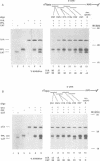
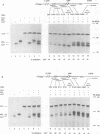
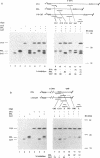
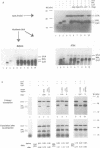
Abstract
We describe a novel experimental approach to investigate mRNA translation. Antisense 2'-O-allyl oligoribonucleotides (oligos) efficiently arrest translation of targeted mRNAs in rabbit reticulocyte lysate and wheat germ extract while displaying minimal non-specific effects on translation. Oligo/mRNA-hybrids positioned anywhere within the 5' UTR or the first approximately 20 nucleotides of the open reading frame block cap-dependent translation initiation with high specificity. The thermodynamic stability of hybrids between 2'-O-alkyl oligos and RNA permits translational inhibition with oligos as short as 10 nucleotides. This inhibition is independent of RNase H cleavage or modifications which render the mRNA untranslatable. We show that 2'-O-alkyl oligos can also be employed to interfere with cap-independent internal initiation of translation and to arrest translation elongation. The latter is accomplished by UV-crosslinking of psoralen-tagged 2'-O-methyloligoribonucleotides to the mRNA within the open reading frame. The utility of 2'-O-alkyloligoribonucleotides to arrest translation from defined positions within an mRNA provides new approaches to investigate mRNA translation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony D. D., Merrick W. C. Analysis of 40 S and 80 S complexes with mRNA as measured by sucrose density gradients and primer extension inhibition. J Biol Chem. 1992 Jan 25;267(3):1554–1562. [PubMed] [Google Scholar]
- Bacon T. A., Wickstrom E. Walking along human c-myc mRNA with antisense oligodeoxynucleotides: maximum efficacy at the 5' cap region. Oncogene Res. 1991;6(1):13–19. [PubMed] [Google Scholar]
- Barabino S. M., Sproat B. S., Lamond A. I. Antisense probes targeted to an internal domain in U2 snRNP specifically inhibit the second step of pre-mRNA splicing. Nucleic Acids Res. 1992 Sep 11;20(17):4457–4464. doi: 10.1093/nar/20.17.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Belsham G. J., Brangwyn J. K. A region of the 5' noncoding region of foot-and-mouth disease virus RNA directs efficient internal initiation of protein synthesis within cells: involvement with the role of L protease in translational control. J Virol. 1990 Nov;64(11):5389–5395. doi: 10.1128/jvi.64.11.5389-5395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J. Dual initiation sites of protein synthesis on foot-and-mouth disease virus RNA are selected following internal entry and scanning of ribosomes in vivo. EMBO J. 1992 Mar;11(3):1105–1110. doi: 10.1002/j.1460-2075.1992.tb05150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. R., Rayner B., Imbach J. L., Paoletti C., Malvy C. Comparative activity of alpha- and beta-anomeric oligonucleotides on rabbit beta globin synthesis: inhibitory effect of cap targeted alpha-oligonucleotides. Biochem Biophys Res Commun. 1989 Oct 16;164(1):311–318. doi: 10.1016/0006-291x(89)91719-1. [DOI] [PubMed] [Google Scholar]
- Boiziau C., Kurfurst R., Cazenave C., Roig V., Thuong N. T., Toulmé J. J. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 1991 Mar 11;19(5):1113–1119. doi: 10.1093/nar/19.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Frank P., Büsen W. Characterization of ribonuclease H activities present in two cell-free protein synthesizing systems, the wheat germ extract and the rabbit reticulocyte lysate. Biochimie. 1993;75(1-2):113–122. doi: 10.1016/0300-9084(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Crum C., Johnson J. D., Nelson A., Roth D. Complementary oligodeoxynucleotide mediated inhibition of tobacco mosaic virus RNA translation in vitro. Nucleic Acids Res. 1988 May 25;16(10):4569–4581. doi: 10.1093/nar/16.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doohan J. P., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Analysis of ribosome pausing during translation of reovirus S1 and S4 mRNAs in virus-infected and vector-transfected cells. J Biol Chem. 1993 Aug 25;268(24):18313–18320. [PubMed] [Google Scholar]
- Goodchild J., Carroll E., 3rd, Greenberg J. R. Inhibition of rabbit beta-globin synthesis by complementary oligonucleotides: identification of mRNA sites sensitive to inhibition. Arch Biochem Biophys. 1988 Jun;263(2):401–409. doi: 10.1016/0003-9861(88)90652-2. [DOI] [PubMed] [Google Scholar]
- Gray N. K., Quick S., Goossen B., Constable A., Hirling H., Kühn L. C., Hentze M. W. Recombinant iron-regulatory factor functions as an iron-responsive-element-binding protein, a translational repressor and an aconitase. A functional assay for translational repression and direct demonstration of the iron switch. Eur J Biochem. 1993 Dec 1;218(2):657–667. doi: 10.1111/j.1432-1033.1993.tb18420.x. [DOI] [PubMed] [Google Scholar]
