Abstract
Purpose
Pancreatic cancer is the fourth leading cause of cancer death in the United States, and new drugs to treat the disease are needed. Pancreatic cancer cells are highly metastatic and exhibit resistance to apoptosis. Small molecules that can restore sensitivity to apoptosis or reduce metastasis would have therapeutic potential against this disease. Manzamine A is an alkaloid isolated from marine sponges that was suspected to have inhibitory activity against the mitogen activated kinase kinase (MEK). Because of this, the effects of Manzamine A were studied in pancreatic cancer cells.
Methods
AsPC-1 cells were treated for 48 h in the presence of various concentrations of Manzamine A and their phenotype, cytotoxicity, cell invasion and susceptibility to apoptosis were observed.
Results
Manzamine A decreased single cell formation, abrogated cell migration and restored the susceptibility of the cells to TRAIL-induced apoptosis in AsPC-1 cells. Its mechanism of action remains unknown, as manzamine A does not inhibit MEK.
Conclusions
Manzamine A appears to have a formerly unrecognized activity in blocking tumor cell invasion as well as in restoring cancer cell susceptibility to apoptosis in vitro and therefore has the potential to be used as an adjuvant to existing cancer therapies.
Keywords: Natural products, Pancreatic cancer, Drug discovery, Mechanism of action
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States, accounting for about ten percent of cancer deaths in the US [1]. The American Cancer Society estimates 42,470 new cases of pancreatic cancer and 35,240 deaths due to pancreatic cancer for 2009 [1]. Because a patient manifests symptoms only after the cancer has metastasized, the prognosis of pancreatic cancer is very negative [1]. Treatment of pancreatic cancer involves surgery, radiation therapy, chemotherapy or a combination of the three, but none of these methods are sufficiently effective. Although combination chemotherapy offers some hope, the prognosis of pancreatic cancer patients under current treatments is still poor, and new drugs to treat the disease are needed.
Pancreatic cancer cells are highly metastatic and very resistant to apoptosis. These characteristics result from the dysregulation of important signal transduction pathways. In pancreatic cancer, constitutive phosphorylation of the Raf-MEK-ERK pathway is a common occurrence [2], contributing to the metastatic potential of the disease by promoting cell dissociation [2] and resistance to apoptosis [3]. Inhibition of this pathway hinders the growth of pancreatic cancer cells [4]. Pancreatic cancer cells also exhibit constitutive activation of the nuclear factor kappa B (NFκB), and its activation correlates with their metastatic potential and resistance to apoptosis [5]. Finally, the constant activation of STAT3 has been shown to promote pancreatic cancer cells growth rate [6], implicating this pathway in the strong metastatic potential exhibited by pancreatic cancer cell lines. Therefore, any potential chemotherapies that can affect these overacting signal transduction pathways would have the potential of being more successful in the treatment of the disease than the currently available ones.
Manzamine A (HB-071) is an alkaloid isolated from sponges of the genera Haliclona sp., Xestospongia sp. and Pellina sp. [7-9]. It has been reported to have anti-tumor, insecticidal, antibacterial, anti-malarial, and anti-inflammatory activities [10-15]. To test if the anti-tumor properties of Manzamine A extended to pancreatic cancer cell lines, AsPC-1 pancreatic adenocarcinoma cells were treated for 48 h in the presence of various concentrations of Manzamine A and their phenotype, cytotoxicity, cell invasion and susceptibility to apoptosis were observed. Since Manzamine A was suspected to act upon the MEK/ERK signaling pathway based on cytoblot screening, the MEK inhibitor U0126 was used as a positive control at a previously reported concentration [2].
Materials and methods
Reagents
Manzamine A was obtained from the Harbor Branch Oceanographic Institution pure compound library. The material was originally isolated from a sponge of the genus Haliclona as described in US patent # 4,895,854. The Manzamine A stock solution was at a concentration of 5 mM in methanol. Methanol, isopropanol and Diff-Quick used in the experiments were purchased from Fisher Scientific, Fair Lawn, NJ. The known MEK inhibitor U0126 was purchased from Calbiochem, San Diego, CA. Tris and sodium chloride for western blotting buffers and 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) used for cell viability assays were purchased from Sigma Chemical Co., St. Louis, MO.
