Abstract
Plants have recently moved into the spotlight with the growing realization that the world needs solutions to energy and food production that are sustainable and environmentally sound. Iron (Fe), copper (Cu), and zinc (Zn) are essential for plant growth and development, yet the same properties that make these transition metals indispensable can also make them deadly in excess. Fe and Cu are most often utilized for their redox properties, while Zn is primarily utilized for is ability to act as a Lewis acid. Here we review recent advances in the field of metal homeostasis, seeking to integrate the findings on uptake, transport, and storage of these three metals.
Introduction
Understanding the fundamentals of plant growth in order to meet the demands for food, fuel and fiber is of the utmost importance. Metals play vital roles in a plant’s life cycle yet there are many impediments to proper metal homeostasis. Here we focus on some of the challenges of maintaining Fe, Zn and Cu homeostasis and the strategies that plants utilize to meet these challenges. These three transition metals, along with Mn, Mo and Ni, comprise the six metal micronutrients considered essential for plants (Table 1). Although reviews often cover each of these transition metals separately, by comparing and contrasting what we have learned about each of these important metals, we can achieve a more integrated picture of how plants manage their ionome.
Table 1.
Essential metal micronutrients for plants
| Element | Biologically Relevant Oxidation States | Lithospherea (mg/kg) | Typical Plantb (mg/kg) | Transporter Family | Examples |
|---|---|---|---|---|---|
| Fe | Fe2+; Fe3+ | 45,000 | 100 | FRD3, NRAMP, OPT, VIT, YSL, ZIP | cytochromes, Fe-S proteins, SOD |
| Mn | Mn2+; Mn3+; Mn4+ | 950 | 50 | CAX, NRAMP, ZIP | water-splitting enzyme in PSII, SOD |
| Mo | Mo4+; Mo6+ | 1.5 | 0.1 | MOT | nitrate reductase, sulfite oxidase, xanthine dehydrogenase, aldehyde oxidase |
| Ni | Ni2+ | 80 | 0.1 | urease | |
| Zn | Zn2+ | 75 | 20 | ZIP, HMA, MTP | RNA polymerase, alcohol dehydrogenase, carbonic anhydrase, SOD |
Fe and Cu both exist in multiple redox states, readily accepting and donating electrons from their d-orbitals. As such, Fe and Cu serve as critical cofactors for components of the electron transport chain in the mitochondrion and in the chloroplast 1. Fe is also found in the center of Fe-S clusters, which act as electron acceptors and donors in a number of key cellular processes including nitrogen fixation, sulfate assimilation, and ethylene biosynthesis 2. The most abundant Cu protein in plants is plastocyanin, a protein that transfers electrons from the cytochrome b6f complex to PSI. Cu is utilized as a cofactor by proteins involved in protection from reactive oxygen species, lignification of the cell wall, pollen formation, proper carbohydrate metabolism, and formation of phenolics in response to pathogen attack 1. Cu is also required by the ethylene receptor for proper signaling 3.
Zn, unlike Fe and Cu, is not redox active. This property, combined with the pronounced Lewis acid characteristics of the Zn2+ ion and the flexibility of the coordination sphere with respect to geometry and the number of ligands, help explain why Zn plays so many different roles inside cells 4. It is required as a cofactor in over 300 enzymes, including RNA polymerase, superoxide dismutase, alcohol dehydrogenase and carbonic anhydrase 5. It also plays key structural roles such as being used to form the finger-shaped DNA binding domain that interacts with the major groove of DNA in the Zn finger family of transcription factors 5,6.
Uptake
The primary source of metals for the plant is the soil (Table 1), making it clear that efficient uptake is essential for life. Even when abundant, metals can be inaccessible in the soil due to their tendency to be present predominantly in an insoluble form. Zn and Cu are primarily insoluble in soils due to adsorption to clay, CaCO3, or organic matter, while Fe is predominantly found as Fe hydroxides 1. Insolubility is particularly pronounced at the higher pH of alkaline soils, which represent approximately 30% of the world’s soils 7.
