Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor with an extremely poor prognosis in spite of multimodal treatment approaches. The estimated median survival in cases with GBM is about 12–16 months. Those patients who survive =3 years after the initial diagnosis are defined as long-term survivors. In this study, we retrospectively analyze 50 consecutive cases of Bulgarian patients with newly diagnosed GBM surgically treated at our institution for a period of 1 year. Four of them survived for more than 36 months after the initial intervention. The histological re-examination revealed features typical of primary GBM in 3 of these cases, which are described in detail in the present paper. A brief review of the relevant literature is also given.
Key Words: Primary glioblastoma multiforme, Long-term survival, Prognostic factors
Introduction
Glioblastoma multiforme (GBM) is the most common and malignant primary brain tumor with an extremely poor prognosis in spite of aggressive treatment, consisting of surgery followed by chemoradiation and adjuvant chemotherapy [1, 2]. The estimated median survival in cases with GBM is about 12–16 months [3, 4]. Those patients who survive =3 years after the initial diagnosis are defined as long-term survivors [5, 7].
Aims
The aims of our study were: (1) to determine the rate of long-term survivors in an unselected group of Bulgarian patients operated on for the first time with histologically confirmed GBM, (2) to verify the histological diagnosis in each of them, and (3) to determine factors associated with favorable clinical outcome in those with established primary GBM.
Material and Methods
We retrospectively analyze 50 consecutive cases of Bulgarian patients with newly diagnosed GBM surgically treated at our institution between January and December 2006. Four of them survived for more than 3 years after the initial intervention. Histological re-examination of the tumor samples revealed features typical of primary GBM in 3 of these cases. The fourth patient was excluded from the study because of the presence of large gemistocytic areas which suggested the diagnosis of a secondary GBM.
Case Reports
Case 1
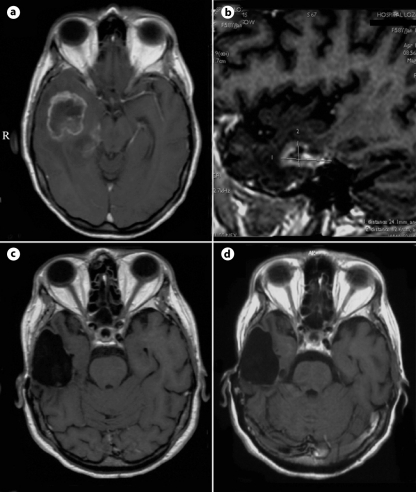
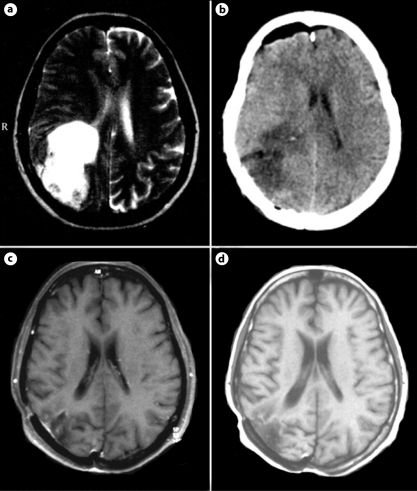
A 57-year-old female with known Crohn's disease was admitted at the clinic with a 10 weeks' history of progressive headache. Physical examination showed signs and symptoms of increased intracranial pressure (ICP) without any focal neurological deficit. The Karnofsky Performance Scale (KPS) score at the time of admission was estimated at 90. The familial history of the patient was unremarkable. Magnetic resonance imaging (MRI) revealed right-sided temporobasal lesion with compression of the adjacent brain structures (fig. 1a). Subtotal excision of the tumor was performed without development of additional neurological deficit. Histological examination revealed typical features of GBM without any areas of differentiation (fig. 2). The patient underwent subsequent conventional external-beam radiotherapy (RT) with a total dose of 60 Gy followed by 2 courses of adjuvant temozolomide (TMZ). A progressive headache occurred 9 months after the initial surgery. MRI revealed a residual tumor at the site of intervention (fig. 1b). The patient was reoperated on with a gross total removal of the lesion. Histological examination showed the same findings as previously. The patient improved after the procedure with regard to increased ICP. Eighteen courses of TMZ were consequently administered. Forty-three months after the diagnosis, the patient is feeling well without neuroimaging evidence of recurrent disease (fig. 1c, d).