Cell culture
The human cancer cell lines Panc-1 (CRL-1469, pancreatic), DLD-1 (CCL-221, colon), A549 (CCL-185, lung), AsPC-1 (CRL-1682, pancreatic), as well as the control cell line Vero (CCL-81, African-green monkey kidney), were obtained from ATCC, grown, aliquotted and maintained in liquid nitrogen. The murine leukemia cell line P388 (0503478) and the multi-drug resistant human ovarian cancer cell line NCI/ADR (0503733) were obtained from NCI Frederick Cancer DCT Tumor repository, grown, aliquotted and maintained in liquid nitrogen. Aliquots of AsPC-1, Panc-1, DLD-1 and Vero were thawed and grown in RPMI-1640 supplemented with 10% Fetal Bovine Serum, 0.11 mg/ml Sodium Pyruvate, 4.5 g/L D-glucose, 18 mM HEPES Buffer, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 0.25 μg/ml amphotericin B, 2 mM L-glutamine and 50 μg/ml gentamicin (Complete RPMI). Aliquots of NCI/ADR, A549 and P388 were grown in Complete RPMI without sodium pyruvate or additional glucose. Cells were maintained in a humidified incubator at 37°C and 5% CO2.
Cytoblot
12,000 AsPC-1 cells per well were plated in 200 μl media/well and allowed to adhere for 24 h in the incubator. At the end of this incubation, 100 μl of medium were removed from each test well and 100 μl of medium containing treatment were added. Cells were incubated for 48 h in the presence of 5 μM and 50 μM of the compounds. Wells were washed with 200 μl/well Tris Buffered Saline (TBS) at 4°C and fixed with 160 μl/well of 3.7% formaldehyde in TBS for at least an hour at 4°C. Cells were permeabilized with 120 μl 100%MeOH (−20°C) for 5 min at 4°C and washed twice at room temperature. 100 μl/well of phosphorylated MEK antibody (pMEK, Santa Cruz Biotechnology sc-7995) diluted 1:100 in 3% milk in TBS were added per well, and the plate was incubated for 1 h at room temperature with mild shaking. Plate was washed twice at room temperature and 100 μl per well of rabbit anti-goat HRP (Calbiochem 401515) diluted 1:1000 were added per well. The plate was then incubated for 1 h at room temperature with mild shaking followed by washing. To visualize the binding, 120 μl of chemiluminescence substrate solution (Amersham Pharmacia) were added per well and incubated for 5 min at room temperature with mild shaking. The samples were read with a plate reader (NOVOstar, BMG Labtech Inc., Durharm, NC) and the data was analyzed in Microsoft Excel. Ninety-one pure compounds were tested in this assay. Each compound was tested in duplicate within a plate, and each test was repeated three times.
Cell viability assay (MTT)
12,000 AsPC-1 cells were plated on a 96-well tissue culture plate. Cells were allowed to adhere for 24 h. At the end of this incubation, 100 μl of medium were removed from each test well and 100 μl of medium containing treatment were added. Treatment consisted of a range of concentrations from 0.6255 to 50 μM of Manzamine A or media with methanol. The cells were then incubated for either 72 (standard cytotoxicity assay) or 48 h at 37°C and 5% CO2. After this incubation, 75 μl 5 mg/ml MTT were added to each well. The cells were then incubated for 3 h at 37°C. The plates were centrifuged for 10 min at 800 rpm. The supernatant was removed and 200 μl acidified isopropyl alcohol (1:500 solution of hydrochloric acid to isopropanol) were added to each well. The plates were shaken for 15 min. The absorbencies of these solutions were measured at 570 nm with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC). The resulting absorbencies were plotted using Microsoft Excel.
Morphological changes
AsPC-1 cells were grown alone, in the presence of 1.25, 2.5, 5 and 10 μM Manzamine A and in the presence of 20 μg/ml U0126 for 48 h. Images were captured using a Nikon digital camera attached to an Olympus light microscope. Alternatively, cells were cultured as stated above, stained with Diff-Quick (Fisher Scientific), and then photographed.
