Abstract
Remembering events from the personal past (autobiographical memory) and inferring the thoughts and feelings of other people (mentalizing) share a neural substrate. The shared functional neuroanatomy of these processes has been demonstrated in a meta-analysis of independent task domains (Spreng, Mar & Kim, 2009) and within subjects performing both tasks (Rabin, Gilboa, Stuss, Mar, & Rosenbaum, 2010; Spreng & Grady, 2010). Here, we examine spontaneous low-frequency fluctuations in fMRI BOLD signal during rest from two separate regions key to memory and mentalizing, the left hippocampus and right temporal parietal junction, respectively. Activity in these two regions was then correlated with the entire brain in a resting-state functional connectivity analysis. Although the left hippocampus and right temporal parietal junction were not correlated with each other, both were correlated with a distributed network of brain regions. These regions were consistent with the previously observed overlap between autobiographical memory and mentalizing evoked brain activity found in past studies. Reliable patterns of overlap included the superior temporal sulcus, anterior temporal lobe, lateral inferior parietal cortex (angular gyrus), posterior cingulate cortex, dorsomedial and ventral prefrontal cortex, inferior frontal gyrus, and the amygdala. We propose that the functional overlap facilitates the integration of personal and interpersonal information and provides a means for personal experiences to become social conceptual knowledge. This knowledge, in turn, informs strategic social behavior in support of personal goals. In closing, we argue for a new perspective within social cognitive neuroscience, emphasizing the importance of memory in social cognition.
Keywords: Autobiographical memory, theory of mind, mentalizing, resting-state functional connectivity, fMRI, default mode network
“We think about people and their intentions; talk about them; look for and remember them.”
Daniel Gilbert, 2006
1. Introduction
Within psychology, the study of memory and the study of social cognition have largely been undertaken in parallel, with little communication or collaboration between the two lines of research. Yet recent neuroscience evidence has moved these two topics of investigation onto intersecting paths. Specifically, there is growing evidence that the recall of personally experienced events (autobiographical memory) and inferring the mental states of others (mentalizing or theory-of-mind) share a neural substrate. This intersection has opened a new avenue of empirical investigation into the cognitive neuroscience of thought.
The idea that recollection and mentalizing share a neural basis was sparked by Buckner and Carroll (2007), who observed that neuroimaging studies of autobiographical memory and what is known as theory of mind appear to show similar patterns of activation. This observation, however, was not based on any systematic or empirical survey of the literature. To address this issue, Spreng and colleagues (2009) conducted a set of voxel-based quantitative meta-analyses of functional neuroimaging data (Laird et al., 2005). This method revealed an overlap between reliable patterns of activity associated with autobiographical memory and mentalizing. This overlap included the medial prefrontal and posterior cingulate cortex, frontal pole, inferior frontal gyrus, medial temporal lobe (parahippocampus, amygdala), superior temporal sulcus and middle temporal gyrus, and the angular gyrus (Figure 1A). Importantly, regions with shared activity far outweighed the unique, non-overlapping, activations.
Figure 1.
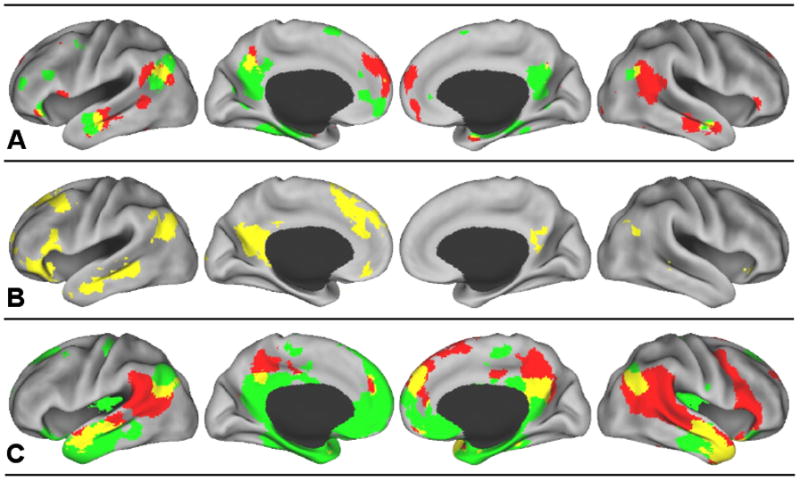
A) Re-presentation of results from the meta-analysis by Spreng and colleagues (2009). Green = autobiographical memory. Red = theory-of-mind. Yellow = overlap between autobiographical memory and theory-of-mind. Results maps were derived based on foci of interest, comprising statistically significant peak activation locations from multiple studies. Localization probability distributions for the foci are modeled at the center of 3-D Gaussian functions, where the Gaussian distributions are summed across the experiments to generate a map of inter-study consistencies that estimate the likelihood of activation for each focus. The foci were modeled using a full-width half-maximum value of 12 mm3. We then compared the summary of observations against a null distribution, determined through 5000 permutations of randomly generated foci identical in number to those being tested. The false discovery rate method was employed to correct for multiple comparisons at p < .05 and subjected to a cluster threshold of 100 mm3 (Laird, et al., 2005). In total, 20 autobiographical memory and 31 theory of mind neuroimaging studies with an equivalent number of peak activation foci were included (for details, see Spreng et al., 2009).