- Gupta K. C. Antisense oligodeoxynucleotides provide insight into mechanism of translation initiation of two Sendai virus mRNAs. J Biol Chem. 1987 Jun 5;262(16):7492–7496. [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Iribarren A. M., Sproat B. S., Neuner P., Sulston I., Ryder U., Lamond A. I. 2'-O-alkyl oligoribonucleotides as antisense probes. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7747–7751. doi: 10.1073/pnas.87.19.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H. E., De Groot N., Hochberg A. A., Hentze M. W. Effect of heparin contained in preparations of small cytoplasmic RNAs on cell-free translation. J Biol Chem. 1991 Jan 25;266(3):1921–1925. [PubMed] [Google Scholar]
- Kean J. M., Murakami A., Blake K. R., Cushman C. D., Miller P. S. Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger RNA. Biochemistry. 1988 Dec 27;27(26):9113–9121. doi: 10.1021/bi00426a008. [DOI] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1986 May;83(9):2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Characterization of ribosome-protected fragments from reovirus messenger RNA. J Biol Chem. 1976 Jul 25;251(14):4259–4266. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm G. M., Blencowe B. J., Sproat B. S., Iribarren A. M., Ryder U., Lamond A. I. Antisense probes containing 2-aminoadenosine allow efficient depletion of U5 snRNP from HeLa splicing extracts. Nucleic Acids Res. 1991 Jun 25;19(12):3193–3198. doi: 10.1093/nar/19.12.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Liebhaber S. A., Cash F. E., Shakin S. H. Translationally associated helix-destabilizing activity in rabbit reticulocyte lysate. J Biol Chem. 1984 Dec 25;259(24):15597–15602. [PubMed] [Google Scholar]
- Lingelbach K., Dobberstein B. An extended RNA/RNA duplex structure within the coding region of mRNA does not block translational elongation. Nucleic Acids Res. 1988 Apr 25;16(8):3405–3414. doi: 10.1093/nar/16.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monia B. P., Lesnik E. A., Gonzalez C., Lima W. F., McGee D., Guinosso C. J., Kawasaki A. M., Cook P. D., Freier S. M. Evaluation of 2'-modified oligonucleotides containing 2'-deoxy gaps as antisense inhibitors of gene expression. J Biol Chem. 1993 Jul 5;268(19):14514–14522. [PubMed] [Google Scholar]
- Nygård O., Nilsson L. Translational dynamics. Interactions between the translational factors, tRNA and ribosomes during eukaryotic protein synthesis. Eur J Biochem. 1990 Jul 20;191(1):1–17. doi: 10.1111/j.1432-1033.1990.tb19087.x. [DOI] [PubMed] [Google Scholar]
- Oh S. K., Sarnow P. Gene regulation: translational initiation by internal ribosome binding. Curr Opin Genet Dev. 1993 Apr;3(2):295–300. doi: 10.1016/0959-437X(93)90037-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. K., Scott M. P., Sarnow P. Homeotic gene Antennapedia mRNA contains 5'-noncoding sequences that confer translational initiation by internal ribosome binding. Genes Dev. 1992 Sep;6(9):1643–1653. doi: 10.1101/gad.6.9.1643. [DOI] [PubMed] [Google Scholar]
- Pantopoulos K., Johansson H. E., Hentze M. W. The role of the 5' untranslated region of eukaryotic messenger RNAs in translation and its investigation using antisense technologies. Prog Nucleic Acid Res Mol Biol. 1994;48:181–238. doi: 10.1016/S0079-6603(08)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Pieles U., Sproat B. S., Lamm G. M. A protected biotin containing deoxycytidine building block for solid phase synthesis of biotinylated oligonucleotides. Nucleic Acids Res. 1990 Aug 11;18(15):4355–4360. doi: 10.1093/nar/18.15.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieles U., Sproat B. S., Neuner P., Cramer F. Preparation of a novel psoralen containing deoxyadenosine building block for the facile solid phase synthesis of psoralen-modified oligonucleotides for a sequence specific crosslink to a given target sequence. Nucleic Acids Res. 1989 Nov 25;17(22):8967–8978. doi: 10.1093/nar/17.22.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E. Regulation of eukaryotic protein synthesis by initiation factors. J Biol Chem. 1993 Feb 15;268(5):3017–3020. [PubMed] [Google Scholar]
- Russell J. E., Liebhaber S. A. Double-stranded RNA triggers generalized translational arrest in Xenopus oocytes. Biochem Biophys Res Commun. 1993 Jul 30;194(2):892–900. doi: 10.1006/bbrc.1993.1905. [DOI] [PubMed] [Google Scholar]
- Sankar S., Cheah K. C., Porter A. G. Antisense oligonucleotide inhibition of encephalomyocarditis virus RNA translation. Eur J Biochem. 1989 Sep 1;184(1):39–45. doi: 10.1111/j.1432-1033.1989.tb14987.x. [DOI] [PubMed] [Google Scholar]
- Sartorius C., Franklin R. M. Hybridization arrest of cell-free translation of the malarial dihydrofolate reductase/thymidylate synthase mRNA by anti-sense oligodeoxyribonucleotides. Nucleic Acids Res. 1991 Apr 11;19(7):1613–1618. doi: 10.1093/nar/19.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Shakin-Eshleman S. H., Liebhaber S. A. Influence of duplexes 3' to the mRNA initiation codon on the efficiency of monosome formation. Biochemistry. 1988 May 31;27(11):3975–3982. doi: 10.1021/bi00411a013. [DOI] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell. 1988 Jan 15;52(1):39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Stripecke R., Hentze M. W. Bacteriophage and spliceosomal proteins function as position-dependent cis/trans repressors of mRNA translation in vitro. Nucleic Acids Res. 1992 Nov 11;20(21):5555–5564. doi: 10.1093/nar/20.21.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]