Sensitivity to TRAIL-induced apoptosis
12000 AsPC-1 cells were plated on a 96-well tissue culture plate at a volume of 200 μl/well, allowing for twelve replicate wells per treatment. Cells were allowed to adhere for 24 h. At the end of this incubation, 100 μl of medium were removed from each test well and 100 μl of medium containing treatment were added. Treatment consisted of media alone, media with methanol, 10 μM Manzamine A or 20 μg/ml U0126. The cells were then incubated for 48 h at 37°C and 5% CO2. After this incubation, half of the wells of each treatment were incubated with 100 ng/ml Super Killer TRAIL (Alexis, San Diego, CA) for 18 h at 37°C and 5% CO2. At the end of this incubation, cells were washed and stained with Annexin V-PE antibody and 7-Amino-actinomycin D (7-AAD) and analyzed by flow cytometry using a BD FACSCanto. The resulting percentages were plotted using Microsoft Excel.
Western blotting
AsPC1 cells were treated with 1.25, 2.5, 5, and 10 μM Manzamine A for 48 h, then lysed using ice cold Radio Immunoprecipitation (RIPA) buffer containing phosphatase and protease inhibitors (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 5 μg/ml Aprotinin, 5 μg/ml Leupeptin, 1% Triton x-100, 1% Sodium deoxycholate, 0.1% SDS, 2 mM Na3VO4, 1 mM NaF). Protein concentration was quantitated using the Lowry method (BCA assay, Pierce, Rockford IL). Twenty μg of protein per sample were run in a pre-cast denaturing 15% SDS-PAGE gel (Biorad, Hercules CA), which was then transferred to a polyvinylidene difluoride (PVDF) membrane, and blocked with 5% non-fat milk in Tris-buffered saline containing Tween-20 (TBST) buffer for one hour at room temperature. After repeated washing, the membrane was incubated with primary antibody overnight at 4°C, followed by repeated washing and a one hour incubation with horseradish peroxidase conjugated secondary antibody at room temperature. Detection of proteins was done with chemiluminescence (Amersham Biosciences, Piscataway, NJ), followed by exposure to X-ray film. The X-ray film was subjected to a densitometry analysis using the NIH image J software. The density was normalized against the density of the actin for each sample. The antibodies used were anti-pMEK, and anti-MEK2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-MEK 1 (Cell Signaling Technologies, Beverly, MA) and anti-β actin (Sigma Chemical Co., St. Louis, MO). Secondary antibodies were obtained from Jackson Immunobiologicals (West Grove, PA) and Calbiochem (San Diego, CA).
In vitro cell invasion assay
The invasiveness of AsPC-1 cells alone, treated with Manzamine A, or treated with U0126 as a positive control, was evaluated in 24-well transwell chambers, as described by Fu et al. in 2003 [16]. Briefly, the upper and lower culture compartments of each well were separated by polycarbonate membranes (8-μm pore size). To determine baseline migration, 2.5×104 cells in 0.5 mL of complete medium containing 5% FBS were placed into the upper compartment of uncoated wells (BD Discovery Labware, San Diego, CA), and 0.75 mL of complete medium containing 10% FBS were placed into the lower compartment. In parallel, to assess the ability of the same cells to penetrate a collagen matrix, the experiment was repeated using upper compartments coated with 100 μg/cm2 of collagen matrix (BD Discovery Labware, San Diego, CA). The transwell chambers were incubated for 48 h at 37°C in 95% air and 5% CO2. Cell penetration through the membrane is detected by staining the cells that made it through the porous membrane into the plate with a Diff-Quik stain kit and observed and photographed at 100× magnification on a light microscope.
Statistics
Statistical analysis of the data sets to determine mean, standard deviation, and standard error of the mean was performed using Microsoft Excel. Data sets were compared using the Student’s T Test. A p value ≤0.05 was considered significant. Outliers were detected through the Grubbs Test.