B) Re-presentation of results from the within-subjects multivariate analysis by Spreng and Grady (2010). Yellow = overlap between autobiographical memory, imagining the future, and theory-of-mind.
C) Results of the rsfcMRI analysis from the current study. Green = Regions correlated with the left hippocampus (p < .001, uncorrected). Red = Regions correlated with the right temporoparietal junction (p < .001, uncorrected). Yellow = overlapping regions correlated with both the left hippocampus and the right TPJ (p < .000001, conjoint probability). Overlap images at thresholds of p < .01, p < .001, p < .0001 (uncorrected) and FWE p < .05 (corrected) are available as supplementary material.
These meta-analyses were based on separate groups of studies, with participants performing either memory or mentalizing tasks. An essential next step was to examine whether this overlap would also be observed when the same individuals engaged in both tasks. Two subsequent neuroimaging studies took this approach and found similar areas of neural overlap, despite using different subject populations, different MRI scanners, and different analytic methods (Rabin, Gilboa, Stuss, Mar, & Rosenbaum, 2010; Spreng & Grady, 2010). Both studies involved the recollection of a personally experienced event and the inference of another’s mental state in response to photographs1. Consistent with the meta-analysis, within-subject performance on matched autobiographical memory and theory-of-mind tasks resulted in a shared pattern of brain activity, including the medial prefrontal and posterior cingulate cortex, frontal pole, inferior frontal gyrus, medial temporal lobe, superior temporal sulcus, middle and inferior temporal gyri, as well as the angular gyrus (Figure 1B shows the results of Spreng & Grady, 2010).
In this article we undertake a critical examination of where the functional neuroanatomy of autobiographical memory and mentalizing overlap, along with providing some conjecture about what this overlap might mean. Toward these aims, we draw upon the above-mentioned studies that attempted to answer this question using very different methods (i.e., meta-analysis and within-subject analysis). These are used to inform a new analysis exploiting resting-state functional connectivity. Resting state functional connectivity analyses of magnetic resonance imaging data (rsfcMRI) detect patterns of low-frequency neural activity during rest. These patterns are based on the correlation of spontaneous BOLD fluctuations in a given seed with all other voxels, revealing distinct and dissociable functional-anatomic networks (Biswal et al., 1995; Fox & Raichle, 2007; Vincent et al., 2008).
To examine the functional overlap between autobiographical memory and theory of mind, we took a conservative approach, selecting one seed from autobiographical memory that did not overlap with mentalizing. Similarly, the seed we selected from mentalizing did not overlap with autobiographical memory. These points of non-overlap were based on the two within-subject studies that directly contrasted autobiographical memory and mentalizing (Rabin, et al., 2010; Spreng & Grady, 2010). The first seed region, the left hippocampus (lHC), is a specialized region for the binding together of information that can later be consciously retrieved or reconstructed (Moscovitch, Nadel, Winocur, Gilboa & Rosenbaum, 2006; Buckner, 2010). The second seed, the right temporal parietal junction (rTPJ), is reliably involved in the inference of others’ mental states (Mar, 2011; Saxe & Kanwisher, 2003). The specificity of the rTPJ’s function, however, is not clear; it is also involved in other attentional processes (Corbetta, Patel, & Shulman, 2008; Hein & Knight, 2008; Mitchell, 2008; 2009). Using rsfcMRI, we identified the intrinsic functional network associated with each seed.