Fig. 1.
MRI scans of case 1. Axial contrast-enhanced T1-weighted image before the first intervention (a); sagittal contrast-enhanced T1-weighted scan at the second admission (b); axial contrast-enhanced T1-weighted controls 4 months later (c), and at the end of the study (d).
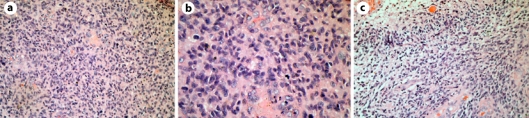
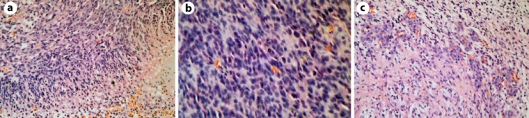
Fig. 2.
Histological findings of case 1. The tumor displays marked hypercellularity (a, H&E ×200), many mitoses (b, H&E ×400), pseudopalisading necroses and microvascular proliferation (c, H&E ×200).
Case 2
A 52-year-old male was referred to our department with a 4 weeks' history of progressive headache. Except signs and symptoms of increased ICP, the neurological status of the patient was rated normal. The KPS score was calculated at 90. The familial history revealed that the father suffered from colorectal carcinoma. MRI showed an intraparenchymal lesion situated in the right temporoparietal region with compression of the lateral ventricle and dislocation of the midline structures to the left (fig. 3a). Subtotal tumor removal was performed in emergency because of the development of extensive intratumoral hemorrhage soon after the admission. The neurological condition of the patient gradually improved after the procedure. Histological examination revealed 'classic' GBM (fig. 4). After the surgery, the patient underwent chemoradiation in standard regimens followed by 4 cycles of adjuvant TMZ. He was symptom free for 4 months, but then the headache reappeared. MRI revealed local progression of the residual tumor (fig. 3b). Subtotal excision of the lesion was performed with the same histological result. The patient's headache improved after the procedure. Twenty-five courses of TMZ were consequently administered. At the end of the study (January 31, 2010), the patient is feeling well with the only complaint of slight left arm paresis (follow-up = 43 months).
Fig. 3.
MRI images of case 2. Axial contrast-enhanced T1-weighted scan at the first admission (a); axial contrast-enhanced T1-weighted image before the second surgery (b); axial contrast-enhanced T1-weighted scans 6 months later (c), and at the end of the study (d).
Fig. 4.
Histological findings of case 2. The tumor shows hypercellularity, prominent cellular polymorphism, and mitoses (a, H&E ×400). Pseudopalisading necroses (b, H&E ×400) and microvascular proliferation (c, H&E ×400) are also seen.
Case 3
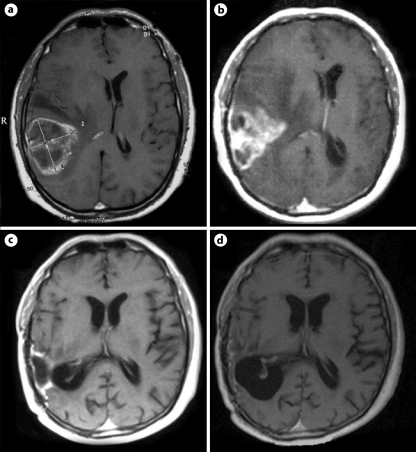
A 56-year-old female presented with a 4 weeks' history of progressive headache. Physical examination revealed signs and symptoms of increased ICP, slight left-sided hemiparesis, and left homonymous hemianopsia. KPS score was estimated at 80. The patient's familial history indicated that her mother had died of a brain tumor of unknown histology. MRI showed a hetero-intense lesion situated in the right parieto-occipital region with compression of the occipital horn of the lateral ventricle (fig. 5a). Gross total tumor removal was performed without subsequent neurological deterioration (fig. 5b). Histology confirmed the diagnosis of a primary GBM (fig. 6). The patient underwent RT with concomitant TMZ 2 months after surgery, followed by 10 cycles of adjuvant TMZ. No tumor regrowth was noted on further MRI controls (follow-up = 39 months; fig. 5c, d). At the end of the study, the patient's only complaint is persistent left homonymous hemianopsia.