Results
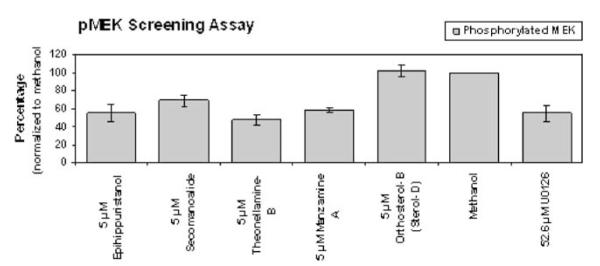
In an effort to find small molecule inhibitors of pMEK in our library of marine natural products, a screening was performed using antibodies against phosphorylated MEK and comparing it to the known MEK inhibitor U0126. U0126 was shown to exhibit its inhibitory effects against MEK at a concentration of 20 μg/ml, which when expressed in molarity is equivalent to 52.6 μM. This concentration of U0126 was used as a positive control in all assays. The results of five compounds tested at a concentration of 5 μM are shown in Fig. 1. Manzamine A was one of the hits from that screening effort.
Fig. 1.
Screening Assay for Inhibitors of Phosphorylated MEK. 12,000 AsPC-1 cells per well were plated and allowed to adhere overnight. Cells were then treated with either 5 μM or 50 μM compounds, vehicle control or 52.6 μM U0126 as a positive control for 48 h. Cells were then fixed, permeabilized and labeled with an antibody specific for phosphorylated MEK. Binding was visualized by chemiluminescence and the data normalized to vehicle control. Each compound was tested in duplicate within a plate, and each test was repeated three times. 91 compounds were assayed. A sampling of compounds that exhibited pMEK inhibition at 5 μM is shown. The data for each compound shown is the average of 3 experiments ± standard deviation
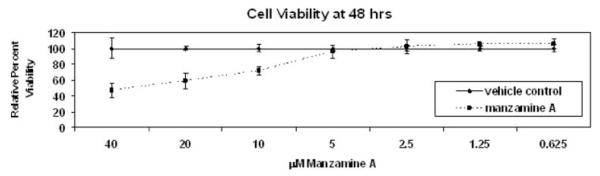
The cytotoxicity of Manzamine A was determined in diverse cancer cell lines using an MTT assay for 72 h. The results are shown in Table 1. In AsPC-1 cells, treatment with 4.17±1.41 μM Manzamine A for 72 h is necessary to obtain 50% cell death (IC50). Overall, Manzamine A is not very cytotoxic, with most IC50 concentrations lying in the 1–6 μM range. Since most of the prior publications showing the effects of U0126 were done at 48 h of treatment, viability of AsPC-1 cells after treatment with different concentrations of Manzamine A was also ascertained at 48 h. With this shorter incubation, 40 μM of Manzamine A were required to induce 50% cytotoxicity in AsPC-1 cells (Fig. 2).
Table 1.
Cytotoxicity of Manzamine A against cancer cells
| IC50 μM | ||||||
|---|---|---|---|---|---|---|
| Panc-1 | AsPC-1 | DLD-1 | Vero | NCI/ADR | A549 | P388 |
| 5.4±1. | 4.17±1.41 | 3.33±1.71 | 3.42±0.07 | 4.37±0.84 | 6.05±0.48 | 1.37±0.99 |
Fig. 2.
Cytotoxicity of Manzamine A in AsPC-1 cells after 48 h of incubation. Cells were plated, allowed to adhere for 24 h, and treated with different concentrations of Manzamine A for 48 h. MTT was added for 4 h and processed as described in materials and methods. Changes in color due to metabolization of MTT by live cells were measured by reading absorbances in a plate reader. Numbers were normalized against vehicle control. The average of 3 experiments ± standard deviation is shown
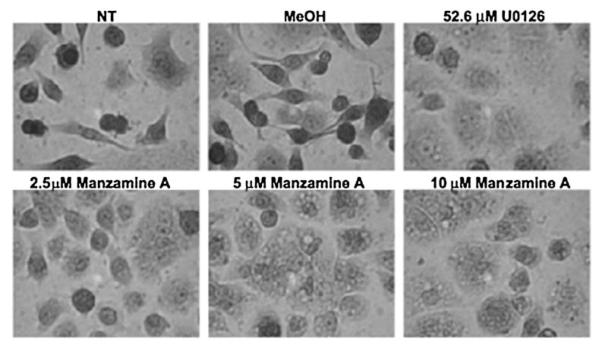
AsPC-1 cells have been shown to grow in single cell formation and to form aggregates in the presence of pMEK inhibitors [2]. Therefore, cell morphology was observed after 48 h of treatment with different concentrations of Manzamine A or the controls (Fig. 3). Treatment of AsPC-1 cells with 5 and 10 μM Manzamine A caused AsPC-1 to lose their single cell formation morphology similar to that caused by 52.6 μM U0126. The changes on morphology were less apparent at lower concentrations of Manzamine A.