Our goal was to examine whether these two intrinsic functional networks would overlap, despite the fact that each seed was selected from non-overlapping regions that dissociated autobiographical memory from mentalizing. More importantly, we were interested in whether these potentially overlapping regions would resemble those identified using task-driven observations of brain function (i.e., meta-analysis and within-subject analysis). This approach provides a test of whether a shared neural substrate exists for these two cognitive domains. It should be emphasized, however, that because this analysis relies on correlations between low-frequency BOLD fluctuations during periods of stimulus-independent thought, the functional networks revealed for each seed cannot be associated with a particular cognitive domain. Instead, any shared intrinsic connectivity of the lHC and the rTPJ may reflect the brain’s potential to integrate information across cortex in preparation for behavior (c.f. Raichle, 2010). Shared resting state functional connectivity could provide evidence that the brain is intrinsically “wired” to provide preferential access to information from memory to social cognitive processes. This intrinsic connectivity might, then, facilitate functional interaction during behavior. With the rsfcMRI results situated within a theoretically motivated context based on previous empirical research, this analysis adds an important piece of information to the current discussion of how memory and mentalizing relate. In the discussion, we speculate on the significance of functional overlap.
2. Results
Although the seeds were chosen from non-overlapping regions based on previous analyses, it was important to determine that there was no intrinsic association between the two areas we chose. Confirming this hypothesis, the correlation between the lHC and rTPJ seeds was not statistically significantly different from zero, mean z′ = −.03, t(69) = −1.01, p > .30. By choosing unrelated seeds, there is no a priori reason to expect that the functional networks associated with each seed would overlap in any region. In spite of this lack of association between the two seeds, we found the lHC and rTPJ to be functionally connected to a number of shared regions of the brain, at p < .001, uncorrected (Figure 1C). The largest regions of overlap include bilateral portions of the lateral temporal and parietal lobes: specifically, posterior superior temporal sulcus and part of the angular gyrus, and bilateral superior temporal gyrus extending anteriorly to the temporal poles. We found additional overlap in medial structures, including the posterior cingulate, dorsomedial and ventral prefrontal cortex, and the amygdala. The extent of overlap was observed across a number of different thresholds from p < .01 to p < .0001 (uncorrected). Even at a relatively much higher threshold of independent FWER corrections of p < .05, overlap was observed on the lateral surfaces and in posterior cingulate cortex (Supplemental Figure 1). These regions correspond well to those areas of overlap identified by the previous meta-analysis and within-subjects analysis (Table 1). The three different approaches converged in identifying an overlap in the superior temporal sulcus, anterior temporal lobe, lateral inferior parietal cortex (angular gyrus), posterior cingulate cortex, dorsomedial and ventral prefrontal cortex, the inferior frontal gyrus and the amygdala.
Table.
Regions of conjunction
| Region | Approximate MNI coordinate | source | ||
|---|---|---|---|---|
| x | y | z | ||
| Superior temporal sulcus | −56 | −10 | −16 | abc |
| 56 | −6 | −14 | ac | |
| Lateral parietal cortex (angular gyrus) | −46 | −64 | 30 | abc |
| 50 | −62 | 26 | abc | |
| Posterior cingulate cortex | 0 | −58 | 32 | abc |
| Ventromedial prefrontal cortex | 2 | 50 | −18 | bc |
| Dorsomedial prefrontal cortex | −4 | 46 | 22 | abc |
| Inferior frontal gyrus | −44 | 32 | −14 | ab |
| Anterior temporal lobe | −46 | 14 | −30 | bc |
| Amygdala | 22 | −4 | −20 | ac |
Brain regions showing conjunction across methods. MNI coordinates represent the approximate location of greatest overlap. Sources are a = meta-analysis, b = within subject fMRI analysis, and c = resting state functional connectivity analysis overlap between the left hippocampus and the right temporal parietal junction as seed regions.
We also observed a great deal of variability in the relation between the lHC and rTPJ seeds across participants, z′ SD = .24. Figure 2 depicts the data for three prototypical subjects, displaying BOLD time course data and corresponding whole brain conjunction images (each map thresholded at z′ > .20). Some participants exhibited a positive correlation between the two regions (Figure 2, Subject 1), and others displayed no correlation (like the overall sample; Figure 2, Subject 2) or a negative correlation (Figure 2, Subject 3). As Figure 2C illustrates, the higher the correspondence between the left hippocampus and rTPJ, the greater the overlap observed for the two functional networks. However, even in the single participant where BOLD activity in these two seeds was uncorrelated, we still see quite substantial areas of overlap. This suggests that our group-level overlap was not driven solely by those who demonstrate high synchrony between the two seeds. Indeed, even Subject 3, who exhibits a negative correlation between the lHC and rTPJ, shows regions of overlap that are consistent with the group results. The behavioral significance of this individual variability in association is unknown, however, and might prove to be a fruitful topic of future investigation.