Acidification of the soil
To overcome the challenge of insolubility in alkaline soils, plants can utilize ATPase activity to extrude protons into the rhizosphere to decrease the pH of the soil under metal limiting conditions 8. As the pH of the soil decreases, the increased concentration of protons helps to generate free metal. For example, Fe3+ is released from insoluble oxides with the concomitant formation of water:
A unit drop in pH increases the solubility of Fe by a thousand fold 9. The ATPase(s) responsible for proton extrusion and soil acidification under Fe deficiency has not yet been confirmed, but it is likely to be a member of the AHA family 10. In Arabidopsis, AHA1, AHA2, and AHA7 are all expressed in the roots and are upregulated under Fe deficiency 11,12. Acidification of the soil would also result in an increase in the solubility of Zn and Cu, by encouraging cation exchange and releasing the divalent metals from insoluble chelates with soil particles. ATPase activity also allows for the establishment of a negative membrane potential, along the order of −100 to −250mV, which serves to drive cation uptake 10.
Reduction
Once freed from insoluble chelates, the transition metals are more accessible for uptake. However, the transporters in many plants have a specific affinity for a particular oxidation state of each metal. Many plants address this problem by utilizing a reduction-based strategy for metal uptake. While Zn is always found in the +2 oxidation state under physiological relevant conditions, both Fe and Cu need to be reduced for uptake by their respective transporters, IRT1 and COPT1 13. For example, Fe is transported into the cell in the divalent form, despite being present in the soil primarily in the trivalent form. To accommodate this, Fe3+ is reduced to Fe2+ by the ferric chelate reductase FRO2, which transports electrons from NADH bound to cytoplasmic binding sites across the membrane via heme to reduce Fe3+ 14. Consistent with this role, FRO2 is expressed in the plasma membrane and shows increased accumulation and activity under Fe deficiency 14,15. When induced by Fe deficiency, FRO2 is also able to reduce Cu, but it is not yet known if it serves as the primary reductase for Cu. Cu uptake transporters have affinity for Cu+, yet Cu+ is readily oxidized to Cu2+ in aerated soil, suggesting the requirement for a reductase 13,16. FRO3 is upregulated under Cu deficiency but is not plasma membrane localized 17,18.
Chelation
In contrast to the reduction-based strategy, grasses primarily utilize a chelation-based strategy for Fe uptake. This strategy employs the release of chelators into the rhizosphere, known as phytosiderophores, to bind Fe3+ for transport into the plant 19,20. Phytosiderophores are synthesized from methionine and are usually referred to collectively as belonging to the mugineic acid family (MAs). Expression of the genes involved in MA biosynthesis are up-regulated under Fe deficiency 21, resulting in increased release of MAs. In barley, MAs are also thought to play a role in mobilizing Zn in addition to Fe 22. Suzuki et al. (2006) showed that roots of Zn-deficient barley plants have increased expression of genes involved in the biosynthesis of MAs, and that there is increased secretion of MAs from the roots of these plants. These authors also showed that Zn-deficient barley is not deficient in Fe, as had previously been suggested to explain phytosiderophore release under Zn deficiency. Furthermore, using a Positron-Emitting Tracer Imaging System (PETIS) to follow the movement of radiolabeled Zn, they showed that more Zn is taken up when plants are supplied with Zn(II)-MAs than with Zn2+, suggesting that MAs secreted as a result of Zn deficiency are effective in absorbing Zn from the soil. In rice, however, similar experiments showed phytosiderophores appear to play a role in the distribution of Zn within the plant rather than in the absorption of Zn from the soil 23. To date, there is no suggested role for phytosiderophores in Cu uptake.
Transporters involved in metal uptake from the soil
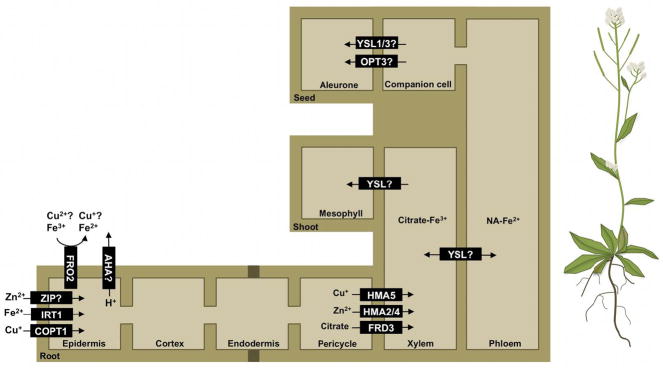
While ions are able to diffuse into the space between the cell wall and the plasma membrane (the apoplast) of some cells in the root, apoplastic transport is blocked by the impermeable Casparian strip in the endodermal layer. At this point, metals must be actively transported across the plasma membrane into the symplastic space where they can then move via plasmadesmata to the inner root cell layers for subsequent loading into the vasculature24 (Figure 1).
