Abstract
Depressive symptoms and alcohol use are frequently positively associated during adolescence. This study aimed to assess the heritability of each phenotype across adolescence; to assess potential shared liabilities; to examine changes in the nature of shared liabilities across adolescence; and to investigate potential causal relationships between depressive symptoms and alcohol use. We studied a longitudinally assessed sample of adolescent Finnish twins (N = 1,282) to test hypotheses about genetic and environmental influences on these phenotypes within and across ages, using data from assessments at ages 12, 14, and 17.5 years. The heritability of depressive symptoms is consistent across adolescence (~40–50%), with contributions from common and unique environmental factors. The heritability of alcohol use varies across time (a2 = .25–.44), and age 14 alcohol use is heavily influenced by shared environmental factors. Genetic attenuation and innovation were observed across waves. Modest to moderate genetic (rA = .26–.59) and environmental (rC = .30–.63) correlations between phenotypes exist at all ages, but decrease over time. Tests for causal relationships between traits differed across ages and sexes. Intrapair MZ difference tests provided evidence for reciprocal causation in girls at ages 14 and 17.5. Formal causal models suggested significant causal relationships between the variables in both boys and girls. The association between depressive symptoms and alcohol use during adolescence is likely due to a combination of shared genetic and environmental influences and causal influences. These influences are also temporally dynamic, complicating efforts to understand factors contributing to the relationship between these outcomes.
Keywords: Depressive symptoms, Alcohol use, Genetics, Shared liability, Structural equation modeling
Introduction
Adolescence is an important period to understand the development of depressive symptomatology and alcohol use. Internalizing disorders commonly manifest during adolescence, with lifetime prevalence of adolescent depression around 14% (Office of Applied Studies 2005). Scores on depression scales, indicating subclinical levels of depression, steadily increase across adolescence (Angold and Costello 2006). Additionally, most individuals begin experimenting with alcohol during their teens (Johnston et al. 2006). Several studies have documented co-occurrence of these behaviors. For example, Crum et al. (2008) recently reported that high levels of depressed mood during childhood were significantly associated with earlier alcohol use onset, alcohol use problems in adolescence, and adult alcohol dependence. A study of Norwegian teenagers (Strandheim et al. 2009) found that, among females, symptoms of anxiety and depression were related to more frequent intoxications. A previous report of the present sample (Sihvola et al. 2008) found that early-onset (age 5–14) depressive disorders were associated with alcohol use frequency and recurrent intoxication, and other types of addictive substance use, at age 17.5. A study of young adults identified a causal association from alcohol misuse to depression (Fergusson et al. 2009).
Alcohol use can moderate depressive symptoms: among a sample of Finnish adolescents undergoing outpatient treatment for depression, higher levels of alcohol use adversely affected the course of depression (Meririnne et al. 2010). Another study suggested that individuals’ motivations for alcohol use might be predictive of the relationship between drinking and depressive symptoms: Grant et al. (2009) found that college students who exhibited “coping” drinking motivations—i.e., used alcohol to avoid or alleviate sadness or anxiety—consumed more alcohol with increasing levels of negative affect than did those with drinking motivations such as experiential enhancement or social conformity.
These studies suggest that the relationship between internalizing disorders and alcohol use is complex, but the presence of one phenotype typically exacerbates difficulties with the other. Importantly, epidemiological studies do not address the possibility that non-causal factors could contribute to the association between these phenotypes. In other words, depressive symptoms might lead to self-medicating alcohol use (Neighbors et al. 1992), and/or alcohol use might lead to depressive symptomatology, but the association might also be due to a common set of biological and/or environmental factors influencing both phenotypes.
Few genetically informative epidemiological studies have been undertaken to investigate potential non-causal mechanisms underlying the association between internalizing behavior and alcohol use, particularly among adolescents. A Norwegian study of twins aged 18–25 found that shared genetic influences fully accounted for the correlation between alcohol use and depressive symptoms among males; among females, this correlation was due to either shared environmental influences or a combination of shared genetic and environmental factors (Tambs et al. 1997). Kendler et al. (1993) reported genetic correlations between alcoholism and major depression (MD) ranging from 0.4 to 0.6 among adult female twins. Results from a study of adult male twins (Lyons et al. 2006) suggest that, while genetic correlations between alcoholism and MD could not be ruled out, a reciprocal-causation model provided the best fit to the data.
Here, we present an analysis of the genetic and environmental factors influencing depressive symptoms and alcohol use across adolescence, as assessed in a longitudinal study of Finnish twins. The longitudinal design enables us to investigate changes in genetic and environmental influences on each phenotype across adolescence, as well as the degree to which alcohol use and depressive symptoms are influenced by shared genetic and environmental influences over time. We addressed several primary questions: (i) to what degree are depressive symptoms and alcohol use influenced by genetic and environmental factors at different ages (12, 14, and 17.5) during adolescence; (ii) at each age, is there evidence of a shared liability to depression and alcohol use, as manifested by genetic and/or environmental correlation between the phenotypes; (iii) if a shared liability exists, does it change across adolescence; and (iv) is there evidence of a causal relationship between phenotypes?
Methods
Sample
FinnTwin 12 (FT12) is an ongoing longitudinal study with the purpose of investigating genetic and environmental influences on health-related behaviors. From 1994 to 1998, Finnish families with twins born between 1983 and 1987 were identified from Finland’s population registry and enrolled in the study. FT12 consists of two stages: the first used questionnaires to assess all twins and parents at baseline (87% participation rate, N = 2,724 families), with follow-up of all twins at ages 14 and 17.5. The second stage consisted of intensive assessments of a subsample of this group; 28% of the subsample consisted of twins selected because of parental scores on the Malmö-modified Michigan Alcoholism Screening Test (Seppa et al. 1990) and assumed to be at increased risk for alcohol problems (Dick et al. 2005; Rose et al. 2004). The current analyses were based on the “intensive” subsample, as data on depressive symptoms and alcohol use were not available for the full sample at all three time points.