Figure 2.
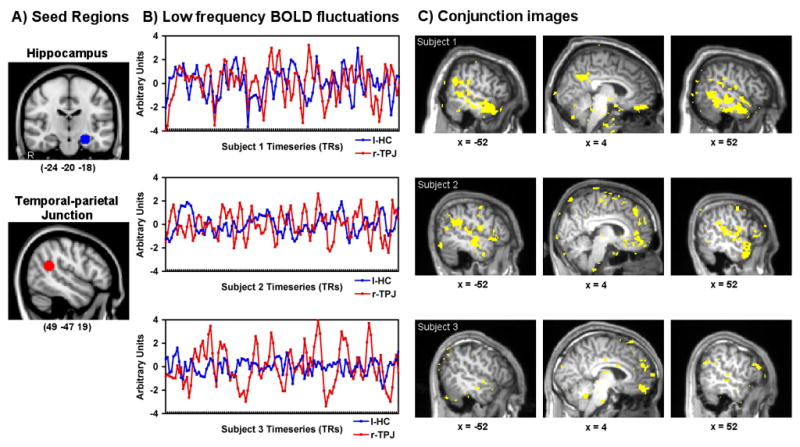
Resting-state functional connectivity seed regions are depicted in the left hippocampus and right TPJ. Three subjects’ low frequency BOLD fluctuations for the lHC and rTPJ are shown over 120 timepoints. Individual subject conjunction images show voxels that are correlated with both the lHC and rTPJ at z′ >.20 for each. The top row shows a high degree of correspondence between hippocampal and rTPJ low frequency fluctuations (z′ = .22) and a conjunction of functional connectivity in the midline and lateral structures. The middle row depicts a single subject with a null correlation similar to that as the population average (z′ = −.03) and corresponding conjunction image. The third row depicts an out of phase relationship between the hippocampus and rTPJ BOLD signal fluctuations (z′ = −.24) and corresponding voxels that are positively correlated with each seed. Note the difference in lateral temporal lobe activity.
3. Discussion
In this article we extended previous work on the neural overlap between autobiographical memory and mentalizing by approaching this question using rsfcMRI. Based on previous within-subjects neuroimaging work, we selected two regions that significantly dissociated the two processes (Rabin et al., 2010; Spreng & Grady, 2010): (1) the lHC for autobiographical memory, and (2) the rTPJ for mentalizing. Our results confirmed that spontaneous low-frequency BOLD signal fluctuations in these two areas were not correlated with one another (although considerable variability across subjects was observed), emphasizing the independence of these two regions. In spite of this independence, the functional networks associated with each seed overlapped in a number of regions. Moreover, these regions are largely consistent with the previously observed overlap derived from meta-analysis (Figure 1A) and that observed for within-subjects studies (Figure 1B; Figure 3 and Table 1 show a direct comparison). In light of the fact that this is the third unique method to confirm some shared neural substrate for both autobiographical memory and mentalizing, this shared network appears to be a reliable observation worthy of more direct study.
Figure 3.
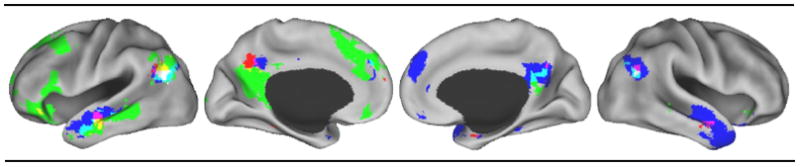
A “conjunction of conjunctions” across studies displaying the areas of overlap identified by the three separate analyses. Red = meta-analysis overlap of autobiographical memory and theory of mind. Green = task-evoked within-subject overlap of autobiographical memory, prospection and theory of mind. Blue = rsfcMRI overlap of lHC and rTPJ spontaneous activity. Yellow = overlap of meta-analysis and task-evoked within-subject results. Purple = overlap of meta-analysis and rsfcMRI results. Light blue = overlap of task-evoked within-subject and rsfcMRI results. White = Overlap of all three methods.