Figure 1. Intercellular metal transport.
Fe, Zn, and Cu are taken up into the symplast by transporters in the epidermis. Reduction of Fe and possibly Cu by FRO2 and acidification of the soil by an AHA contribute to increased metal uptake. Metals can then travel through the symplastic space to the vasculature, bypassing the waxy Casparian strip on the endodermis. Transport into the xylem is still not fully characterized but is thought to involve members of the HMA family and the citrate effluxer FRD3. In the xylem, metals are carried to the shoot through the transpiration stream where they are unloaded into the shoot, most likely by a member of the YSL family. YSLs may also translocate metals to the phloem, where they can then be delivered to the seed. Dark brown boxes represents the Casparian strip.
As we think about metals moving from the rhizosphere into the plant, it is important to note the challenge for researchers of tracking metal transport due to the limits of metal imaging techniques currently available. Methods such as inductively coupled plasma mass spectrometry (ICP-MS) allow for sensitive metal detection 25 but ICP-MS requires destructive preparation of the samples, limiting its use in following metal localization. As mentioned above, PETIS technology, which uses γ-rays emitted from positrons (β+), has been used to visualize and quantify the uptake and translocation of radiolabeled 52Fe and 62Zn in grasses 22,23,26,27 but such imaging requires specialized equipment and cannot give you cellular and subcellular resolution. Synchrotron X-ray fluorescence (SXRF) also allows the non-destructive spatial visualization of metal abundance at the tissue level and has the great advantage that it can be used to detect multiple metals simultaneously at high resolution 28,29. However, to easily examine metal localization in vivo, one would really like metal-specific fluorophores. Fluorescent small molecules that respond to metal ions in the cell with appropriate selectivity and sensitivity offer the ability to probe the cell biology of metals with spatial and temporal fidelity 30. While a fluorescent sensor has been used for localization of Zn in Arabidopsis roots31, no equivalent sensors exist for Fe and Cu.
Transport of metals into the symplast of the epidermis is carried out via members of several transporter families. Fe is taken up primarily through the high affinity transporter IRT1 32–35. This essential member of the ZIP (ZRT, IRT-like proteins) metal transporter family localizes to the plasma membrane of the root epidermis and is required for seedling survival 33–35. The lethal phenotype of irt1 mutants can be rescued by addition of exogenous Fe, indicating that its primary role is in uptake of Fe 33,34. Like FRO2, IRT1 has been found to be upregulated in response to Fe deficiency, and substantial protein accumulation occurs only under Fe deficiency 36, suggesting both transcriptional and post-translational control 37. The mechanism for this regulation has recently been elucidated, and IRT1 has been shown to be regulated postranslationally by ubiquitination at specific lysine residues which leads to proteosome-mediated degradation 37.
While it has long been thought that grasses solely use the chelation-based strategy for Fe uptake 38, work in recent years has identified orthologs of IRT1 that are upregulated under Fe deficiency in rice and can rescue Fe transport in yeast mutants 26,39. Further overturning the dogma that grasses solely transport chelated Fe, a recent paper has demonstrated that rice is able to take up Fe2+ when nicotianamine (NA) synthesis is compromised 40. NA is the precursor for the synthesis of MAs. This ability for grasses to take up Fe2+ would be particularly advantageous in plants such as rice that are often grown in flooded soils where Fe would be less oxidized and more likely to exist as Fe2+. This finding changes the way we categorize metal uptake strategies, emphasizing a greater plasticity that was originally thought.
Although the essential role of IRT1 is in Fe uptake, it can also transport other divalent metals 41, and irt1 plants have reduced levels of Zn as well as other cations 33,35. Because irt1 plants are able to survive without the addition of excess Zn, it is likely that Zn is primarily taken up into the plant via other transporters. ZIP2 and ZIP5 are two good candidates for Zn uptake as they both localize to the plasma membrane of root epidermal cells 42 and are able to complement Zn uptake mutants in yeast 43,44. It is not yet known, however, which proteins are responsible for Zn uptake.