Fig. 5.
MRI and computed tomography scans of case 3. Axial T2-weighted image before the initial intervention (a); control computed tomography scan on the second postoperative day (b); axial contrast-enhanced T1-weighted images 18 months later (c), and at the end of the study (d).
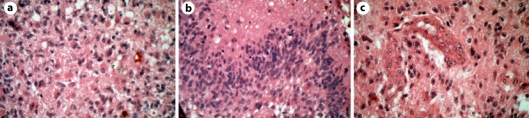
Fig. 6.
Histological findings of case 3. The tumor displays pseudopalisading necroses (a, H&E ×200), hypercellularity with frequent mitotic figures (b, H&E ×400), and microvascular proliferation (c, H&E ×200).
Discussion
The 3-year survival rate in cases with GBM varies between different authors. In 1999, Scott et al. [6] reported on 689 unselected patients with histologically confirmed GBM. Fifteen of them (2.2%) were classified as long-term survivors. In 2004, Ohgaki et al. [8] published a population-based study of 715 newly diagnosed GBM cases. The authors claimed that survival rate at 3 years was 1.2%, despite the fact that not all of the cases were followed until death. However, more recent studies demonstrate increased long-term survival probabilities. For example, in 2008, Filippini et al. [9] reported on a long-term survival rate of 7% in 676 consecutive patients with histologically confirmed primary GBM. One year later, Sonoda et al. [10] and Glas et al. [11] estimated long-term survival rates of 14.6 and 18.5%, respectively. According to our data, the long-term survival probability in Bulgarian patients with newly diagnosed GBM is 8%.
A number of studies have been addressed to define factors capable of predicting which GBM patient will become a long-term survivor. We consider that all these factors can be classified into the following 3 groups:
Patient-Dependent Factors
Most authors agree that young age at presentation is a predictor of long-term survival in patients with GBM [6, 12, 13, 14, 15, 16, 17]. For example, in 1993, Chandler et al. [12] estimated a mean age of 39.2 years in a group of 22 long-term survivors. This finding was confirmed by many other subsequent studies: Archibald et al. [13] (1994) – 37.7 years, Morita et al. [14] (1996) – 39.2 years, Scott et al. [6] (1999) – 43.5 years, Burton et al. [15] (2002) – 39 years, McLendon and Halperin [16] (2003) – 40.2 years, and Steinbach et al. [17] (2006) – 42.0 years. Interestingly, in 2007, Krex et al. [7] indicated a median age of 51 years which was significantly higher from that reported before. However, it seems that patient age >60 years is more likely to be associated with poor clinical outcome [18, 19]. The mean age of our long-term survivors is 55 years (range, 52–57 years), which does not correspond with the literature.
The true impact of gender on the survival is not clearly estimated. Some authors claim that female gender is a predictor of long-term survival in patients with GBM. For example, in 2004, Shinojima et al. [20] described 6 cases with a survival of more than 5 years. All 6 were women, with a mean age of 44.2 years. However, other authors reported equal or even inverse sex distribution [5, 16]. In our study, females outnumbered males by a ratio of 2:1.
It is proven that a KPS score =80 before surgery correlates with improved GBM survival. In 1999, Scott et al. [6] demonstrated that long-term survivors had a higher mean KPS score compared with controls. On the other hand, in 2009, Chaichana et al. [19] found that a KPS score =80 is associated with unfavorable clinical outcome. These findings are supported by many other reports [9, 20, 21]. All 3 patients included in our study had KPS scores of =80 at the time of admission, which is comparable with the literature.
Although the clinical presentation of GBM may not differ significantly between long-term and short-term survivors, it has been demonstrated that absence of major neurological deficit prior to surgery is associated with better prognosis [19]. Furthermore, a longer duration of signs and symptoms is more likely to be found in long-term survivors – 52 versus 7.2 weeks [21]. In our study, the leading patient complaints were associated with increased ICP. The mean symptom duration was 6 weeks (range, 4–10 weeks), which is not typical for long-term survivors.