Fig. 3.
Manzamine A abrogates cell dissociation in AsPC-1 cells. Cells were plated, allowed to adhere for 24 h, and treated with different concentrations of Manzamine A, methanol as a vehicle control or U0126 as a positive control for 48 h. Cells were fixed and stained with Diff-Quick, placed under a microscope and photographed under a 40× objective. Experiment was repeated 3 times. One representative experiment is shown
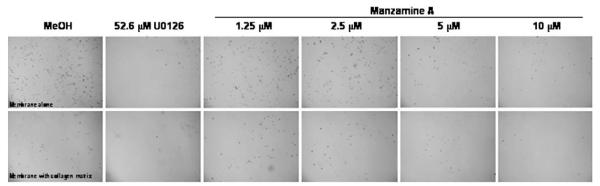
Next, the ability of Manzamine A to reduce in vitro cell migration of AsPC-1 cells was tested. As shown in Fig. 4, cells cultured with media alone or the vehicle control migrated well in the presence or absence of a collagen matrix. Treatment with either 52.6 μM U0126 or 10 μM Manzamine A prevented migration of AsPC-1 cells in the presence or absence of a collagen matrix.
Fig. 4.
Manzamine A inhibits in vitro cell migration. Cells were trypsinized and placed on top of inserts containing a collagen matrix or a membrane alone in media containing 5%FBS. Inserts were placed on top of a plate with media containing 10% FBS to create a serum gradient. Cells on top inserts were treated with different concentrations of Manzamine A, methanol as a vehicle control or U0126 as a positive control 48 h. Inserts were removed and plate was placed under a microscope and photographed under a 40× objective. Experiment was repeated 3 times. One representative experiment is shown
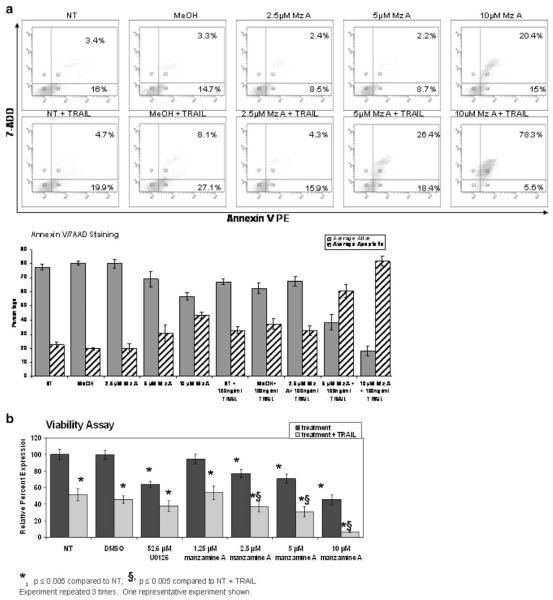
Annexin V staining was used to determine if Manzamine A sensitized pancreatic cancer cells to TRAIL-mediated apoptosis (Fig. 5a). 7 aminoactinomycin D (7AAD), a non-permeable nucleic acid stain, was used in conjunction with Annexin V. When evaluating the data, the lower left quadrant contains both annexin V and 7AAD negative cells, which correspond with live cells, the lower right quadrant contains early apoptotic cells designated by being Annexin V positive but 7AAD negative, the upper right quadrant contains late apoptotic or necrotic cells (positive for both stains) and the upper left quadrant contains debris. To summarize the data, the percentages of cells in the right quadrants were added and graphed against those in the lower left quadrant. As shown in Fig. 5a, treatment of AsPC-1 cells with 5 and 10 μM Manzamine A induced an average of 31% and 43% apoptosis respectively. Treatment with 100 ng/ml super killer TRAIL induced 33% apoptosis. However, cells treated with super killer TRAIL antibody in addition to 10 μM Manzamine A exhibited 82% induction of TRAIL-mediated apoptosis. The experiment also was performed using an MTT assay. Treatment with 5 and 10 μM Manzamine A resulted in a 29% and a 54% loss of viability respectively. Treatment with 52.6 μM U0126 resulted in a 36% loss of viability while treatment with TRAIL alone caused a 54% loss of viability. However, the combination of 52.6 μM U0126 in combination with TRAIL induced 62% loss of viability while the combination of TRAIL with 5 μM manzamine A resulted in a 79% loss of viability and with 10 μM resulted in a 93% loss of viability (Fig. 5b).