Unlike Fe and Zn, Cu is not taken up primarily as Cu2+, but instead is transported as Cu+ by COPT1 45, a member of the COPT family 46. COPT proteins are the Arabidopsis orthologs of the yeast transporter CTR. COPT1 can complement yeast ctr1 mutants, is upregulated under Cu deficiency in plants and mutant plants show decreased Cu accumulation as well as upregulation of genes that respond to Cu limitation 45,47. Cu2+ is more commonly found in the soil than Cu1+13. Uptake of Cu2+ may be mediated by a member of the ZIP family, a transporter family known to preferentially transport divalent cations. Both ZIP2 and ZIP4 are known to be upregulated by Cu deficiency and can complement ctr1 yeast mutants for Cu uptake 48, but further research with loss of function mutants is needed to test the involvement of these transporters in Cu uptake.
As mentioned above, Fe is primarily taken up as chelated MA complexes in grasses. The transporter responsible for Fe(III)-MA uptake, YS1 (yellow stripe 1), was originally identified in maize 49 using the ys1 mutant that is defective for phytosiderophore uptake. YS1 is expressed in the roots in response to Fe deficiency and localizes to the plasma membrane, as would be expected for an uptake transporter 50. In rice, OsYSL15 (yellow stripe like) is the primary Fe-MA uptake transporter 51. Members of the YSL family also are involved in metal distribution within the plant as will be discussed below.
Transport between tissues
Although initial uptake into the plant is clearly critical, many of the essential roles of metals are in photosynthetic shoot tissue. Metals must therefore be transported through the plant from uptake at the roots to the tissues where they are required. Once within the root epidermal cell after uptake from the soil, ions can move through symplastic passages from the epidermis to the pericycle to be loaded into the xylem 24. Metals must be actively loaded into the xylem and transported by the transpiration stream to shoot tissue 52. Some tissues such as the seed are not fed by the transpiration stream and must rely on the phloem for nutrients. Other tissues such as developing leaves do not yet have fully differentiated xylem and must receive the necessary metals through the phloem, which differentiates more quickly 52. Proper loading and unloading of the vasculature is essential for metal transport in the plant.
Root to shoot transport
The transporter that loads Fe into the xylem is not yet known, but Fe is most likely transported chelated to other molecules. Candidates include citrate and NA. NA is found throughout the plant kingdom and as mentioned earlier, is the precursor for formation of phytosiderophores in grasses. The pH of the xylem favors the chelation of Fe to citrate rather than NA 52, and it is known that Fe exists as Fe3+-citrate chelates in the xylem 53. FRD3, a citrate transporter that localizes to the plasma membrane of the pericycle and the vascular cylinder 54, has been recently shown to efflux citrate into the xylem and is required for Fe transport to the shoot 53,55. frd3 plants show reduced citrate levels in the xylem as well as the shoot, and they hyperaccumulate Fe in the root, emphasizing the necessity of FRD3 for long distance transport of Fe. Rice also relies on a FRD3-like gene, OsFRDL1, for efficient translocation of Fe to the shoot 56. Fe is thought to be unloaded from the vasculature into developed tissue through yet unknown mechanisms.
Zn is effluxed into the xylem for long distance transport by the heavy metal transporters HMA2 and HMA4, which localize to the plasma membrane of the root and shoot vasculature 57. hma2hma4 mutants show decreased shoot Zn and increased root Zn, supporting their role in xylem loading 57. HMA4 was originally identified as a gene with increased expression in the Zn hyperaccumulator Arabidopsis halleri 58,59. Recent work has uncovered the mechanism of this increased expression, demonstrating that, surprisingly, the increase in expression is not due to trans factors but rather to a triplication of the gene and changes to cis regulatory elements driving HMA4 expression 60. The group also demonstrated that HMA4 is the primary means of Zn shoot hyperaccumulation in A halleri, showing that plants that hyperaccumulate Zn in the shoot show higher levels of HMA4 expression, and knockdown of HMA4 by RNAi abrogated the hyperaccumulation in the shoot. Interestingly, the group also showed a separation between the ability to accumulate elevated levels of Zn in the shoot and the ability to tolerate these levels. By expressing AhHMA4 in A thaliana under control of the A halleri endogenous promoter, the group was able to show that these transformed plants recapitulated the Zn distribution patterns of A halleri and showed increased shoot Zn levels, but they also showed signs of Zn toxicity. This emphasizes that additional genes are required in hyperaccumulators to confer tolerance to high levels of metals. Hyperaccumulators have been discussed in depth elsewhere 61,62,63. The ligand for transport of Zn to the shoot is likely to be NA or organic acids 8.