Tumor-Dependent Factors
There is an agreement that patients with secondary GBM survived significantly longer than those with primary GBM [8, 22]. According to Ohgaki et al. [8], this can be explained by the younger age of the cases with secondary GBMs rather than a reflection of different biological behavior. All 3 cases described here fulfilled the accepted criteria for primary GBM: a short clinical history (less than 3 months) and typical histopathological findings of GBM at the first biopsy.
In 1993, Simpson et al. [23] failed to find any influence of tumor size prior to surgery on GBM survival. On the contrary, in 2004, Jeremic et al. [24] demonstrated that patients who had tumors of 4 cm in size or less did better when compared to those with larger lesions. However, both authors agree that frontal tumor location is associated with better survival, probably because of the possibility for more extensive surgical removal. The tumor was right-sided in all long-term survivors described here. The frontal lobe was not involved in any of the cases.
The classical histological predictors of long-term survival include: presence of giant cells, focal oligodendroglial differentiation, and absence of small anaplastic elements [7, 16, 20, 25, 26, 27, 28]. In 2004, Shinojima et al. [20] were able to detect giant cells exclusively in long-term survivors. In 2005, Deb et al. [27] identified areas of oligodendroglial differentiation in 2 of 6 tumors investigated. According to McLendon and Halperin [16], intermediate fibrillary elements are more common and small anaplastic elements are less common in long-term survivors. The histological re-evaluation revealed typical features of primary GBM in 3 of our long-term survival cases. One patient was excluded from the study because of the presence of large gemistocytic areas which suggested the diagnosis of a secondary GBM.
It is obvious that routine histopathological grading has limited capacity to predict GBM survival. Currently, efforts are focused on detecting molecular markers of long-term survival [1, 29]. It has been demonstrated that TP53 overexpression is more likely and MDM2 overexpression is less likely to be found in long-term survivors [30]. In 2002, Burton et al. [15] demonstrated 19q chromosomal loss exclusively in long-term survival cases. Interestingly, EGFR overexpression was not detected in any of the long-term survivors described by Deb et al. [25]. The authors found TP53 positivity in 4 of the 5 tumors investigated. In 2005, Korshunov et al. [31] described 46 copy number aberrations strongly associated with GBM outcome. Using array-based comparative genomic hybridization analysis, the authors found 26 copy number aberrations associated with short-term and 20 – with long-term survival. In 2007, Krex et al. [7] demonstrated MGMT hypermethylation in 28 of 36 tumors (77.7%) investigated. They also found TP53 mutations in 9 of 31 cases (29.0%) and EGFR overexpression in 10 of 38 cases (26.3%). Only 2 of 32 cases (6.3%) carried a combined 1p and 19q deletion. Recently, Carro et al. [32] identified 2 genes – C/EBP-β and STAT3, the inactivation of which was strongly associated with long-term survival. Unfortunately, no patient in the present study had been investigated in search for molecular predictors.
Treatment-Dependent Factors
The actual therapeutic approach in the cases with GBM consists of surgery followed by chemoradiation and adjuvant chemotherapy [33, 34]. Most authors agree that a more aggressive treatment is associated with prolonged survival [1, 2, 4, 6, 9, 18, 22, 24]. Interestingly, in 2001, Sabel et al. [28] reported on a 69-year-old patient who survived for more than 17 years after the diagnosis of giant cell GBM. The patient had not undergone any postoperative RT or adjuvant chemotherapy and died without clinical evidence of GBM recurrence.
It seems that gross total tumor removal is not essential for long-term survival. For example, in 1993, Chandler et al. [5] found such a radical excision in only 2 of 22 long-term survivors investigated. This finding was confirmed later by McLendon and Halperin [15]. In our study, gross total tumor excision was achieved in one patient and subtotal in the other two, who were consequently reoperated.