Fig. 5.
Manzamine A restores sensitivity to apoptosis in AsPC-1 cells. a Cells were plated, allowed to adhere for 24 h, and treated with different concentrations of Manzamine A or methanol as a vehicle control for 48 h. In addition to this treatment, some cells received 100 ng/ml super killer TRAIL for 18 h. Cells were then trypsinized, labeled with Annexin V antibody and 7AAD stain and analyzed by flow cytometer. Experiment was repeated 3 times. Dot plots for one representative experiment are shown, as well as a graph containing the average of 3 experiments ± standard deviation. b Cells were plated, allowed to adhere for 24 h, and treated with different concentrations of Manzamine A, methanol as a vehicle control or U0126 as a positive control for 48 h. In addition to this treatment, some cells received 100 ng/ml super killer TRAIL for 18 h. MTT was added for the last 4 h of incubation and processed. Changes in color due to metabolization of MTT by live cells were measured by reading absorbances in a plate reader. Numbers were normalized against vehicle control. The average of 3 experiments ± standard deviation is shown
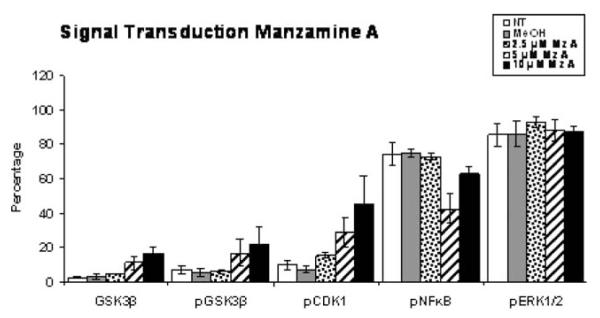
Finally, the effects of Manzamine A upon the MEK1/2 signal transduction pathway were ascertained. As shown in Fig. 6, while 52.6 μM U0126 causes an obvious inhibition of Erk phosphorylation in AsPC-1 cells, treatment with Manzamine A failed to show a reduction in Erk phosphorylation. However, a recent publication reported that Manzamine A binds glycogen synthase kinase 3 beta (GSK3β)in an enzymatic assay [17], providing a plausible mechanism of action. GSK3β shares structure similarity with ERK [18], affects proliferation and apoptosis and its activity has been associated with survival of pancreatic cancer cells [19]. Therefore, intracellular staining of phosphorylated GSK3β and its associated downstream proteins was carried out and analyzed by flow cytometry. As shown in Fig. 7, treatment of AsPC-1 cells with Manzamine A increased the expression of phosphorylated GSK3β, thus increasing the inactive form of this molecule. That this decrease affected GSK3β signaling was confirmed by the increase of expression of phosphorylated cyclin D1 and a decrease of phosphorylated p65 NFκB (Fig. 7), both of which are expected effects of the inhibition of GSK3β signaling.
Fig. 6.
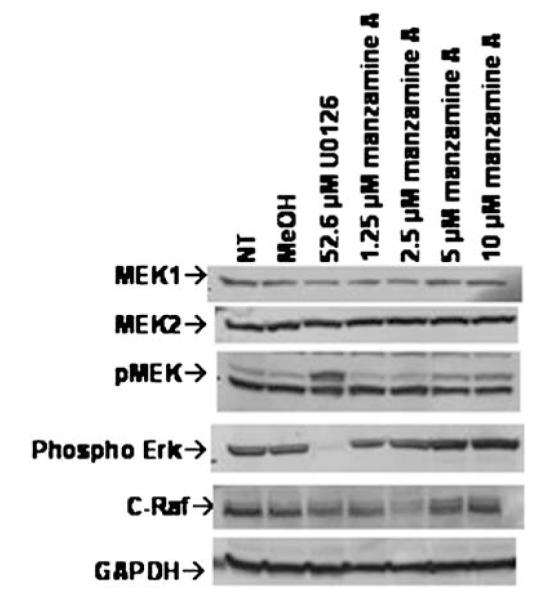
Manzamine A does not exert its effects on AsPC-1 cells through the pMEK/Erk signaling pathway. Cells were plated, allowed to adhere for 24 h, and treated with different concentrations of Manzamine A, methanol as a vehicle control or U0126 as a positive control for 48 h. At the end of that incubation protein was isolated and subjected to western blotting. Experiment was repeated three times. One representative experiment is shown
Fig. 7.