Efflux of Cu into the vasculature is also thought to occur through an HMA family transporter. Work has implicated HMA5 in Cu efflux, showing that HMA5 is predominantly expressed in the root and is strongly and specifically induced by Cu. hma5 mutants overaccumulate Cu in the root, suggesting a compromised efflux system when HMA5 is absent64. Further evidence in support of the role of HMA5 in Cu translocation from the roots to the shoots come from a study of natural variation in copper tolerance among Arabidopsis accessions65. Cu is likely chelated to NA for translocation from the root to the shoot, based on the biochemical properties of NA and the phenotypes of the chloronerva mutant of tomato that cannot synthesize NA 52.
Shoot to seed transport
Fe2+ and Zn2+ are thought to be transported in the phloem as NA chelates 66, and, although the transporters involved in phloem loading and unloading are not fully known, they are thought to include members of the YSL group, a subfamily of the oligopeptide transporter (OPT) family of transporters 52. One of the better characterized members of this subfamily is YSL1, which localizes to the shoot vasculature as well as the siliques, pollen grains, and the developing seeds67. ysl1 mutants accumulate reduced Fe in the seeds, and these seeds show germination defects on low Fe soil, suggesting a role for YSL1 in metal loading of the seed 67. YSL3 is also expressed in the shoot vasculature as well as in the pollen. A recent study on the double mutant, ysl1ysl3, demonstrated that these both of these transporters play a role in Fe, Cu, and Zn remobilization from leaf tissue 68. Seeds accumulate these metals at reduced levels in the absence of YSL1 and YSL3. In addition, YSL2 has been shown to complement Fe and Cu uptake yeast mutants when supplemented with NA 69, although a second group failed to see complementation by YSL2 70. In further support of a role in transporting complexes into the vasculature, both groups found that YSL2 localizes to the lateral plasma membrane 69,70. Furthermore, the rice ortholog, OsYSL2, also localizes to the vasculature and has been shown to transport Fe2+-NA when expressed in Xenopus laevis oocytes 71.
Although OPT proteins are named for their ability to transport oligopeptides, a recent study has demonstrated that OPT3 may serve a role in transporting Fe 72, and while it has not been established what form of Fe is transported, it is likely to be chelated to NA or an oligopeptide. This group showed that opt3-2 mutants have reduced seed Fe content and abrogated seedling growth under Fe deficient conditions, suggesting a role of OPT3 in seed Fe loading. Although yeast studies with OPT3 have suggested that it can transport Cu as well 48, OPT3 does not seem to play a role in Zn or Cu loading as opt3-2 seeds actually accumulate increased levels of these two metals 72.
Intracellular transport
Once transported to the proper tissue, metals must be properly distributed at the subcellular level to ensure sufficient levels to the necessary compartments while safely storing metals under times of excess (Figure 2).
Figure 2. Intracellular metal transport.
Fe and Zn are transported into the vacuole by VIT1 and MTP1/3 respectively, and Fe is remobilized from the vacuole by NRAMP3/4. Transport into the chloroplast is best characterized for Cu, which is transported into the chloroplast by HMA1, PAA1 and possibly PIC1. PAA2 is thought to transport Cu across the thylakoid membrane. Transport of Fe into the chloroplast is known to require reduction by FRO7 and may involve transport by PIC1. Very little is known about transport in and out of the mitochondria, thought ATM3 is well established as an Fe-S exporter.