What does this shared set of brain region represent? How are we to interpret this overlap and similarity? For one, we do not think that autobiographical memory and mentalizing should be considered identical in any way. Although we have observed an overlap, there are also a great number of non-overlapping regions, and the two cognitive processes entail some obvious differences. One possible explanation of the overlap is that a single process supports both personally re-experiencing the past and inferring the mental states of another. This has been proposed by a number of other theorists, with this underlying process taking the form of self-projection (Buckner & Carroll, 2007; Mitchell, 2009) or scene-construction (Hassabis & Maguire, 2007), for example. While such an explanation is possible, it is by no means necessary. It is completely possible for a single region to serve different functions depending upon the co-activated regions and the task at hand (Hein & Knight, 2008; Bressler & McIntosh, 2007). The fact that the regions identified largely comprise heteromodal association cortex is consistent with the possibility of functional flexibility. Below, we speculate on the possible significance of the functional overlap, which may include the facilitation of social conceptual knowledge and the role of memory in strategic social behavior.
Many of the overlapping regions are also implicated in semantic memory (Binder, Desai, Graves, & Conant, 2009; Martin, 2007). Both autobiographical memory and mentalizing draw upon conceptual knowledge related to actions, relationships, and the self. This alone, however, does not explain the shared patterns of functional connectivity at rest, which were observed in the absence of task-evoked brain activity. Instead, the shared pattern of resting state functional connectivity may demonstrate the neural potential for the acquisition and updating of semantic information. Semantic memory is built from experience. The functional neuroanatomy illustrated here may be critical for the transfer of information from experience to the formation of social conceptual information, which in turn guides social behavior, both implicitly and explicitly. We hypothesize that the overlap observed is a type of distributed integration zone, where information from autobiographical memory and mentalizing processes is synthesized and interacts. A network such as this could provide a means for information in memory, particularly autobiographical experiences, that are drawn upon when making social judgments, inferences or simulations.
In real-world social situations, we possess a rich mental history of past interactions that can influence our current social processing. With respect to an individual we know well or have met only once, our expectations about that person will be based on previously acquired information, which will shape how we interpret his or her behavior. An early study by Cohen (1981) provides a useful illustration of how this might take place. In this study, participants were told that a woman was either a waitress or a librarian before watching a video of her going about her day. Once the video was over, participants exhibited better recall of those behaviors and attributes that were consistent with the stereotype of either a waitress or a librarian (depending on previous knowledge). Here we see that even a tiny bit of information about a person can influence how we process his or her behavior. Real-world social perception is likely to be shaped by memory to an even larger degree, based on the fact that we possess an elaborated personal history of interactions with individuals and not just knowledge of their occupation.
The function of memory is not simply to recall the past, but to construct flexible models of the future (Buckner, 2010; Schacter, Addis, & Buckner, 2008). The hippocampus, by connecting discrete memories, is involved in the acquisition and application of conceptual knowledge during decision-making (Kumaran et al., 2007; Kumaran, Summerfeild, Hassabis & Maguire, 2009). The adaptive advantage of formulating conceptual knowledge is that it arms one with generalizations that apply to any number of related situations, including those yet to be experienced in the future. One may learn principles or generalizations from present and past experiences and apply them to any and all relevant future experiences. This flexible process seems very well suited for navigating the complexities of the social environment, which is unstructured and labile. Moreover, the cost of making inaccurate predictions in the social domain are high, and trial and error learning in adults is unsuitably slow. Successful social navigation results in the formation of professional and intimate relationships; failure in the social domain spells social rejection and isolation.
We use our memory of social experiences to make predictions about our peers and to calibrate our own behavior accordingly. When making social judgments, about whether a person will enjoy accompanying us to a party, for example, we can draw upon a wealth of memories. In doing so, we might think back to the last party we attended together and recall how one friend made a comment that belied her annoyance at the noise and the behavior of the other guests. Choosing another guest based on this memory will help us avoid another potentially awkward situation.