Because of the complicated structure of our twin model, we did not assess qualitative gender effects, and therefore excluded opposite-sex twin pairs from our analyses. Thus, only monozygotic (MZ) and same-sex dizygotic (DZ) twin pairs were included. Complete or partial data were available for 169 female MZ pairs, 170 male MZ pairs, 138 female DZ pairs, and 165 male DZ pairs; the total sample consisted of 1,282 individuals. Zygosity was determined using a well-validated questionnaire completed by both co-twins at baseline, as described elsewhere (Kaprio et al. 1995). Confirmation of zygosity came from genetic testing of DNA from twin pairs participating in the ongoing fourth wave of data collection. The ethics committee of the Helsinki and Uusimaa University Hospital District, Finland, and the institutional review board of Indiana University approved data collection.
Measures
Depressive symptoms were assessed using the Children’s Depression Inventory (CDI) (Kovacs 1991) at ages 12 and 14, and a subscale of the General Behavior Inventory (GBI) (Depue 1987) at age 17.5. The CDI consists of 27 questions addressing depressive symptoms such as disturbed mood, interpersonal behaviors, and vegetative functioning. Each item has three options, corresponding to no symptoms, mild symptoms, or definite symptoms (or never, sometimes, and often), scored from 1 to 3, with three points assigned to the highest level. The subscale of the GBI consists of 10 items corresponding approximately to criteria A for a Major Depressive Episode in the DSM-IV-TR (American Psychiatric Association 2000). Each item has four options (never, sometimes, often, very often), resulting in a possible score of 40. Cronbach’s alpha values were good for this sample: α = 0.84 for age 12 CDI, α = 0.84 for age 14 CDI, and α = 0.89 for age 17.5 GBI.
Measures of alcohol use differed across ages. At age 12, participants were asked whether they had drunk alcohol without adults present. At age 14, participants were asked how frequently they consumed alcohol, with options: never/don’t drink, less than once per month, 1–2 times per month, or once per week or more. At age 17.5, they were also asked about drinking frequency, with nine options: never, once per year or less, 2–4 times per year, once every 2 months, once per month, twice per month, once per week, twice per week, or daily. The age 17.5 measure was collapsed into four groups to parallel the age 14 responses for twin modeling.
Statistical analysis
Phenotypic correlations of ordinalized data (see below) were conducted in MPlus Version 5 (Muthen and Muthen 1998–2007) and the significance corrected to account for the correlated nature of twins. Twin correlations and tests for gender differences, corrected for twinship, were conducted in SAS 9.1.3. Twin modeling was conducted in Mx (Neale et al. 2006) using the raw ordinal data option. Because it is not currently possible to simultaneously model ordinal and continuous variables in Mx, and alcohol use measures were ordinal, the depression scales were ordinalized into four categories at each age by subdividing raw scores into approximately equal ranges. In twin modeling, liability to measured phenotypes can be attributed to several latent sources of variance: additive genetic factors (A), non-additive genetic factors (D), shared environment (C), and unique environment (E). The C variance component represents environmental exposures and experiences shared by both members of a twin pair, and contributes to twins’ increased phenotypic similarity irrespective of zygosity. Environmental factors unique to one twin are accounted for by the E component; these factors generally reduce twin similarity for a given phenotype. This component also includes measurement error. Estimates of each variance component are calculated by comparing the phenotypic correlation between MZ twins, who share all their genes, to DZ twins, who share half of their genes on average identical by descent. Here, phenotypic correlation between MZ twins was higher than between DZ twins in most cases (Table 1), suggesting that phenotypic variation is at least partially attributable to genetic influences.
Table 1.
Polychoric cross-twin correlations within and across phenotypes and ages are presented
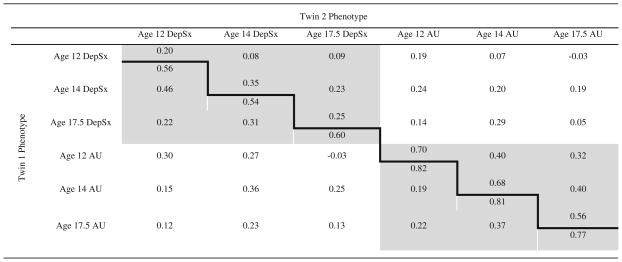 |
Monozygotic cross-twin correlations are below the bold line and dizygotic cross-twin correlations are above. Cross-twin within-trait correlations are shaded; cross-twin cross-trait correlations are not
DepSx depressive symptoms, AU alcohol use
In order to estimate genetic and environmental influences on each phenotype, as well as the genetic and environmental covariance across time, we fit a Cholesky decomposition model with six variables: depression at ages 12, 14, and 17.5; and alcohol use at ages 12, 14, and 17.5. Variables were ordered by age; within age, depression was the first variable, since depressive symptoms were evident prior to alcohol use initiation for most individuals. The C and E pathways follow the same pattern. The full model allows for genetic influences (A1) that impact both outcomes (depressive symptoms and alcohol use), at all time points; genetic influences that don’t impact depressive symptoms at 12, but influence all other variables (A2); genetic influences that become evident at age 14 (A3), influence both variables and carry through to age 17.5, etc. This is an atheoretical model from which submodels are fit to determine the best-fitting model for the data.
Models were estimated using a full information maximum likelihood estimation method (Neale et al. 2003). The fit of nested models was assessed as a function of the change in the value of twice the log likelihood of the data, which is distributed as a χ2 statistic with degrees of freedom equal to the difference in the number of parameters estimated between models. A significant Δχ2 indicates a significant deterioration in model fit, which would result in rejection of the nested model. We also used the Akaike Information Criterion (Akaike 1987) (AIC) to select models. A lower AIC value indicates a better balance between the explanatory power of a model and parsimony.