Chloroplast
Nearly 90% of the Fe in the plant is localized to the chloroplast, where it is required for use in the electron transport chain and the synthesis of chlorophyll, heme, and Fe sulfur clusters 24. In addition, Cu and Zn or Fe are required in the chloroplast as cofactors for superoxide dismutases (SODs), which catalyze the conversion of superoxides to hydrogen peroxide, preventing cellular damage by the extremely reactive hydroxyl radical species normally produced from the electron transport chain 73. Recent work has shown that the two chloroplastic Fe-SODs, FSD2 and FSD3, are required to protect the chloroplast from ROS damage during chloroplast development 74. CuZn-SODs are the other type of SOD found in the chloroplast and under limiting conditions of metals, plants can make either CuZn-SOD or Fe-SOD 13,75. The mechanism of this fluidity of metal use as cofactors involves differential control of Cu enzymes by microRNAs 76,77, which are, in turn, regulated by the transcription factor SPL7 (squamosa promoter binding protein like)78. Interestingly, transcripts encoding essential proteins requiring Cu such as plastocyanin are not targeted for degradation by the microRNAs, while CuZn SOD transcripts are targeted for degradation under Cu-limiting conditions. This allows plants to allocate Cu to the most essential functions under Cu deficiency while using other metals for enzymes that carry out equivalent functions. This type of flexibility has also been observed in Chlamydomonas where expression of the heme utilizing cytochrome c6 is induced under Cu deficiency while the Cu-dependent plastocyanin is actively degraded 79.
While the necessity of metals in the chloroplast has been clearly established, the transporters responsible are still not all identified. The permease PIC1 localizes to the inner chloroplast envelope and is critical for chloroplast development 80. PIC1 was able to complement a yeast Fe transport mutant as well as a yeast Cu transport mutant, suggesting it may also transport these metals in plants, although whether PIC1 can transport Fe in the plant has yet to be shown. An alternative role for PIC1 has been suggested as it has also been identified as a component of a protein translocation complex 81. In addition to the requirement for a transporter, it has been recently shown that reduction of Fe by FRO7 is required for uptake into the chloroplast 82. fro7 mutants have reduced Fe in the chloroplast and show photosynthetic defects, including perturbed photosystem components and compromised electron transport. Most importantly, FRO7 is required for seedling survival under Fe-limiting conditions. The requirement for reduction at the chloroplast 82 along with the identification of a reductase in the mitochondrial proteome 83, the abundance of other FROs 17, and the transport of Fe as Fe3+-citrate chelates 53 raises the possibility that Fe must be reduced at each of the membranes that it must cross.
Although the chloroplastic transporters for Fe and Zn are not yet determined, more is known about Cu transport into the chloroplast. Previous work identified PAA1 (HMA6) and PAA2 (HMA8), two members of the Cu-transporting PIB-type ATPase family, as critical for Cu delivery to plastocyanin in the chloroplast 84,85. PAA1 localizes to the inner chloroplast envelope, and PAA2 localizes to the thylakoid membrane. Cu transport into the chloroplast is not completely abolished in paa1paa2 mutants, suggesting the action of other transporters. In addition to PAA1, another family member that is a possible candidate is HMA1, which localizes to the chloroplast envelope and shows increased ATPase activity in the presence of Cu and Zn 86,87. Found to be a Ca+/heavy metal pump in yeast, HMA1 may play a specialized role in Cu delivery to superoxide dismutase, as hma1 mutants show reduced chloroplastic CuZn SOD activity but normal plastocyanin content 86,87.
Mitochondria
Fe and Cu must also be transported into the mitochondria as well to function in the respiratory electron transport chain and synthesis of Fe sulfur clusters. As with the chloroplast, the mitochondrial transporters for these proteins have not yet been identified. While no importer has been identified, STA1/AtATM3, an ABC transporter orthologous to the yeast ATM1p, has been implicated in the export of Fe sulfur clusters and can rescue yeast atm1 mutants 88. While the other two ATM proteins in Arabidopsis, ATM1 and ATM2, also localize to the mitochondria, they are not able to rescue the mutant and most likely do not play a role in Fe sulfur cluster export in plants 89. Little more is known for Cu transport. The Cu chaperone Cox17 has been implicated in Cu delivery within the mitochondria 90 but no transporter has been identified yet. Zn is most likely transported by a ZIP that localizes to the mitochondria, but as of yet no ZIP transporters have been assigned this function.
