurodegenerative disorder in the clinical setting of mild cognitive impairment when the other more ‘classic’ features of AD and DLB have not yet become manifest.
Disclosure Statement
Dr. Bliwise has served on the Advisory Board for Takeda Pharmaceuticals and has been on the Speaker's Bureau for Takeda Pharmaceuticals and Boehringer-Ingelheim. Mr. Mercaldo reports no disclosures. Dr. Avidan has served on the Advisory Board for Takeda Pharmaceuticals and has been on the Speaker's Bureau for Takeda Pharmaceuticals, Sanovian, Cephalon, and Pfizer. Dr. Boeve has received grant support from Myriad Pharmaceuticals and honoraria from General Electric Healthcare. Ms. Greer reports no disclosures. Dr. Kukull reports no disclosures.
Acknowledgements
The authors express their appreciation to Dr. Jeffrey Cummings for his encouragement with this study. This work was supported by the following grants: R01 NS-050595; U01 AG-016976; P50 AG-025688; P50 AG-16574; R01 AG-15866, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research program of the Mayo Clinic Foundation.
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 2.Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004;6(suppl 1A):S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 3.Harper DG, Stopa EG, McKee AC, et al. Dementia severity and Lewy bodies affect circadian rhythms in Alzheimer's disease. Neurobiol Aging. 2004;25:771–781. doi: 10.1016/j.neurobiolaging.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer's Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 6.Lau DT, Mercaldo ND, Harris AT, et al. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord. 2010;24:56–63. doi: 10.1097/WAD.0b013e31819d6ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 13.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team R . A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. http://www.R-project.org. [Google Scholar]
- 15.Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51:363–370. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 16.Boeve BF, Silber MH, Ferman TH, et al. Association of REM sleep behaviors disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Dis. 2001;16:622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 17.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 18.Grace JB, Walker MP, McKeith IG. A comparison of sleep profiles in patients with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry. 2000;15:1028–1033. doi: 10.1002/1099-1166(200011)15:11<1028::aid-gps227>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Boddy F, Rowan EN, Lett D, et al. Subjectively reported sleep quality and excessive daytime somnolence in Parkinson's disease with and without dementia, dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22:529–535. doi: 10.1002/gps.1709. [DOI] [PubMed] [Google Scholar]
- 20.Bliwise DL, Watts RL, Watts N, et al. Disruptive nocturnal behavior in Parkinson's disease and Alzheimer's disease. J Geriatr Psychiatry Neurol. 1995;8:107–110. doi: 10.1177/089198879500800206. [DOI] [PubMed] [Google Scholar]
- 21.Rye DB, Bliwise DL. Movement disorders specific to sleep and the nocturnal manifestations of waking movement disorders. In: Watts RL, Koller WC, editors. Movement Disorders: Neurologic Principles and Practice. ed 2. New York: McGraw Hill; 2004. pp. 855–890. [Google Scholar]
- 22.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 23.Rye DB. Parkinson's disease and RLS: the dopaminergic bridge. Sleep Med. 2004;5:317–328. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a Parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 25.Gjerstad MD, Boeve B, Wentzel-Larsen T, et al. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008;79:387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 26.Bliwise DL, Hughes M, McMahon P, et al. Observed sleep/wakefulness and severity of dementia in an Alzheimer's disease special care unit. J Gerontol A Biol Sci Med Sci. 1995;50A:M303–M306. doi: 10.1093/gerona/50a.6.m303. [DOI] [PubMed] [Google Scholar]
- 27.Prinz PN, Vitaliano PP, Vitiello MV, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 28.Rao V, Spiro JR, Samus QM, et al. Sleep disturbances in the elderly residing in assisted living: findings from the Maryland Assisted Living Study. Int J Geriatr Psychiatry. 2005;20:956–966. doi: 10.1002/gps.1380. [DOI] [PubMed] [Google Scholar]
- 29.Tractenberg RE, Singer CM, Kaye JA. Characterizing sleep problems in persons with Alzheimer's disease and normal elderly. J Sleep Res. 2006;15:97–103. doi: 10.1111/j.1365-2869.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tractenberg RE, Singer CM, Kaye JA. Symptoms of sleep disturbance in persons with Alzheimer's disease and normal elderly. J Sleep Res. 2005;14:177–185. doi: 10.1111/j.1365-2869.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Bliwise DL, Pour Ansari F, et al. Daytime sleepiness and functional impairment in Alzheimer's disease. Am J Geriatr Psychiatry. 2007;15:620–626. doi: 10.1097/JGP.0b013e3180381521. [DOI] [PubMed] [Google Scholar]
- 32.Bliwise DL, Bevier WC, Bliwise NG, et al. Systematic 24-hour behavioral observations of sleep/wakefulness in a skilled care nursing facility. Psychol Aging. 1990;5:16–24. doi: 10.1037//0882-7974.5.1.16. [DOI] [PubMed] [Google Scholar]
- 33.Endeshaw YW, Ouslander JG, Schnelle JF, et al. Polysomnographic and clinical correlates of behaviorally observed daytime sleep in nursing home residents. J Gerontol A Bio Sci Med Sci. 2007;62:55–61. doi: 10.1093/gerona/62.1.55. [DOI] [PubMed] [Google Scholar]
- 34.Bliwise DL, He L, Ansari FP, et al. Quantification of electromyographic activity during sleep: a phasic electromyographic metric (PEM) J Clin Neurophysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- 35.Bliwise DL, Rye DB. Elevated PEM (phasic electromyographic metric) rates in rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep. 2008;31:853–857. doi: 10.1093/sleep/31.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]