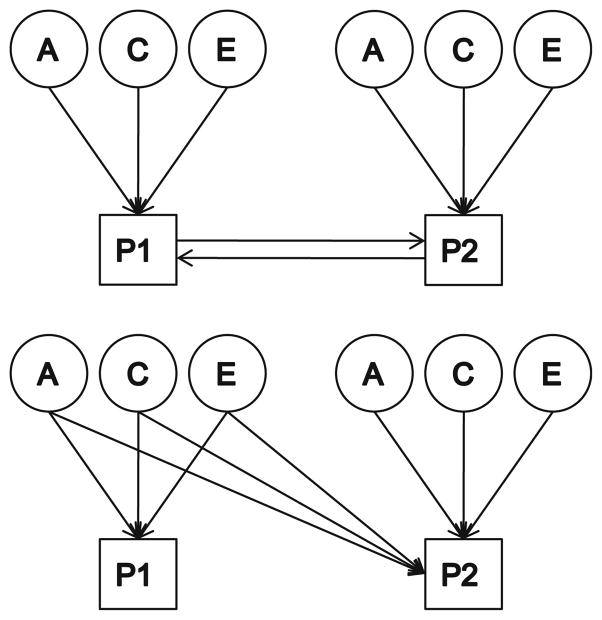
In order to test for causality, we conducted two different sets of analyses. The first is the cross-sectional MZ intrapair differences model (Pietilainen et al. 2007; De Moor et al. 2008), in which the phenotypic difference between twins for one trait is regressed onto the phenotypic difference in the other trait. This test is conducted only for MZ twins to control for genetic influences. Significant positive regression coefficients would be consistent with a causal hypothesis, indicating that differences in depressive symptoms within a twin pair are associated with differences in alcohol use between the co-twins (or vice versa). Because reciprocal causation is feasible, each phenotype was regressed onto the other. In addition, we tested formal cross-sectional reciprocal causation twin models, asking whether either or both causal paths could be dropped. Since causal models are not nested within Cholesky models, fit statistics for these models are informally compared (Fig. 1).
Fig. 1.
A within-wave reciprocal causation model is depicted at top, and a within-wave shared liability model on the bottom. Only Twin 1’s phenotypes are depicted. P1, P2 phenotype 1, phenotype 2, T1, T2 time 1, time 2, A additive genetic factors, C shared environmental factors, E unique environmental factors
Results
Descriptive statistics
Analyzed separately by gender, measures of depressive symptoms and alcohol use did not differ by zygosity at any age (data not shown). CDI scores at age 12 were comparable in males (mean = 35.12) and females (mean = 35.20), with no significant gender difference (Z = −0.2, p = 0.84). At age 14, female CDI scores (mean = 33.74) were significantly higher than male scores (mean = 32.73), (Z = −2.18, p = 0.03). At age 17.5, GBI scores differed significantly (Z = −7.03, p < 0.0001) between the genders, with scores higher among females (mean = 16.33) than among males (mean = 13.66).
At age 12, only a small proportion (6.2%) of individuals reported drinking without adults present, and there were no gender differences (Z = −0.61, p = 0.54). Trends toward more frequent drinking among females were observed at age 14, (Z = −2.06, p = 0.04), and 38.8% of the sample reported using alcohol. No gender differences in drinking frequency were observed at age 17.5 (Z = −1.52, p = 0.13), and 88.9% of the sample reported using alcohol.
Polychoric correlations of ordinal measures of alcohol use and depressive symptoms within and across ages are available in Table 2. Longitudinal estimates suggested that earlier drinking tended to correlate with later depressive symptoms more strongly than did earlier depressive symptoms with later drinking.
Table 2.
Phenotypic polychoric correlations between phenotypes, statistical significance corrected for the correlated nature of twins
| Age of depressive symptoms | Age of alcohol use |
||
|---|---|---|---|
| 12 (N = 1,280) | 14 (N = 1,154) | 17.5 (N = 904) | |
| 12 (N = 1,216) | 0.294*** | 0.098* | 0.067 |
| 14 (N = 700) | 0.201** | 0.252*** | 0.134** |
| 17.5 (N = 901) | 0.151* | 0.145*** | 0.138*** |
p < 0.05,
p < 0.01,
p < 0.001
Shared liability (Cholesky) twin modeling
Model fitting statistics are provided in Table 3. Variance could be constrained to be equal across genders without a loss in model fit (Model 2 in Table 3); subsequent models incorporated this constraint. We next tested whether an AE (Model 3) or a CE model (Model 4), where shared environmental or genetic factors, respectively, are excluded across both phenotypes, provided an adequate fit. Both the AE and CE model fit the data significantly worse than did the ACE model, and were rejected.
Table 3.
Model fitting results
| Model # | Model | Comparison | Δdf | Δχ2 | p-value | ΔAIC |
|---|---|---|---|---|---|---|
| 1 | ACE (full model) | n/a | (6009) | (10796.21) | n/a | (−1221.799) |
| 2 | Constrain variance across genders | 2 vs. 1 | 63 | 72.005 | 0.204 | −53.986 |
| 3 | AE model | 3 vs. 2 | 21 | 42.249 | 0.004 | 0.249 |
| 4 | CE model | 4 vs. 2 | 21 | 48.016 | 0.001 | 6.016 |
| 5 | Drop factor loadings from C1, C3, and C5 | 5 vs. 2 | 12 | 9.686 | 0.643 | −14.314 |
| 6 | Drop all C-factor loadings onto depressive symptoms | 6 vs. 5 | 3 | 9.215 | 0.027 | 3.215 |
| 7 | Drop A correlations between phenotypes | 7 vs. 5 | 9 | 18.86 | 0.026 | 0.86 |
| 8 | Drop A correlations across ages within phenotypes | 8 vs. 5 | 6 | 51.378 | 0 | 39.378 |
| 9 | Drop E correlations across ages | 9 vs. 5 | 12 | 20.69 | 0.055 | −3.31 |
| 10 | Drop E correlations between phenotypes | 10 vs. 5 | 9 | 6.540 | 0.685 | −11.460 |
| 11 | Drop all E correlations | 11 vs. 5 | 15 | 25.023 | 0.05 | −4.977 |
Model selection proceeded as described in the “Methods”
Because preliminary analyses of depression and alcohol use modeled separately indicated that an AE model fit depression data (but not alcohol-use data) well and an ADE did not fit better than an AE model for depression (data not shown), we tested submodels in which shared environmental factor loadings on depression varied (Models 5–6 in Table 3). We found that three “C” factors sufficiently explained the data, and that these factors loaded onto both depression and alcohol use phenotypes. Subsequent models were nested within Model 5.
