Abstract
The patient was a 63-year-old man who was referred to our hospital as an emergency case with the chief complaint of abdominal pain. An abdominal CT revealed a right retroperitoneal tumor of 15 cm and retroperitoneal bleeding. After a transcatheter arterial embolization was performed, the patient was transferred to our department. There was no infective focus. The white blood cell (WBC) count (37,820/μl, normal range <8 pg/ml) and the serum granulocyte colony-stimulating factor (G-CSF) level (2,670 pg/ml, normal range <8 pg/ml) were high. Bone marrow biopsy revealed little fat, significant hyperplasia, and predominantly increased neutrophils, but no findings of bone metastasis or bone marrow involvement. A G-CSF-producing tumor was diagnosed and right nephrectomy and retroperitoneal tumorectomy were performed. However, the tumor had infiltrated into the inferior vena cava, diaphragm and abdominal wall, and only part of the tumor could be removed. In histopathological tests, hematoxylin-eosin staining showed malignant fibrous histiocytoma-like findings mixed with those for well-differentiated liposarcoma, and the case was diagnosed as dedifferentiated liposarcoma. Preoperative 18F-FDG-PET computed tomography showed diffuse 18F-FDG uptake throughout the bone marrow and elevated uptake at the tumor site. However, since bone biopsy and bone scintigraphy indicated no bone metastasis or bone marrow involvement, we concluded that PET/CT imaging gave false-positive results in the bone marrow. This is the first report of PET/CT imaging of a G-CSF-producing tumor in a urological disease. The imaging results may be useful for differential diagnosis for this tumor in patients with high WBC counts without infection.
Key Words: Retroperitoneal dedifferentiated liposarcoma, Granulocyte colony-stimulating factor, Spontaneous rupture, PET/CT imaging
Introduction
Carcinomas producing granulocyte colony-stimulating factor (G-CSF) are extremely malignant and have a poor prognosis [1, 2]. These carcinomas are rare and are mostly found in the lung [2]. Thus, a liposarcoma producing G-CSF is particularly rare and has only been described in a few case reports [1, 3]. Here, we present a case of retroperitoneal dedifferentiated liposarcoma producing G-CSF. The tumor also showed spontaneous rupture, which has not been previously reported for retroperitoneal liposarcoma.
Positron emission tomography using 18F-fluorodeoxyglucose (18F-FDG-PET) is used to detect tumors based on elevated glucose metabolism in carcinoma cells. It has also been shown that 18F-FDG uptake by bone marrow is correlated with the peripheral blood neutrophil count [4], since uptake reflects increased marrow metabolism. We performed 18F-FDG-PET with computed tomography (PET/CT) for imaging of the G-CSF-producing tumor and found high 18F-FDG uptake by the tumor itself and diffuse uptake throughout the bone marrow. The findings for the bone marrow were shown to be false-positive. These are the first PET/CT images of a G-CSF-producing tumor in a urological disease.
Case Report
The patient was a 63-year-old man with a history of hypertension and no specific family history. He had suffered pain in the right back since the middle of July 2008. He was taken to the Emergency Department of our hospital due to significant aggravation of the pain in late July 2008. An abdominal CT imaging (fig. 1) revealed a non-fatty, non-fibrotic, and non-calcified right retroperitoneal tumor of 15 cm in size and retroperitoneal hemorrhage. Hemorrhagic shock occurred due to spontaneous rupture of the retroperitoneal tumor during the examination, and an emergency transcatheter arterial embolization was conducted. The nutrient vessels were the superior and inferior suprarenal arteries, inferior phrenic artery, renal capsular artery, and the first lumbar artery.
Fig. 1.