RT with concomitant and adjuvant TMZ has become the standard of care for patients with newly diagnosed GBM [34]. In a study of 39 long-term survivors, Hottinger et al. [35] found that all patients had received RT. Interestingly, concomitant TMZ was administered only in 18% of them. It seems that combination between RT and nitrosourea may increase long-term survival rate. For example, in 2009, Sonoda et al. [10] published a study of 123 consecutive GBM patients initially treated by maximum tumor resection followed by RT and intravenous injection of nimustine hydrochloride. Using this strategy, the authors reported on a long-term survival rate of 14.6%. Subsequent chemoradiation in standard regimens was performed in two of the patients included in our study; the other patient underwent RT alone. All patients received at least 10 courses of adjuvant TMZ until the end of the study period (range, 10–29 cycles).
Conclusion
This is the first clinical study to determine the rate of long-term survivors in an unselected group of Bulgarian patients with newly diagnosed GBM. Further prospective trials with a large number of patients are needed to determine the factors associated with favorable clinical outcome in cases with primary GBM.
Acknowledgement
The authors wish to thank the Stay Foundation – Sofia, Bulgaria, (www.stay.eu.com) for supporting this research project.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 2.Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, Yan H, Adamson DC. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets. 2009;13:701–718. doi: 10.1517/14728220902942348. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Jenkins RB. Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Front Biosci. 2000;5:213–231. doi: 10.2741/smith. [DOI] [PubMed] [Google Scholar]
- 4.Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, Büyükberber S. Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: a long-term analysis. Tumori. 2009;95:191–197. doi: 10.1177/030089160909500210. [DOI] [PubMed] [Google Scholar]
- 5.Chandler KL, Prados MD, Malec M, Wilson CB. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993;32:716–720. doi: 10.1227/00006123-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Scott JN, Rewcastle NB, Brasher PM, Fulton D, MacKinnon JA, Hamilton M, Cairncross J, Forsyth P. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol. 1999;46:183–188. [PubMed] [Google Scholar]
- 7.Krex D, Klink B, Hartmann C, von Deimling SM, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G. Long-term survival with glioblastoma multiforme. Brain. 2007;130(Pt 10):2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 8.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lütolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 9.Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D, Farina R, Farinotti M, Fariselli L, Finocchiaro G, Giombini S, Pollo B, Savoiardo M, Solero CL, Valsecchi MG. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79–87. doi: 10.1215/15228517-2007-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonoda Y, Kumabe T, Watanabe M, Nakazato Y, Inoue T, Kanamori M, Tominaga T. Long-term survivors of glioblastoma: clinical features and molecular analysis. Acta Neurochir (Wien) 2009;151:1349–1358. doi: 10.1007/s00701-009-0387-1. [DOI] [PubMed] [Google Scholar]
- 11.Glas M, Happold C, Rieger J, Wiewrodt D, Bähr O, Steinbach JP, Wick W, Kortmann RD, Reifenberger G, Weller M, Herrlinger U. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27:1257–1261. doi: 10.1200/JCO.2008.19.2195. [DOI] [PubMed] [Google Scholar]
- 12.Chandler KL, Prados MD, Malec M, Wilson CB. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993;32:716–720. doi: 10.1227/00006123-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Archibald YM, Lunn D, Ruttan LA, Macdonald DR, Del Maestro RF, Barr HW, Pexman JH, Fisher BJ, Gaspar LE, Cairncross JG. Cognitive functioning in long-term survivors of high-grade glioma. J Neurosurg. 1994;80:247–253. doi: 10.3171/jns.1994.80.2.0247. [DOI] [PubMed] [Google Scholar]
- 14.Morita M, Rosenblum MK, Bilsky MH, Fraser RA, Rosenfeld MR. Long-term survivors of glioblastoma multiforme: clinical and molecular characteristics. J Neuro Oncol. 1996;27:259–266. doi: 10.1007/BF00165483. [DOI] [PubMed] [Google Scholar]