Vacuole
The vacuole may serve as key cell compartment where metals can be stored in times of excess while providing a source during times of deficiency. Fe is known to be transported into the vacuole by the transporter VIT1, which has been recently shown to be critical for proper localization of Fe in the seed 28. Remobilization of Fe from the vacuole is thought to be mediated by the actions of NRAMP3 and NRAMP4 91, which have been shown to be upregulated under Fe deficiency and both localize to the vacuolar membrane 91,92. While single mutants of either NRAMP3 or NRAMP4 show no phenotype, nramp3nramp4 mutants show a 90% lethality rate when grown on Fe deficient soils, suggesting that these proteins are functionally redundant and required for Fe mobilization under Fe deficiency 91.
Zn has been shown to be transported into the vacuole by members of the MTP (Metal Tolerance Protein) family, also referred to as CDF (cation diffusion facilitator) proteins. Both MTP1 and MTP3 localize to the vacuolar membrane 93–96 and overexpression of MTP1 or MTP3 confers resistance to high levels of Zn 93,94. Loss of expression of MTP1 or MTP3 confers Zn hypersensitivity, further supporting a role for these transporters in Zn vacuolar loading. The transporter(s) responsible for Zn remobilization from the vacuole is not yet known. A proteomic analysis of a vacuolar membrane-enriched fraction from rice roots identified two transition metal transporters, ZIP2 and COPT5, making these candidates for transporting Cu into the vacuole, but there is as of yet no functional data to go along with this localization 97.
Toxicity
The same qualities that make these transition metals so essential as cofactors can also make them highly toxic within the cell. For example, free Fe can generate high levels of oxygen and hydroxyl free radicals through the Fenton reaction 98. The main strategies that the plant employs to combat this toxicity are sequestration and chelation to carrier molecules.
In addition to sequestration within the vacuole, Fe has been shown to be stored in plastids in ferritin, a protein nanocage that can store up to 4500 atoms of Fe3+ in its interior as an Fe oxide mineral 99. In animals, ferritin is the primary storage form for Fe, but recent work has suggested that in plants the role of ferritin is solely to deal with excess Fe and prevent oxidative damage 100, much like the detoxifying role of ferritin in bacteria 101 and Clamydomonas 102,103. Of the four ferritins found in Arabidopsis, only FER2 is found in the seed while FER1, FER3, and FER4 are expressed in shoot tissue and FER1 is found in the root 104. When FER2 or FER1, FER3 and FER4 were knocked out, Fe levels of the seed and shoot, respectively, remained unchanged, while there was clear evidence of oxidative stress 100. This suggests that, unlike in animals, most plants use ferritin primarily to detoxify Fe rather than as a major storage unit. Some plants, however, do use ferritin as a storage unit, and an exciting new finding has shown that oceanic diatoms use ferritin to safely store Fe for later use 105. This finding is remarkable not only because it identified ferritin in a lineage that had not previously been known to have ferritin, but it also demonstrated that its presence in these diatoms conferred a competitive growth advantage over other oceanic diatoms that did not possess ferritin 105.
Many transition metals are complexed with carriers as another strategy to prevent toxicity. In animals, Fe, Cu, and Zn are all found associated with carrier molecules, notably transferrin for Fe and albumin for Cu and Zn 1. In plants, there is no known chaperone for Zn or Fe, although a group recently identified an Fe chaperone in humans 106. In plants, Fe is often found chelated to NA, citrate, or phytosiderophores 52. Cu is found bound to chaperones that deliver it to particular compartments or proteins where it will be used 13. CCH binds Cu+ and is thought to recycle Cu from senescing tissue 107. Another Cu chaperone, CCS1, is thought to deliver Cu to superoxide dismutase in the chloroplast 108. Yet another, COX19, increases under Cu treatment or induction of ROS production and may deliver Cu to cytochrome c oxidase 109. We should also point out that metallothioneins and phytochelatins can both bind metals and probably function in protecting plants from Cu and Cd toxicity 110,111.