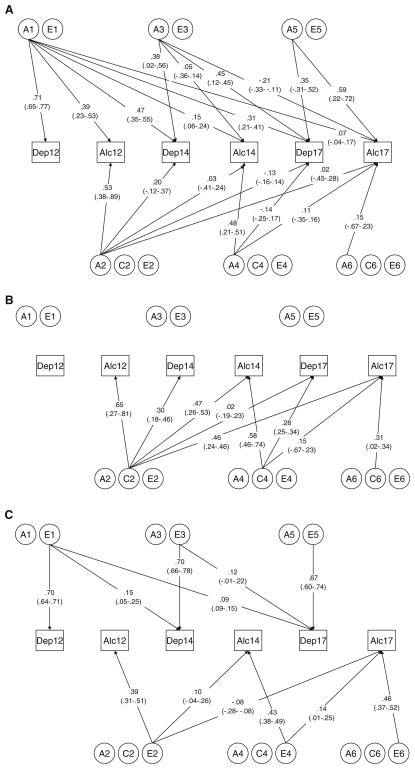
Next, we tested whether genetic (“A”) and unique environmental (“E”) factors were specific to one phenotype (i.e., depression or alcohol use), and whether they were time-specific (i.e., affecting phenotypes only at age 12, 14, or 17.5). We found that genetic factors were neither phenotype- nor time-specific (Models 7 and 8, respectively): six genetic factors loaded onto both depression and alcohol use phenotypes across time. Models 9–11 tested the effects of E factors. Removing cross-time E correlations (Model 9) results in a nearly significant (p = 0.055) deterioration in fit, while removing cross-trait E correlations (Model 10) had no such effect. Dropping all E correlations (Model 11) resulted in a significantly (p = 0.05) worse model fit. Thus, Model 10 was selected as the final model, wherein three latent unique environmental factors influenced depressive symptoms (E1, E3, and E5), and three different latent unique environmental factors influenced alcohol use (E2, E4, and E6). To avoid over-fitting, we did not test whether additional individual paths could be dropped from the model. Final path estimates and confidence intervals from the best-fitting model are depicted in Fig. 2a–c. Variance components from the best-fitting model are presented in Table 4; genetic and environmental covariances are available in Supplementary Material.
Fig. 2.
Factor loadings from the final model, along with 95% confidence intervals, are depicted for genetic factors (a), shared environmental factors (b), and unique environmental factors (c)
Table 4.
Variance components (95% CI) from best-fitting modela
| A | C | E | |
|---|---|---|---|
| Age 12 depressive symptoms | .51 (.42–.60) | 0 (n/a) | .49 (.41–.58) |
| Age 12 alcohol use | .43 (.13–.90) | .42 (.07–.67) | .15 (.08–.23) |
| Age 14 depressive symptoms | .40 (.38–.56) | .09 (.07–.25) | .51 (.42–.55) |
| Age 14 alcohol use | .25 (.24–.46) | .56 (.35–.70) | .19 (.14–.25) |
| Age 17.5 depressive symptoms | .45 (.28–.58) | .08 (.03–.18) | .47 (.37–.59) |
| Age 17.5 alcohol use | .44 (.43–.45) | .33 (.18–.51) | .23 (.18–.31) |
Total might not sum to 1 due to rounding
A genetic variance, C shared environmental variance, E unique environmental variance
−2LL = 10884.621, df = 6093, AIC = −1301.379
Genetic and environmental influences on depressive symptoms
The heritability of depressive symptoms remains relatively static across adolescence. At age 12, depressive symptoms are moderately heritable (a2 = .51). This estimate decreases at age 14 (a2 = .40) and increases by age 17.5 (a2 = .45). Genetic correlations across ages are moderate to high (rA = .46–.74). Genetic influences on depressive symptoms that are evident at age 12 (i.e., factor A1) account for 54% of the total genetic variance in depressive symptoms at age 14, and 21% at age 17.5. Genetic factors that become influential on depressive symptoms at age 14 (A3) still account for nearly 45% of the total genetic variance at age 17.5. Only a small proportion of the total variance can be attributed to shared environmental factors at ages 14 (c2 = .09) and 17.5 (c2 = .08); the model suggests there are no significant shared environmental influences on depressive symptoms at age 12. The shared environmental correlation between depression at age 14 and 17.5 is low (rC = .08). The total contribution of unique environmental influences to depressive symptoms is also quite stable at different ages (e2 = .49, .51, and .47 at ages 12, 14, and 17.5, respectively). Unique environmental correlations across depressive symptoms at different ages are low (rE = .13–.20). Unique environmental influences on depressive symptoms at each age are strongly driven by time-specific factors (E3 at age 14, and E5 at age 17.5), with only modest contributions from environmental factors relevant at earlier ages.