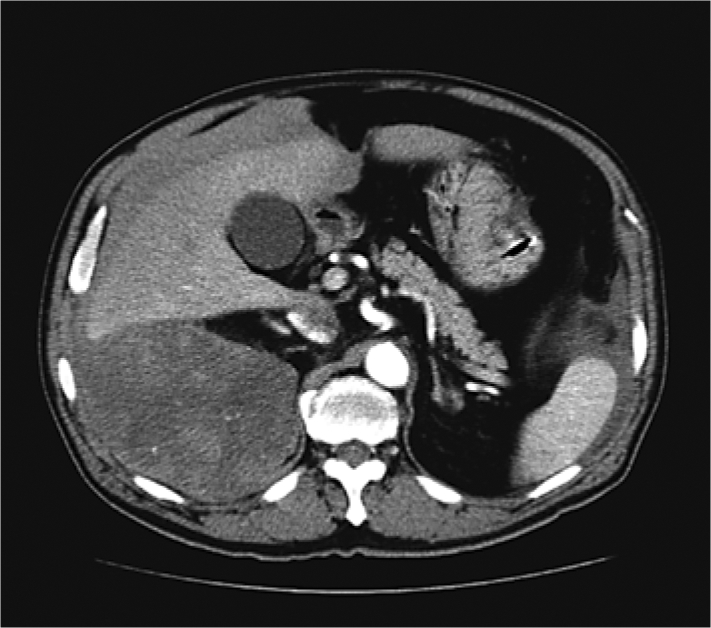
CT imaging shows a non-fatty, non-fibrotic, and non-calcified right retroperitoneal tumorous lesion of 15 cm in size with no clear boundary and retroperitoneal hemorrhage caused by spontaneous rupture.
Three days after admission, the patient was transferred to our department for a more detailed examination of the retroperitoneal tumor. A routine peripheral hematological examination indicated a high white blood cell (WBC) count of 37,820/μl and a strong left-shift of the differential leukocyte count, with a neutrophil count of 34,794/μl. However, no infection appeared to be present based on physiological and biochemical findings, and endocrinological tests showed no abnormal findings. Since the WBC count remained high after the transfer to our department, further tests were carried out at the Department of Hematological Internal Medicine, based on the suspicion of a G-CSF-producing tumor or a comorbid hematological disease.
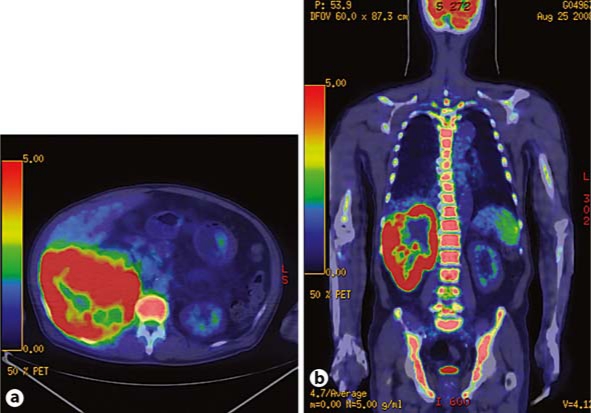
In parallel, PET/CT imaging was conducted for further systemic examination. Elevated uptake of <18F-FDG correlated with the tumor site (maximum standardized uptake value, SUVmax, was 18.5) and the case was diagnosed as malignant. PET/CT imaging also indicated diffuse uptake throughout the bone marrow, and particularly elevated uptake in the vertebral bone (SUVmax: 10.5) (fig. 2). However, bone scintigraphy showed no bone metastasis, and bone marrow biopsy revealed little fat, significant hyperplasia, and increased neutrophils, but no findings of metastasis or bone marrow involvement.
Fig. 2.
PET/CT was performed after an intravenous injection of 260 MBq 18F-FDG. a Axial fused PET/CT imaging shows elevated uptake of 18F-FDG at the site of the tumor (SUVmax: 18.5), indicating that the legion was malignant. b Whole-body coronal fused PET/CT imaging shows diffuse uptake throughout the bone marrow, with especially high uptake in the vertebral bone (SUVmax: 10.5).