- 15.Burton EC, Lamborn KR, Feuerstein BG, Prados M, Scott J, Forsyth P, Passe S, Jenkins RB, Aldape KD. Genetic aberrations defined by comparative genomic hybridization distinguish long-term from typical survivors of glioblastoma. Cancer Res. 2002;62:6205–6210. [PubMed] [Google Scholar]
- 16.Mc Lendon RE, Halperin EC. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003;98:1745–1748. doi: 10.1002/cncr.11666. [DOI] [PubMed] [Google Scholar]
- 17.Steinbach JP, Blaicher HP, Herrlinger U, Wick W, Nägele T, Meyermann R, Tatagiba M, Bamberg M, Dichgans J, Karnath HO, Weller M. Surviving glioblastoma for more than 5 years: the patient's perspective. Neurology. 2006;66:239–242. doi: 10.1212/01.wnl.0000194221.89948.a0. [DOI] [PubMed] [Google Scholar]
- 18.Nieder C, Astner ST, Molls M, Grosu AL. Analysis of long-term survivors of glioblastoma multiforme in a single institution with aggressive local retreatment protocol. Anticancer Res. 2007;27:2993–2996. [PubMed] [Google Scholar]
- 19.Chaichana K, Parker S, Olivi A, Quiñones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 20.Shinojima N, Kochi M, Hamada J, Nakamura H, Yano S, Makino K, Tsuiki H, Tada K, Kuratsu J, Ishimaru Y, Ushio Y. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg. 2004;101:219–226. doi: 10.3171/jns.2004.101.2.0219. [DOI] [PubMed] [Google Scholar]
- 21.Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, MacKinnon JA, Sutherland G, Cairncross JG, Forsyth P. Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci. 1998;25:197–201. doi: 10.1017/s0317167100034016. [DOI] [PubMed] [Google Scholar]
- 22.Mineo JF, Quintin-Roue LB, Buburusan V, Besson G. Glioblastomas: clinical study and search for prognostic factors. Neurochirurgie. 2002;48:500–509. [PubMed] [Google Scholar]
- 23.Simpson, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive radiation therapy oncology group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 24.Jeremic B, Milicic B, Grujicic D, Dagovic A, Aleksandrovic J, Nikolic N. Clinical prognostic factors in patients with malignant glioma treated with combined modality approach. Am J Clin Oncol. 2004;27:195–204. doi: 10.1097/01.coc.0000055059.97106.15. [DOI] [PubMed] [Google Scholar]
- 25.Kozak KR, Moody JS. Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro Oncol. 2009;11:833–841. doi: 10.1215/15228517-2008-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naydenov E, Bussarsky V, Nachev S, Hadjidekova S, Toncheva D. Long-term survival of a patient with giant cell glioblastoma: Case report and review of the literature. Case Rep Oncol. 2009;2:103–110. doi: 10.1159/000228545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deb P, Sharma MC, Mahapatra AK, Agarwal D, Sarkar C. Glioblastoma multiforme with long term survival. Neurol India. 2005;53:329–332. [PubMed] [Google Scholar]
- 28.Sabel M, Reifenberger J, Weber RG, Reifenberger G, Schmitt HP. Long-term survival of a patient with giant cell glioblastoma. Case report. J Neurosurg. 2001;94:605–611. doi: 10.3171/jns.2001.94.4.0605. [DOI] [PubMed] [Google Scholar]
- 29.Sulman EP, Guerrero M, Aldape K. Beyond grade: molecular pathology of malignant gliomas. Semin Radiat Oncol. 2009;19:142–149. doi: 10.1016/j.semradonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Burton EC, Lamborn KR, Forsyth P, Scott J, O'Campo J, UyeharaLock J, Prados M, Berger M, Passe S, Uhm J, O'Neill BP, Jenkins RB, Aldape KD. Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors. Clin Cancer Res. 2002;8:180–187. [PubMed] [Google Scholar]
- 31.Korshunov A, Sycheva R, Golanov A. Genetically distinct and clinically relevant subtypes of glioblastoma defined by array-based comparative genomic hybridization (array-CGH) Acta Neuropathol. 2006;111:465–474. doi: 10.1007/s00401-006-0057-9. [DOI] [PubMed] [Google Scholar]
- 32.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, Califano A, Iavarone A. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, Osti MF, De Paula U, Lanzetta G, Tombolini V, Maurizi Enrici R. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 34.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 35.Hottinger AF, Yoon H, DeAngelis LM, Abrey LE. Neurological outcome of long-term glioblastoma survivors. J Neurooncol. 2009;95:301–305. doi: 10.1007/s11060-009-9946-9. [DOI] [PubMed] [Google Scholar]