Another possibility is that we could simply draw upon our semantic knowledge about this individual in order to predict his or her behavior, rather than personally re-experiencing past events. I do not need to project myself back into time and “remember” that my friend did not have a good time at the last party we attended, I can simply “know” that she does not enjoy loud parties. In other words, semantic memory may play just as large a role in social cognition as the recollection of specific events. Semantic information can be acquired much more easily, and through many more routes, than drawing upon the recollections of many specific events. We can also learn about other people indirectly from others, for example, as in the study by Cohen (1981) mentioned previously. Semantic social information is likely to underpin the mental models of others that we employ to interpret and predict behavior. We are extremely fast and accurate in making judgments about people (Carney, Colvin & Hall, 2007). Part of this speed comes from building efficient and accurate representations of others that then allow for understanding, cooperating with, or exploiting people’s motives, feelings and thoughts. Single trial learning and knowing the source of information (and its accuracy) are critical for building individual person representations; these representations facilitate mentalizing and allow for flexible and adaptive behavior. Neuroimaging researchers have begun to tackle the topic of social semantic knowledge, with promising results (Zahn et al., 2007; Simmons, Reddish, Bellgowan, & Martin, 2010).
It is important to point out that the neuroimaging studies that reveal a neural overlap for autobiographical memory and mentalizing do not rely upon tasks that adequately capture the complex, repeated, and long-term nature of real-world social processing we have just discussed. However, work with brain-damaged patients corroborates our inferences regarding the importance of memory for successful social interactions. For example, patient M.L. has been characterized in clinical reports as having a profound impairment of autobiographical memory, but also co-occuring deficits in self-regulation. These deficits are attributed to a focal lesion to the right uncinate fasciculus, a band of white matter fibers connecting the medial temporal lobes with ventral frontal cortex (Levine et al., 1998). The inability to regulate one’s own behavior impairs social cognition and strategic social navigation. Consistent with this idea, it was reported that M.L. had difficulty in knowing how to behave around family members and friends after the injury and had to relearn socially acceptable behavior (Levine et al., 1998). Over time, he was only able to assume increased parenting responsibilities under structured routines and with assistance from his wife (Levine et al., 1998). In spite of his social gains, however, he remains impaired in the self-regulation of his behavior in unstructured situations (Levine et al., 1999). Levine et al. suggest that the ability to remember the past, and the corollary of predicting the future, can facilitate the inhibition of inappropriate actions arising from immediate environmental stimuli (1999). Indeed, the ability to maintain a long view during interpersonal exchanges can facilitate the inhibition of inappropriate actions that could damage one’s relationships or reputation and enhance the influence of delayed cooperative rewards on immediate decisions (Boyer, 2008). For example, one inhibits an angry outburst at a colleague due to the importance of maintaining a long-term cordial working relationship.
Work with M.L. and other amnesiacs appears to contradict a study by Rosenbaum and colleagues (2007), which demonstrated that two patients incapable of autobiographical memory could successfully pass a battery of mentalizing tasks (one of whom was M.L.). Upon closer examination, however, no necessary contradiction exists. Autobiographical memory might be less important for momentary, spontaneous, mental inference tasks that employ strangers as targets. This does not negate the possibility that memory of personal experiences is important for real-world situations involving repeated interactions with known individuals. This is not currently captured in the mental inference tasks employed by Rosenbaum and colleagues (2007), such as the Reading the Mind-in-the-Eyes Task (Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). However, adult patient populations with profound impairments in theory of mind reasoning, such as adults with schizophenia (Corcoran & Frith, 2003) and high functioning autism and Asperger’s syndrome (Adler, Nadler, Eviatar & Shamay-Tsoory, 2010), demonstrate a shared impairment in autobiographical recollection. Distinguishing the more perceptual forms of social cognition tapped by theory-of-mind tasks from more complex real-world social interactions faced by psychiatric and brain-damaged patients is an important, and under-recognized, goal for future research. This challenge will likely necessitate the development of cognitive and clinical measures that effectively assess the integration of past experience with social reasoning.
One recent neuroimaging study of healthy adults adopted this promising approach, illustrating how knowledge of our selves and our past experiences influences mental inferences. Perry, Hendler and Shamay-Tsoory (2010) examined brain activity when subjects made emotional judgments of people deemed similar or dissimilar to themselves (e.g. How would Joe feel about losing his wallet?). In the case of similar others, Perry et al. (2010) found pronounced involvement of the hippocampus, but critically, only when the emotional event had occurred in the subject’s own life. The authors suggest that we project our memories onto people we see as similar in order to better understand and empathize with them.