Based on all the above findings and the high serum G-CSF level of 2,670 pg/ml (normal <8 pg/ml), the cause of the increased WBC count was determined to be a G-CSF-producing tumor. A malignant tumor was suspected and right nephrectomy and retroperitoneal tumorectomy were conducted in mid-September. However, since the tumor had infiltrated into the inferior vena cava, diaphragm and abdominal wall already, only partial resection of the tumor was performed.
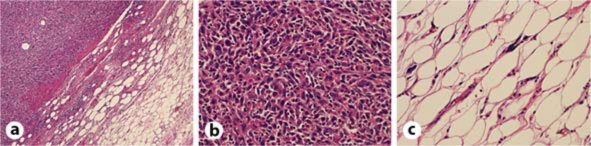
The resected tissue was yellowish-white and fragile. Histopathological tests using hematoxylin-eosin staining showed findings for malignant fibrous histiocytoma mixed with those for well-differentiated liposarcoma (fig. 3). The tumor was diagnosed as dedifferentiated liposarcoma based on the diagnostic criteria of the World Health Classification (WHO) [5, 6]. The postoperative peripheral WBC count was 21,000/μl and the serum G-CSF level was 1,170 pg/ml, both of which showed a transient decrease. With informed consent from the patient, only symptomatic therapy, but no chemotherapy or radiotherapy, was carried out while observing the prognosis. The patient died in February 2009. The final peripheral WBC count was 145,800/μl.
Fig. 3.
Histological diagnosis with hematoxylin-eosin showing malignant fibrous histiocytoma-like findings mixed with those for well-differentiated liposarcoma, leading to the diagnosis of dedifferentiated liposarcoma. a Well-differentiated liposarcoma components and malignant fibrous histiocytoma-like findings with a distinct border. b Malignant fibrous histiocytoma-like findings. c Well-differentiated liposarcoma components.
Discussion
Liposarcomas are morphologically subdivided into five main subgroups: well-differentiated, myxoid, round cell, pleomorphic, and dedifferentiated [6]. Dedifferentiated liposarcoma was first recognized by Evans in 1979 [7] and was defined in 2002 as a tumor with a clear-cut and well-differentiated component clearly separated from or occupying a large area beside a poorly differentiated component [5, 6]. Among 78 cases of retroperitoneal liposarcoma, Fabre-Guillevin et al. [6] described 52 cases of dedifferentiated liposarcoma with a differentiated component with features of malignant fibrous histiocytoma (36 undifferentiated pleomorphic sarcoma; 14 fibrosarcoma; 1 myxofibrosarcoma; 1 osteosarcoma; and/or 4 leiomyosarcoma), 21 of well-differentiated liposarcoma, 2 of myxoid liposarcoma, 2 of round cell liposarcoma, and 1 of pleomorphic liposarcoma. The cases of dedifferentiated liposarcoma had local recurrence and metastasis at rates of about 40 and 20%, respectively, and a poorer clinical outcome compared to well-differentiated liposarcoma [6, 7].
G-CSF-producing carcinomas are rare and mostly found in the lung. The production of G-CSF has been reported in cases of hepatocellular carcinoma, pancreatic cancer, and gastric cancer [8, 9, 10]. G-CSF-producing carcinomas show aggressive growth and a very poor prognosis [8, 9, 10]. There have only been a few previous case reports of a G-CSF-producing liposarcoma [1, 3]. Six cases of liposarcoma accompanied by leukocytosis have been described in the English literature [1]. However, the G-CSF level has only been evaluated in 3 cases: in an upper arm dedifferentiated liposarcoma described by Sakamoto et al. [1], in a mesenteric pleomorphic liposarcoma reported by Nakamura et al. [3], and in the current case. In all 3 cases, the G-CSF level and peripheral WBC counts were correlated with the clinical course. The G-CSF level and WBC count showed a transient postoperative decrease in our patient, but this was followed by an aggressive clinical course and the WBC count was 145,800/μl at the time the patient died. No hematological diseases such as leukemia or a leukemoid reaction were observed and bone marrow biopsy only showed hyperplasia. These findings led to the diagnosis of a G-CSF-producing retroperitoneal dedifferentiated liposarcoma.