Genetic and environmental influences on alcohol use
The heritability of alcohol use is more dynamic across development. At age 12, genetic influences account for 43% of the total variance; this decreases to a2 = .25 at age 14, and rebounds to a2 = .44 at age 17.5. Genetic influences on alcohol use that are evident at age 12 (factors A1 and A2) account for < 10% of the total genetic variance in depressive symptoms at age 14, and < 2% at age 17.5. Similarly, genetic factors that become influential on alcohol use at age 14 (A3 and A4) account for< 15% of the total genetic variance at age 17.5. Factor A5 accounts for over 80% of the total genetic variance in alcohol use at age 17.5, and also influences contemporaneous depressive symptoms. The genetic correlations between alcohol use across ages are consistently low (rA = .05–.16). Alcohol use is strongly influenced by shared environmental factors, and the proportion of variance attributable to these factors is dynamic: c2 = .42, .56, and .33 at ages 12, 14, and 17.5, respectively. Shared environmental correlations across ages are high (rC = .63–.80). Unique environmental factors account for only a modest proportion of the total variance in alcohol use, but become more influential across development: e2 = 0.15 at age 12, .19 at age 14, and .23 at age 17.5. Unique environmental correlations are modest, ranging from rE = −.16–.24. As with depressive symptoms, genetic innovation influencing alcohol use is observed at each age; in addition, both shared and unique environmental influences are due largely, though not entirely, to age-specific factors.
Relationships between depressive symptoms and alcohol use
Genetic correlations between depressive symptoms and alcohol use are generally positive, but decrease with increasing age, from rA = .59 at age 12, to rA = .29 at age 14, and rA = .26 at age 17.5. Within-age shared environmental correlations between the phenotypes were moderate (rC = .30–.63), yet shared environmental factor loadings onto depression variables are quite low. Unique environmental correlations between phenotypes could be set to 0.
In several cases, genetic correlations between phenotypes across time were substantial. Depressive symptoms at age 12 and alcohol use at 14 were modestly genetically correlated (rA = .29), as were age 12 depressive symptoms and age 17.5 alcohol use (rA = .11); alcohol use at 12 was moderately correlated with depressive symptoms at age 17.5 (rA = .43). However, cross-phenotype correlations between ages 14 and 17.5 were weakly negative: rA = −.11 for depressive symptoms at 14 and alcohol use at 17.5. The genetic correlation between alcohol use at 14 and depressive symptoms at 17.5 was near 0 (rA < −.01). Shared environmental correlations across time were derived from very low covariances and varied widely (rC = .08–.82).
Causal models
Parameter estimates and significance values for the MZ intrapair differences regressions are provided in Table 5. For males, phenotypic differences in one trait did not predict phenotypic differences in the other trait at any age. For females, there was no association between trait differences at age 12. However, at age 14, intrapair differences in one trait did significantly (p = 0.0052 or p = 0.0183, depending on which variable was used as the independent variable) and positively predict differences in the other. At age 17.5, the association remained significant (p = 0.0075 or p = 0.0137). These results are consistent with causation between traits.
Table 5.
Unstandardized parameter estimates and p-values for MZ intrapair differences regressions
| Age | Dependent variable | Independent variable | Boys |
Girls |
||
|---|---|---|---|---|---|---|
| B | p-value | B | p-value | |||
| Age 12 | Alcohol use | Depressive Sx | −0.0143 | 0.8129 | 0.0184 | 0.7521 |
| Depressive Sx | Alcohol use | −0.4148 | 0.8273 | 0.7074 | 0.7469 | |
| Age 14 | Alcohol use | Depressive Sx | 0.0513 | 0.2500 | 0.1339 | 0.0183 |
| Depressive Sx | Alcohol use | 0.9550 | 0.2497 | 2.1808 | 0.0052 | |
| Age 17.5 | Alcohol use | Depressive Sx | 0.0305 | 0.4255 | 0.0771 | 0.0075 |
| Depressive Sx | Alcohol use | 0.1951 | 0.5131 | 0.8229 | 0.0137 | |
We next tested causal twin models and compared fit statistics to those of corresponding bivariate Cholesky models (see Fig. 1). For these analyses, the only submodels tested were those investigating the significance of the causal pathways (i.e., AE/CE models, etc., were not fit). Within each wave, we tested whether causal paths in either direction could be set to zero (dropped), and whether both causal paths could be dropped. Results are summarized in Table 6. In most cases each causal path could be dropped individually (see Table 6 for exceptions), but these paths could not be dropped in tandem. Relevant fit statistics (χ2 and AIC) for the full causal models were quite comparable to the corresponding bivariate Cholesky models at each age, and in every case the difference in AIC between the two models is very small (< 2.00, for a one degree of freedom difference).
Table 6.
Summary of causal model findings, and comparison of fit statistics to corresponding Cholesky fit statistics
| Age 12 |
Age 14 |
Age 17.5 |
||||
|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | |
| Can the causal path from alcohol use to depressive symptoms be dropped? (Δdf = 1) | No, p = 0.042 | Yes, p = 0.473 | Yes, p = 0.333 | No, p = 0.001 | Yes, p = 0.333 | Yes, p = 0.231 |
| Can the causal path from depressive symptoms to alcohol use be dropped? (Δdf = 1) | Yes, p = 0.089 | Yes, p = 0.379 | Yes, p = 0.801 | Yes, p = 0.450 | Yes, p = 0.837 | Yes, p = 0.882 |
| Can both causal paths be dropped? (Δdf = 2) | No, p < 0.001 | No, p = 0.010 | Yes, p = 0.128 | No, p < 0.001 | Yes, p = 0.188 | No, p < 0.001 |
| Full causal model | ||||||
| −2LL | 1750.740 | 1628.995 | 1776.183 | 1800.139 | 1988.678 | 2204.950 |
| AIC | −819.260 | −753.115 | −63.817 | −19.861 | 238.678 | 392.950 |
| Full Cholesky modela | ||||||
| −2LL | 1750.279 | 1628.605 | 1773.966 | 1800.116 | 1987.898 | 2204.416 |
| AIC | −817.721 | −751.395 | −64.034 | −17.884 | 239.989 | 394.416 |
The p-value provided is the result of the test of dropping the causal path(s) indicated. A p < 0.05 indicates that the submodel fits significantly worse than the full model
The full causal model has one degree of freedom more than the full Cholesky model
Discussion
We addressed four primary questions: (i) how much of the variance in depressive symptoms and alcohol use can be explained by genetic and environmental factors across different ages during adolescence; (ii) at each age, does genetic and/or environmental correlation exist between these phenotypes; (iii) is the nature of any shared liability stable or dynamic; and (iv) is there evidence of a causal relationship between phenotypes? These issues will be addressed in turn.