We would be remiss to discuss the overlapping regions of autobiographical memory and mentalizing without commenting on their similarity to the default network (Buckner, Andrews-Hanna & Schacter, 2008). First observed to be deactivated during a number of visually demanding tasks (Shulman et al., 1997), the regions that comprise this network have since been associated with a number of cognitive functions, including narrative comprehension (Mar, 2011), prospection (Andrews-Hanna et al., 2010; Spreng et al., 2009; Spreng & Grady 2010), forming associations (Bar et al., 2007), and stimulus-independent thought and mind-wandering (Mason et al., 2007; Christoff et al., 2009). All of these functions are consistent with our hypothesis that the regions comprise a distributed integration zone, where information from our past experiences interacts to form a working context that frames social processes and behavior. Future research will need to dissociate the specific roles of regions within the network as well as examine how regions facilitate the functional integration of information. One key region may be the posterior cingulate, which has already shown much promise in serving as a central network hub (Andrews-Hanna et al., 2010; Buckner et al., 2009; Fransson & Marrelec, 2008; Hagmann et al., 2008).
To conclude, we do not only reason about others and remember our past experiences independently: more often, we use our past experiences in order to reason about others. There has been a growing trend to realize a more broad and integrated cognitive neuroscience that examines the relationships between processes rather than just the neural correlates of single processes. The relation between memory and social cognition is one topic that seems ripe for this type of investigation, with many preliminary studies showing a great deal of promise. Further work establishing the structure of this relation as well as explicit tests of updating social conceptual knowledge, will provide us with a window into how “I remember you,” how I interpret your behavior, and how I act when we are together.
4. Experimental Procedure
4.1 Participants
70 right-handed young adults (Mage = 23.5, SD = 2.5; 37 women) with normal or corrected-to-normal vision and no history of psychiatric, neurological, or other medical illness participated in the present study. All participants were compensated and gave written informed consent prior to participation, in accordance with the guidelines of the institutional review board of Harvard University.
4.2 MRI Data Collection
Brain imaging data were acquired with a 3.0T Siemens TimTrio MRI scanner with a 32-channel phased-array whole-head coil. Anatomical scans were acquired using a T1-weighted multi-echo volumetric MRI (TR = 2200ms; TE’s = 1.54, 3.36, 5.18, 7.01ms; 7° flip angle; 1.2 mm isotropic voxels). The BOLD functional scan was acquired with a T2*-weighted EPI pulse sequence (TR = 3000ms; TE = 30ms; 85° flip angle; 47 axial slices parallel to the plane of the anterior commissure–posterior commissure; 3.0 mm isotropic voxels). Six minutes and 12 seconds of BOLD data were acquired in a darkened room with participants’ eyes open.
4.4 MRI Preprocessing & Analysis
All fMRI data were preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). The first 4 volumes were excluded from analyses to allow for T1-equilibration effects. Data were corrected for slice-dependent time shifts and for head motion within and across runs using a rigid body correction. Images were then spatially normalized to the standard space of the Montréal Neurological Institute (MNI) atlas, yielding a volumetric time series resampled at 2mm cubic voxels. After standard preprocessing, resting-state data were subjected to additional preprocessing steps described previously (Vincent et al., 2008; Van Dijk et al., 2009) and briefly here. First, a temporal low-pass filter was applied to the atlas-aligned BOLD data, retaining signal with frequency range less than 0.08 Hz. Data were then spatially smoothed with a Gaussian kernel, FWHM = 6 mm. Then, sources of variance of non-interest were removed from the data by regressing the following nuisance variables (in addition to first temporal derivatives of each): the 6 motion parameters obtained during the motion correction procedure, the mean whole-brain signal, the mean signal from the lateral ventricles, and the mean signal from a region within the deep cerebral white matter.
To analyze whole brain patterns of intrinsic low-frequency BOLD correlations, the mean BOLD signal time-course for each participant was extracted from two seed ROIs with a radius of 8mm in the lHC (MNI: −24 −20 −18) and rTPJ (MNI: 49 −47 19). The coordinates were taken as the average of peak voxels from two direct contrasts between autobiographical memory and theory of mind (Rabin, et al., 2010; Spreng & Grady, 2010) after coordinates reported in Talairach space (Rabin, et al., 2010) were converted to MNI using the Lancaster transformation (Laird et al., 2010; Lancaster et al., 2007). The correlation coefficient for the seeds’ time-courses with the time-course for every other voxel in the brain was computed using Pearson’s product-moment formula. These values were then converted to z-values using Fisher’s r-to-z transformation. Whole-brain voxel-wise z-maps were then subjected to random-effects analyses to asses statistical significance across participants at the group level using t-tests performed in SPM2 (threshold p < .001, uncorrected). Overlap images at thresholds of p < .01, p < .001, p < .0001 (uncorrected) and FWER p < .05 (corrected) are presented in supplementary Figure 1. The reliability of the correlations between the two seed regions was determined with a simple t-test on the r-to-z computed correlation values. Cortical surfaces are displayed on a partially inflated brain (population average landmark surface: PALS-B12) using CARET software (Van Essen, 2005).