Spontaneous rupture of the liposarcoma also occurred in our case. This is an extremely rare clinical presentation, with the description of spontaneous rupture of a renal liposarcoma by Mazuch et al. [11] being the only related case. Therefore, this is the first report of spontaneous rupture of a retroperitoneal liposarcoma. The rapid aggressive growth of the G-CSF-producing tumor may have caused the rupture, and palliative surgery was performed because of the rupture.
PET/CT is useful for the evaluation of malignant lesions. In cases of carcinoma, diffuse 18F-FDG uptake in bone marrow is often indicative of bone metastasis or bone marrow involvement [12]. In our case, PET/CT showed diffuse 18F-FDG uptake throughout the bone marrow, which indicated multiple bone metastases. Morooka et al. [2] described 2 cases of G-CSF-producing lung cancer that showed diffuse 18F-FDG uptake throughout the bone marrow. These PET/CT findings were similar to the findings in our case. Morooka et al. [2] and Goshen et al. [12] commented that diffuse elevated 18F-FDG uptake in bone marrow may be helpful in the diagnosis of a G-CSF-producing tumor. Bone metastasis is a differential diagnosis, and in our case bone scintigraphy and bone marrow biopsy were useful for ruling out bone metastasis.
Murata et al. [4] showed that 18F-FDG uptake by bone marrow is strongly correlated with the WBC count (especially neutrophil count) and suggested that 18F-FDG uptake by bone marrow reflects marrow metabolism, which is mainly regulated by granulocyte progenitors and stimulated by endogenous hematopoietic growth factors. Kazama et al. [13] found increased 18F-FDG uptake in patients treated with G-CSF and showed that the increased uptake returned to the pretreatment value approximately 1 month after discontinuation of G-CSF. The serum G-CSF level is likely to be higher in patients with a G-CSF-producing tumor than in those treated with G-CSF. Thus, for a G-CSF-producing tumor, at least 1 month is needed before a PET/CT evaluation after removal of the primary lesion.
Other conditions associated with diffuse 18F-FDG uptake in the bone marrow include marrow hyperplasia resulting from hemolytic/iron-deficiency/blood-loss anemia. In addition, Van de Weile et al. [15] examined the relationship between bone marrow 18F-FDG uptake and levels of serum cytokines. In patients with non-small cell lung carcinomas, which are known to produce cytokines, 18F-FDG uptake by bone marrow was related to the level of transforming growth factor-beta (TGF-β). Thus, bone marrow 18F-FDG uptake in patients with a G-CSF-producing tumor may also be related to cytokines such as TGF-β.