The heritability of depressive symptoms remains relatively consistent across adolescence (a2 = .41–.51). Shared environmental factors account for little of the total variance in depressive symptoms (c2 = .08–.09), while unique environmental factors are highly influential (e2 = .47–.51). The presence of non-trivial “new” genetic and environmental factor loadings at each successive age is noteworthy. For example, the third genetic factor (A3) accounts for ~41% of the total genetic variance of depressive symptoms at age 14; factor A5 accounts for ~28% of the total genetic variance in depressive symptoms at age 17.5. Thus, although the total heritability of depressive symptoms is relatively consistent across time, the genetic variation underlying depressive symptoms is temporally dynamic. These results differ from a previous longitudinal study of depression in adolescents (O’Connor et al. 1998), wherein the best-fitting model included no novel genetic effects at a second wave. Those participants were aged 10–18 during the first data collection wave, with the second wave of data collected 3 years later. This substantial age variation likely contributes to the discrepancy with the current report, as our analyses clearly suggest dynamic changes across relatively short time periods in adolescence.
Most unique environmental variation affecting depressive symptoms is time-specific: ~95% of the total environmental variance in depressive symptoms at ages 14 and 17.5 explained by time-specific influences. However, the inclusion of cross-time unique environmental correlations provides a better fit than excluding those paths, as demonstrated by the borderline significant p-value of Model 11 (p = 0.05) and the lower AIC of Model 10 relative to Model 11 (Table 3), suggesting some carry-over effects across time.
Our heritability estimates for depressive symptoms are comparable to those in previous adolescent samples. Glowinski et al. (2003) and Rijsdijk et al. (2003) reported heritabilities of 0.40 and 0.44 in adolescent females, respectively; Tambs et al. (1997), assessing young adults aged 18–25, also reported comparable genetic influences (a2 = .25–.48 in males and .45–.56 in females). However, some researchers have reported estimates in excess of 0.80 (Kendler et al. 2008; Scourfield et al. 2003; Thapar and McGuffin 1994); and others have found genetic factors to be far less influential (Eaves et al. 1997; Silberg et al. 1999) than reported here. This variation is likely attributable to a number of factors. Real variation among populations certainly exists and can result in different heritability estimates. The use of disparate measures of depression (e.g., DSM criteria, Achenbach’s internalizing scale [Kendler et al. 2008], CDI, GBI) across studies also contributes to different results. Importantly, the CDI measures a broad range of depressive symptoms (e.g., interpersonal behaviors, mood, self-evaluation), whereas the GBI subscale closely parallels DSM-IV-TR criteria for a major depressive episode. Finally, our multivariate model provides additional power, enabling us to detect shared environmental effects on depressive symptoms that can often be excluded from less powerful univariate models.
The heritability of alcohol use is similar at ages 12 and 17.5 (a2 = .45 and .44, respectively), but far lower at age 14 (a2 = .24). Unique environmental factors account for little of the total variance at any age, but become more influential across time. In contrast to depressive symptoms, shared environmental factors heavily influence alcohol use: c2 is highest at age 14 (.56), but also accounts for ~40% of the total variance at age 12 and ~33% at age 17.5. Previous analyses in Finnish twin samples indicated that from age 14 to 18, genetic influences assume increasing importance as shared environmental influences decrease (Dick et al. 2007). Accordingly, the higher heritability at age 12 than age 14 is interesting. Perhaps very early alcohol use is genetically influenced, whereas by age 14 it is becoming more normative for adolescents to start experimenting, resulting in C’s increased importance. Importantly, the phenotype changes between ages 12 and 14: at age 12, the use of alcohol without adults present is non-normative (< 7% of the sample engaged in this behavior), but by age 14 nearly 40% of the sample uses alcohol. We might expect these measures of alcohol use to be influenced by rather distinct suites of genes. Indeed, genetic innovation is more pronounced for alcohol use than for depressive symptoms: ~90% of the total genetic variance of alcohol use at 14 can be accounted for by novel genetic influences; at age 17.5 this figure is ~85%. However, shared environmental variance at ages 14 and 17.5 is largely influenced by environmental factors that were already relevant at earlier ages. Even more striking is that common environmental influences evident at age 12 account for ~68% of the total shared environmental variance in alcohol use at age 17.5, although they account for less of the total variance at that age.
Previously reported heritability estimates of alcohol use vary widely. One study reported quite low heritability in boys and girls (a2 = .07 and .10, respectively) (Rhee et al. 2003); others report higher estimates (a2 = .72–.96) (Maes et al. 1999; Miles et al. 2005). Our results are intermediate to the polarized previous reports. Again, this might be due to age differences in those studies and/or phenotype assessment variation.
Our results indicate that contemporaneous depressive symptoms and alcohol use are modestly to moderately genetically (rA = .26–.59) and environmentally (rC = .30–.63) correlated during adolescence. The environmental component of this correlation is limited to environmental factors shared by twins. Shared liability exists at each age assessed, although the structure of our final model excludes environmental correlation at age 12. The genetic correlation between phenotypes is strongest at age 12 and steadily decreases; the shared environmental correlation is high at age 14 and decreases at age 17.5, although factor loadings onto depression are quite low. Thus, the genetic and environmental influences on depressive symptoms and alcohol use are developmentally dynamic for each phenotype individually and as they affect both phenotypes. Importantly, these results suggest that a shared liability, in the form of common genetic and/or environmental factors, is at least partially accountable for the phenotypic correlation between depressive symptoms and alcohol use across adolescence.