Supplementary Material
Supplemental Figure. Results of the rsfcMRI analysis at varying thresholds. Green = Regions correlated with the left hippocampus; Red = Regions correlated with the right temporoparietal junction. Yellow = overlapping regions correlated with both the left hippocampus and the right TPJ. A) Both maps independently thresholded at p < .01 (uncorrected); B) p < .001 (uncorrected); C) p < .0001 (uncorrected); and, D) FWER p < .05 (corrected).
Acknowledgments
We thank Randy Buckner, Itimar Khan, Hesheng Liu and Tanveer Talukdar for preprocessing and rsfcMRI tools, Scott Guerin for assistance with data collection, Marina Rain for assistance in preparing this manuscript, and Kathy Gerlach and Dale Stevens for their comments. We gratefully acknowledge that the resting state data were collected with support from NIH Grant MH060941 to Dan Schacter.
Footnotes
Spreng & Grady (2010) also examined imagining a future event in response to photographs
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler N, Nadler B, Eviatar Z, Shamay-Tsoory SG. The relationship between theory of mind and autobiographical memory in high-functioning autism and Asperger syndrome. Psychiat Res. 2010;178:214–216. doi: 10.1016/j.psychres.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus. 2007;17:420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test Revised version: A study with normal adults, and adults with Asperger’s syndrome or high-functioning autism. J Child Psychol Psyc. 2001;42:241–251. [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cer Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends Cog Sci. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Bressler SL, McIntosh AR. The role of neural context in large-scale neurocognitive network operations. In: Jirsa VK, McIntosh AR, editors. Springer Handbook on Brain Connectivity. Springer; New York: 2007. pp. 403–419. [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cog Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney DR, Colvin CR, Hall JA. A thin slice perspective on the accuracy of first impressions. J Res Pers. 2007;41:1054–1072. [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. Person categories and social perception: Testing some boundaries of the processing effects of prior knowledge. J Pers Soc Psychol. 1981;40:441–452. [Google Scholar]
- Corbetta M, Patel GH, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran R, Frith CD. Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol Med. 2003;33:897–905. doi: 10.1017/s0033291703007529. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivitoal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gilbert DT. Los Angeles Times. 2006. Jul 2, If only gay sex caused global warming. [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VC, et al. Mapping the structural core of the human cerebral cortex. Public Library of Science Biology. 2008;7:1479–1493. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cog Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hein G, Knight RT. Superior temporal sulcus—It’s my area: Or is it? J Cog Neurosci. 2008;20:1–12. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Hassabis D, Spiers HJ, Vann SD, Vargha-Khadem F, Maguire EA. Impaired spatial and non-spatial configural learning in patients with hippocampal pathology. Neuropsychologia. 2007;45:2699–2711. doi: 10.1016/j.neuropsychologia.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox M, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutierrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, Tulving E, Stuss DT. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levine B, Freedman M, Dawson D, Black S, Stuss DT. Ventral frontal contribution to self-regulation: Convergence of episodic memory and inhibition. NeuroCase. 1999;5:263–275. [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011 doi: 10.1146/annurev-psych-120709-145406. in press. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Ann Rev of Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cer Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Phil Trans R Soc B. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Perry D, Hendler T, Shamay-Tsoory SG. Projecting memories: The role of the hippocampus in emotional mentalizing. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.08.057. in press. [DOI] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. Common and unique neural correlates of autobiographical memory and theory of mind. J Cog Neurosci. 2010;22:1095–1111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cog Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss D, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in theory of mind. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cog Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PSF, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady C. Patterns of brain activity supporting autobiographical memory, prospection and theory-of-mind and their relationship to the default mode network. J Cog Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J Cog Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner R. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2009;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey E, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Results of the rsfcMRI analysis at varying thresholds. Green = Regions correlated with the left hippocampus; Red = Regions correlated with the right temporoparietal junction. Yellow = overlapping regions correlated with both the left hippocampus and the right TPJ. A) Both maps independently thresholded at p < .01 (uncorrected); B) p < .001 (uncorrected); C) p < .0001 (uncorrected); and, D) FWER p < .05 (corrected).