In conclusion, we encountered a rare case of a G-CSF-producing retroperitoneal dedifferentiated liposarcoma that underwent spontaneous rupture due to rapid aggressive growth. This case is also the first report of PET/CT imaging of a G-CSF-producing tumor in a urological disease. PET/CT imaging of the tumor showed diffuse uptake throughout the bone marrow. In such cases, one needs to be careful to avoid a misdiagnosis of bone metastasis, but imaging may be useful for differential diagnosis of a G-CSF-producing tumor in patients with abnormally high WBC counts in the absence of infection.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Sakamoto A, Matono H, Yoshida T, Tanaka K, Matsuda S, Oda Y, Iwamoto Y. Dedifferentiated liposarcoma with leukocytosis. A case report of G-CSF-producing soft-tissue tumors, possible association with undifferentiated liposarcoma lineage. World J Surg Oncol. 2007;5:131. doi: 10.1186/1477-7819-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morooka M, Kubota K, Murata Y, Shibuya H, Ito K, Mochizuki M, Akashi T, Chiba T, Nomura T, Ito H, Morita T. (18)F-FDG-PET/CT findings of granulocyte colony stimulating factor (G-CSF)-producing lung tumors. Ann Nucl Med. 2008;22:635–639. doi: 10.1007/s12149-008-0146-z. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura A, Tanaka S, Takayama H, Sakamoto M, Ishii H, Kusano M, Onizuka Y, Ota S, Mitamura K. A mesenteric liposarcoma with production of granulocyte colony-stimulating factor. Intern Med. 1998;37:884–890. doi: 10.2169/internalmedicine.37.884. [DOI] [PubMed] [Google Scholar]
- 4.Murata Y, Kubota K, Yukihiro M, Ito K, Watanabe H, Shibuya H. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: measurements by PET/CT. Nucl Med Biol. 2006;33:999–1004. doi: 10.1016/j.nucmedbio.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Dei tos AP, Pedeutour F. Dedifferentiated liposarcoma. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumors: Pathology and Genetics: Tumors of Soft Tissue and Bone. Lyon: International Agency for Research on Cancer; 2002. pp. 38–39. [Google Scholar]
- 6.Fabre-Guillevin E, Coindre JM, Somerhausen Nde S, Bonichon F, Stoeckle E, Bui NB. Retroperitoneal liposarcomas: follow-up analysis of dedifferentiation after clinicopathologic reexamination of 86 liposarcomas and malignant fibrous histiocytomas. Cancer. 2006;106:2725–2733. doi: 10.1002/cncr.21933. [DOI] [PubMed] [Google Scholar]
- 7.Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507–523. doi: 10.1097/00000478-197912000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Araki K, Kishihara F, Takahashi K, Matsumata T, Shimura T, Suehiro T, Kuwano H. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007;27:716–721. doi: 10.1111/j.1478-3231.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 9.Joshita S, Nakazawa K, Sugiyama Y, Kamijo A, Matsubayashi K, Miyabayashi H, Furuta K, Kitano K, Kawa S. Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course. Intern Med. 2009;48:687–691. doi: 10.2169/internalmedicine.48.1900. [DOI] [PubMed] [Google Scholar]
- 10.Yamano T, Morii E, Ikeda J, Aozasa K. Granulocyte colony-stimulating factor production and rapid progression of gastric cancer after histological change in the tumor. Jpn J Clin Oncol. 2007;37:793–796. doi: 10.1093/jjco/hym094. [DOI] [PubMed] [Google Scholar]
- 11.Mazuch J, Striez I, Malcek V, Kuník Z, Pelc J. Spontaneous rupture of a renal liposarcoma. Rozhl Chir. 1989;68:824–829. [PubMed] [Google Scholar]
- 12.Goshen E, Davidson T, Yeshurun M, Zwas ST. Combined increased and decreased skeletal uptake of F-18 FDG. Clin Nucl Med. 2006;31:520–522. doi: 10.1097/01.rlu.0000233080.16944.a4. [DOI] [PubMed] [Google Scholar]
- 13.Kazama T, Swanston N, Podoloff DA, Macapinlac HA. Effect of colony-stimulating factor and conventional- or high-dose chemotherapy on FDG uptake in bone marrow. Eur J Nucl Med Mol Imaging. 2005;32:1406–1411. doi: 10.1007/s00259-005-1890-0. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri N, Wright P, Balan KK. Rhabdomyosarcoma with widespread bone marrow infiltration: beneficial management role of F-18 FDG PET. Clin Nucl Med. 2007;32:787–789. doi: 10.1097/RLU.0b013e318148b434. [DOI] [PubMed] [Google Scholar]
- 15.Van de Wiele C, VandeVyver F, Debruyne C, Philippé J, van Meerbeeck JP. FDG uptake by the bone marrow in NSCLC patients is related to TGF-beta but not to VEGF or G-CSF serum levels. Eur J Nucl Med Mol Imaging. 2008;35:519–522. doi: 10.1007/s00259-007-0628-6. [DOI] [PubMed] [Google Scholar]