Anti-proliferative and anti-metastatic effects of Manzamine A on AsPC-1 cells may be mediated by acting upon GSK3β. Cells were plated, allowed to adhere for 24 h, and treated with different concentrations of Manzamine A and methanol as a vehicle control for 48 h. Cells were then fixed, permeabilized, and labeled with fluorescent antibodies. Fluorescence was analyzed by flow cytometry. Experiment was repeated three times. The average of 3 experiments ± standard deviation is shown
Discussion
Pancreatic cancer cells have high metastatic potential and resistance to apoptosis that makes them highly unresponsive to current treatments, as evidenced by the 5% survival rate. The AsPC-1 cell line is known to have a very aggressive metastatic potential and to be one of the most resistant to apoptosis of the pancreatic cancer cell lines available. Thus, the effects of Manzamine A in this cell line make this compound a potential adjuvant in the treatment in pancreatic cancer. Manzamine A was identified from a screening effort as being a potential as an inhibitor of pMEK. While the lack of involvement of the pMEK pathway in the mechanism of action of Manzamine A was puzzling, the ability of Manzamine A to inhibit GSK3β in AsPC-1 pancreatic cells provides an alternative explanation for the observed sensitization of apoptosis, as inhibition of GSK3β leads to apoptosis of pancreatic cancer cells via the inhibition of NFκB [19]. Inhibition of GSK3β, however, does not explain the loss of single cell formation and loss of migratory ability of AsPC-1 cells treated with manzamine A. These events suggest that GSK3β inhibition is not the sole mechanism of action of manzamine A in AsPC-1 cells. While the ability of Manzamine A to bind GSK3β is being explored in the treatment of Alzheimer’sdisease [17], this is the first report that shows the pro-apoptotic effects and potential anti-metastatic effects suggested by the prevention of migration and loss of single cell formation that Manzamine A has in AsPC-1 pancreatic cancer cells. These are new effects against cancer of this compound as its only known activity was its ability to kill cancer cells (Table 1), which does at concentrations much higher than those desired for chemotherapeutics.
Irrespective of its mechanism of action, the effects that Manzamine A has on AsPC-1 pancreatic cancer cells remain unequivocal. Manzamine A abrogated the single cell formation that correlates with the high metastatic potential of AsPC-1 cancer cells, using a fifth of the concentration needed for U0126 to produce similar results. Manzamine A also prevented cancer cell migration, which could translate in further anti-metastatic effects. Moreover, unlike U0126, Manzamine A is shown here to abrogate the resistance to TRAIL mediated apoptosis which causes the failure of many current therapies. Furthermore, Manzamine A appears to be fairly stable and shows little cytotoxicity on its own at small concentrations. These results suggest Manzamine A has a strong potential to be used in combination therapy in the treatment of pancreatic cancer, and merits further research. Studies are currently underway to address its effects in other in vitro cell lines and an in vivo model for metastatic pancreatic cancer. While a major concern with marine derived compounds is their limited availability, the total synthesis for Manzamine A has been reported [20], and a microbe that can produce Manzamine A through fermentation has been isolated [21]. Thus renewable sources of the compound exist for future studies and widespread use.
Acknowledgements
Funding for this project was provided by NIH/NCI 1R01CA093455-01A1, the State of Florida Center of Excellence in Biomedical & Marine Biotechnology (COE-HRE07), and the Health Resources & Services Administration Center for Sustainable Use of Marine Resources (4C76HF00231-01-04).