As mentioned previously, in order to avoid over-fitting, we limited our model-fitting procedure to tests that address general questions about the genetic and environmental nature of depressive symptoms and alcohol use. As such, a number of genetic and environmental factor loadings remain in the final model even though the confidence intervals around these estimates span 0 (i.e., are not statistically significant). Careful scrutiny of these paths (in Fig. 2a–c) is necessary to fully appreciate the nuances of the genetic/environmental relationships between traits and across time for this population. For example, genetic factor A1 accounts for the majority of significant shared genetic variation across traits and time. In addition, factors A2 and A4 contribute little to the total genetic variance of depressive symptoms, and are largely time-specific. Thus, although model-fitting results clearly indicate that genetic influences are shared across traits and time, much of the variance shared across traits is derived from one genetic factor, and genetic influences are largely time-specific.
Our assessment of causal relationships between depressive symptoms and alcohol use provide suggestion of causality, though the results are not wholly conclusive. Results from the MZ intrapair differences model are consistent with causation, in girls only, at ages 14 and 17. In the formal twin modeling, causal pathways could not be dropped between the two variables, though in most cases it was not possible to determine the direction of causation. Furthermore, fit statistics did not differ substantially between the causal models and the corresponding bivariate Cholesky models, making it difficult to identify a preferred model. In general, twin models have low power to make this discrimination (Heath et al. 1993). It is worth noting that one can also make inferences about potential causal relationships from the longitudinal Cholesky model presented in the “Results”: de Moor et al. (2008) state that, if a causal relationship exists between phenotypes, this would manifest as significant genetic, shared environmental, and unique environmental correlations. However, as demonstrated by Model 10 (Table 3), unique environmental correlations between traits can be dropped from the model. This finding appears inconsistent with causation, though it could also be due to measurement error. In any case, the results suggest that if causal relationships exist between the traits, they might vary between the sexes, and change over time.
To our knowledge, this is the first report of genetic and environmental correlations between depressive symptoms and alcohol use in a longitudinally assessed adolescent sample. As such, it provides a unique insight into the dynamic relation between these behavioral phenotypes. Because very few prior reports of genetic correlations between these phenotypes during adolescence exist, it is difficult to contextualize our findings. This underscores the need for additional studies in genetically informative adolescent samples. The complex, dynamic nature of genetic and environmental influences on alcohol use and depressive symptomatology may explain the inconsistent findings within the literature on the connection between alcohol and depression. Internalizing pathways to alcohol use have not been established and delineated as well as externalizing pathways (Zucker 2008). Our results suggest that this could be because the relationship between these variables is due to a mixture of genetic and environmental factors that differ across time and, as suggested by other studies (Han et al. 1999), though not in the shared liability models tested here, across genders. The potential causal relationship between traits further complicates our understanding of their association. Although our data do not provide definitive conclusions about whether a causal or shared liability model provides a superior fit to the data, our results are not wholly inconsistent with causal paths between traits, which changes over time and across the sexes.
Prior reports relied on stringent diagnostic criteria, and focused almost entirely on adult samples. Importantly, the current study suggests that the shared biological and environmental influences on depressive symptoms and alcohol use are present early in adolescence, and can be identified even at sub-clinical levels of each phenotype. The genetic and shared environmental correlations decrease across time; however, the shared liability between depression and alcohol use, present in adolescence, might establish the foundation for associations between these phenotypes in adulthood even with decreasing genetic and/or environmental correlations. These findings have potential clinical implications: adolescents presenting with only depressive symptoms or alcohol use might be at increased risk for developing the other phenotype in time (Sihvola et al. 2008) due to shared genetic/environmental liability, and/or causal influences.
We acknowledge a number of limitations to the current study. As our analyses were conducted on a sample of Finnish twins, a portion of which were enriched for risk of alcohol problems, the generalizability of our findings is uncertain. In addition, we utilized different phenotypic measures at different ages, which may have implications for the continuity of influences across time, as suggested by the structural equation modeling. Furthermore, our results might not be generalizable to measures of alcohol use other than drinking frequency, such as frequency of intoxication or alcohol abuse or dependence. Our exclusion of twins of opposite gender precluded testing for qualitative gender effects in this study. Despite these limitations, these analyses provide important evidence that shared genetic and environmental factors influence the association between depressive symptoms and alcohol use in a dynamic manner across adolescence.
Supplementary Material
Acknowledgments
This work is supported by The Academy of Finland Centre of Excellence in Complex Disease Genetics, the National Institute on Alcohol Abuse and Alcoholism (AA12502 and AA09203 to RJR, AA15416 to DMD), and the Academy of Finland (100499, 205585, and 118555 to JK). ACE was supported by the National Institute of Mental Health (MH020030).
Footnotes
This work is edited by Valerie Knopik
Electronic supplementary material The online version of this article (doi:10.1007/s10519-010-9400-y) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Alexis C. Edwards, Email: aedwards5@vcu.edu, Department of Psychiatry, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, PO Box 980126, Richmond, VA 23298-0126, USA
Elina Sihvola, Department of Public Health, University of Helsinki, Helsinki, Finland.
Tellervo Korhonen, Department of Public Health, University of Helsinki, Helsinki, Finland, Unit for Child and Adolescent Psychiatry, National Institute for Health and Welfare, Helsinki, Finland.
Lea Pulkkinen, Department of Psychology, University of Jyväskylä, Jyväskylä, Finland.
Irma Moilanen, Department of Psychiatry, University of Oulu, Oulu, Finland.
Jaakko Kaprio, Department of Public Health, University of Helsinki, Helsinki, Finland, Institute for Molecular Medicine, University of Helsinki, Helsinki, Finland, Unit for Child and Adolescent Psychiatry, National Institute for Health and Welfare, Helsinki, Finland.