References
- 1.American Cancer Society; [(accessed 10/01/09)]. All About Pancreatic Cancer. http://www.cancer.org/docroot/CRI/CRI_2x.asp?sitearea=&dt=34. [Google Scholar]
- 2.Tan X, Egami H, Kamohara H, Ishikawa S, Kurizaki T, Yoshida N, Tamori Y, Takai E, Hirota M, Ogawa M. Involvement of the mitogen-activated protein kinase kinase 2 in the induction of cell dissociation in pancreatic cancer. Int J Oncol. 2004;24:65–73. [PubMed] [Google Scholar]
- 3.Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79:355–369. [PubMed] [Google Scholar]
- 4.Motomura W, Tanno S, Takahashi N, Nagamine M, Fukuda M, Kohgo Y, Okumura T. Involvement of MEK-ERK signaling pathway in the inhibition of cell growth by troglitazone in human pancreatic cancer cells. Biochem Biophys Res Commun. 2005;332:89–94. doi: 10.1016/j.bbrc.2005.04.095. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346–354. [PubMed] [Google Scholar]
- 6.Toyonaga T, Nakano K, Nagano M, Zhao G, Yamaguchi K, Kuroki S, Eguchi T, Chijiiwa K, Tsuneyoshi M, Tanaka M. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107–116. doi: 10.1016/s0304-3835(03)00482-8. [DOI] [PubMed] [Google Scholar]
- 7.Edrada RA, Proksch P, Wray V, Witte L, Muller WE, Van Soest RW. Four new bioactive manzamine-type alkaloids from the Philippine marine sponge Xestospongia ashmorica. J Nat Prod. 1996;59:1056–1060. doi: 10.1021/np9604083. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe D, Tsuda M, Kobayashi J. Three new manzamine congeners from amphimedon sponge. J Nat Prod. 1998;61:689–692. doi: 10.1021/np970564p. [DOI] [PubMed] [Google Scholar]
- 9.Ichiba T, Corgiat JM, Scheuer PJ, Kelly-Borges M. 8-Hydroxymanzamine A, a beta-carboline alkaloid from a sponge. Pachypellina sp. J Nat Prod. 1994;57:168–170. doi: 10.1021/np50103a027. [DOI] [PubMed] [Google Scholar]
- 10.Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT. Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J Nat Prod. 2004;67:1314–1318. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousaf M, Hammond NL, Peng J, Wahyuono S, McIntosh KA, Charman WN, Mayer AM, Hamann MT. New manzamine alkaloids from an Indo-Pacific sponge. Pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory diseases. J Med Chem. 2004;47:3512–3517. doi: 10.1021/jm030475b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng J, Hu JF, Kazi AB, Li Z, Avery M, Peraud O, Hill RT, Franzblau SG, Zhang F, Schinazi RF, Wirtz SS, Tharnish P, Kelly M, Wahyuono S, Hamann MT. Manadomanzamines A and B: a novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J Am Chem Soc. 2003;125:13382–13386. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tekwani BL, Hamann MT. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J Nat Prod. 2003;66:823–828. doi: 10.1021/np020592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Holmes MJ, Kara UA. Immune-mediated parasite clearance in mice infected with Plasmodium berghei following treatment with manzamine A. Parasitol Res. 2001;87:715–721. doi: 10.1007/s004360000366. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, Holmes MJ, Higa T, Hamann MT, Kara UA. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob Agents Chemother. 2000;44:1645–1649. doi: 10.1128/aac.44.6.1645-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–889. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 17.Hamann M, Alonso D, Martin-Aparicio E, Fuertes A, Perez-Puerto MJ, Castro A, Morales S, Navarro ML, Del Monte-Millan M, Medina M, Pennaka H, Balaiah A, Peng J, Cook J, Wahyuono S, Martinez A. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of the manzamine alkaloids. Potential for Alzheimer’s disease. J Nat Prod. 2007;70:1397–1405. doi: 10.1021/np060092r. [DOI] [PubMed] [Google Scholar]
- 18.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65:2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 20.Winkler JD, Axten JM. The first total synthesis of Ircinol A, Ircinal A, and Manzamines A and D. J Am Chem Soc. 1998;120:6425–6426. doi: 10.1021/ja981303k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill RT, Hamann M, Peraud O, Kasanah N. Manzamine-producing Actinomycetes. USA: Number PCT/US2003/024238 Patent Application. 2003