Richard J. Rose, Department of Psychological and Brain Sciences, Indiana University, Bloomington, IN, USA
Danielle M. Dick, Email: ddick@vcu.edu, Department of Psychiatry, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, PO Box 980126, Richmond, VA 23298-0126, USA
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4. American Psychiatric Publishing, Inc; USA: 2000. (text revision) [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child Adolesc Psychiatr Clin N Am. 2006;15(4):919–937. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Crum RM, Green KM, Storr CL, Chan YF, Ialongo N, Stuart EA, Anthony JC. Depressed mood in childhood and subsequent alcohol use through adolescence and young adulthood. Arch Gen Psychiatry. 2008;65(6):702–712. doi: 10.1001/archpsyc.65.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- Depue R. General behavior inventory. Department of Psychology, Cornell University; Ithaca: 1987. [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. J Abnorm Child Psychol. 2005;33(2):219–229. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007;10(2):315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff E, Pickles A, Rutter M, Neale MC, Reynolds CA, Erikson MT, Heath AC, Loeber R, Truett KR, Hewitt JK. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. J Child Psychol Psychiatry. 1997;38(8):965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Arch Gen Psychiatry. 2009;66(3):260–266. doi: 10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- Glowinski AL, Madden PA, Bucholz KK, Lynskey MT, Heath AC. Genetic epidemiology of self-reported lifetime DSM-IV major depressive disorder in a population-based twin sample of female adolescents. J Child Psychol Psychiatry. 2003;44(7):988–996. doi: 10.1111/1469-7610.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant VV, Stewart SH, Mohr CD. Coping-anxiety and coping-depression motives predict different daily mood-drinking relationships. Psychol Addict Behav. 2009;23(2):226–237. doi: 10.1037/a0015006. [DOI] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94(7):981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional family data. Behav Genet. 1993;23(1):29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Teen drug use continues down in 2006, particularly among older teens; but use of prescription-type drugs remains high. University of Michigan News and Information Services; Ann Arbor: 2006. [Google Scholar]
- Kaprio J, Rimpela A, Winter T, Viken RJ, Rimpela M, Rose RJ. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67(5):739–753. [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women. A twin study of the causes of comorbidity. Arch Gen Psychiatry. 1993;50(9):690–698. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychol Med. 2008;38(11):1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The child’s depression inventory (CDI) Multi Health Systems; North Tonawanda: 1991. [Google Scholar]
- Lyons MJ, Schultz M, Neale M, Brady K, Eisen S, Toomey R, Rhein A, Faraone S, Tsuang M. Specificity of familial vulnerability for alcoholism versus major depression in men. J Nerv Ment Dis. 2006;194(11):809–817. doi: 10.1097/01.nmd.0000244480.78431.49. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, Neale MC, Eaves LJ. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60(3):293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Meririnne E, Kiviruusu O, Karlsson L, Pelkonen M, Ruuttu T, Tuisku V, Marttunen M. Brief report: excessive alcohol use negatively affects the course of adolescent depression: one year naturalistic follow-up study. J Adolesc. 2010;33(1):221–226. doi: 10.1016/j.adolescence.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Miles DR, Silberg JL, Pickens RW, Eaves LJ. Familial influences on alcohol use in adolescent female twins: testing for genetic and environmental interactions. J Stud Alcohol. 2005;66(4):445–451. doi: 10.15288/jsa.2005.66.445. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 5. Muthen & Muthen; Los Angeles: 1998–2007. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Virginia Institute for Psychiatric and Behavioral Genetics. Virginia Commonwealth University; Richmond: 2003. Mx: statistical modeling. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Department of Psychiatry, Virginia Commonwealth University; Richmond: 2006. [Google Scholar]
- Neighbors B, Kempton T, Forehand R. Co-occurrence of substance abuse with conduct, anxiety, and depression disorders in juvenile delinquents. Addict Behav. 1992;17(4):379–386. doi: 10.1016/0306-4603(92)90043-u. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Genetic contributions to continuity, change, and co-occurrence of antisocial and depressive symptoms in adolescence. J Child Psychol Psychiatry. 1998;39(3):323–336. [PubMed] [Google Scholar]
- Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. Substance Abuse and Mental Health Services Administration; Rockville: 2005. [Google Scholar]
- Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, Oresic M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects—a monozygotic twin study. PLoS One. 2007;2(2):e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60(12):1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Snieder H, Ormel J, Sham P, Goldberg DP, Spector TD. Genetic and environmental influences on psychological distress in the population: General Health Questionnaire analyses in UK twins. Psychol Med. 2003;33(5):793–801. doi: 10.1017/s0033291703007451. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcohol Clin Exp Res. 2004;28(10):1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Scourfield J, Rice F, Thapar A, Harold GT, Martin N, McGuffin P. Depressive symptoms in children and adolescents: changing aetiological influences with development. J Child Psychol Psychiatry. 2003;44(7):968–976. doi: 10.1111/1469-7610.00181. [DOI] [PubMed] [Google Scholar]
- Seppa K, Sillanaukee P, Koivula T. The efficiency of a questionnaire in detecting heavy drinkers. Br J Addict. 1990;85(12):1639–1645. doi: 10.1111/j.1360-0443.1990.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Sihvola E, Rose RJ, Dick DM, Pulkkinen L, Marttunen M, Kaprio J. Early-onset depressive disorders predict the use of addictive substances in adolescence: a prospective study of adolescent Finnish twins. Addiction. 2008;103(12):2045–2053. doi: 10.1111/j.1360-0443.2008.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry. 1999;56(3):225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Strandheim A, Holmen TL, Coombes L, Bentzen N. Alcohol intoxication and mental health among adolescents—a population review of 8983 young people, 13–19 years in North-Trondelag, Norway: the Young-HUNT Study. Child Adolesc Psychiatry Ment Health. 2009;3(1):18. doi: 10.1186/1753-2000-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambs K, Harris JR, Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression. Results from a bivariate analysis of Norwegian twin data. Behav Genet. 1997;27(3):241–250. doi: 10.1023/a:1025662114352. [DOI] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. A twin study of depressive symptoms in childhood. Br J Psychiatry. 1994;165(2):259–265. doi: 10.1192/bjp.165.2.259. [DOI] [PubMed] [Google Scholar]
- Zucker RA. Anticipating problem alcohol use developmentally from childhood into middle adulthood: what have we learned? Addiction. 2008;103(Suppl 1):100–108. doi: 10.1111/j.1360-0443.2008.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.