Remaining questions
Despite the recent advances in understanding metal homeostasis in plants, there are still many questions that remain to be resolved. Plants have clearly overcome the many challenges of metal homeostasis, from uptake to transport to localization to toxicity. Of these, we understand the most about uptake and overcoming toxicity. Transport between tissues and subcellular localization still pose many questions. For example, it is still unclear how the vasculature is unloaded and reloaded in the shoot, a critical step in getting metals to the places where they are required. At the cellular level, the transporters involved in mitochondrial and chloroplastic transport have yet to be fully understood. Given the essential function of Fe and Cu in both of these compartments, it will be of great interest to know what transporters regulate movement of these metals. In addition, it is known that metals exist in multiple reduction states, and many transporters and chelators are specific to a particular valency. It is very likely that yet uncharacterized reductases are present at transition areas where metals must be reduced for transport or binding, and future studies will be needed to identify essential players. Beyond the scope of this review are the many more unanswered questions regarding regulation of metal homeostasis 78,112. Another challenge that that was not discussed in this review is how metalloproteins acquire the correct metal. In the case of Cu, it appears chaperones deliver Cu but is this also true for other metals? Recent work in cyanobacteria documents that the compartment where a metalloprotein folds can determine which metal it binds 113,114.
Given the importance of metals to the survival and proper function of plants, and given the importance of plants to nutrition and energy, it is imperative that research address the many unknowns that remain in the field of metal homeostasis.
Supplementary Material
Acknowledgments
We thank members of the Guerinot laboratory for helpful discussions; we also thank the many laboratories, both cited and not cited in this review due to space limitations, who have contributed to this field of investigation. C.P. is supported by a training grant from the National Institute of General Medical Sciences (T32GM008704). Work in our laboratory is supported by grants from the National Science Foundation (IBN-0344305; IBN-0419695; DBI-0606193), the National Institutes of Health (RO1 GM 078536), the Department of Energy (DE-FG-2-06ER15809) and the National Institute of Environmental Health Sciences (5 P42 ES007373).
References
- 1.Marschner H. Mineral Nutrition of Higher Plants. Academic Press; Boston: 1995. [Google Scholar]
- 2.Balk J, Lobreaux S. Biogenesis of iron-sulfur proteins in plants. Trends Plant Sci. 2005;10:324–331. doi: 10.1016/j.tplants.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Hirayama T, et al. RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;97:383–93. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 4.Lim NC, Freake HC, Bruckner C. Illuminating zinc in biological systems. Chem Eur J. 2005;11:38–49. doi: 10.1002/chem.200400599. [DOI] [PubMed] [Google Scholar]
- 5.Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 6.Ciftci-Yilmaz S, Mittler R. The zinc finger network of plants. Cell Mol Life Sci. 2008;65:1150–60. doi: 10.1007/s00018-007-7473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan BB, Gruissem W, Jones RL. Biochemistry and Molecular Biology of Plants. Vol. 1367. American Society of Plant Physiologists; Rockville: 2000. [Google Scholar]
- 8.Palmgren MG, et al. Zinc biofortification of cereals: problems and solutions. Trends Plant Sci. 2008;13:464–73. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmgren MG. PLANT PLASMA MEMBRANE H+-ATPases: Powerhouses for Nutrient Uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 11.Colangelo EP, Guerinot ML. The essential bHLH protein FIT1 is required for the iron deficiency response. Plant Cell. 2004;16:3400–3412. doi: 10.1105/tpc.104.024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinneny JR, et al. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–945. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- 13.Puig S, Andres-Colas N, Garcia-Molina A, Penarrubia L. Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ. 2007;30:271–90. doi: 10.1111/j.1365-3040.2007.01642.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–7. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- 15.Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003;133:1102–10. doi: 10.1104/pp.103.025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crichton RR, Pierre JL. Old iron, young copper: from Mars to Venus. Biometals. 2001;14:99–112. doi: 10.1023/a:1016710810701. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee I, Campbell NH, Ash JS, Connolly EL. Expression profiling of the Arabidopsis ferric chelate reductase (FRO) gene family reveals differential regulation by iron and copper. Planta. 2006;223:1178–1190. doi: 10.1007/s00425-005-0165-0. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee I. PhD thesis. University of South Carolina; 2007. Iron Uptake and Homeostasis in Arabidopsis. [Google Scholar]
- 19.Curie C, Briat JF. Iron transport and signaling in plants. Annu Rev Plant Biol. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- 20.Haydon MJ, Cobbett CS. Transporters of ligands for essential metal ions in plants. New Phytol. 2007;174:499–506. doi: 10.1111/j.1469-8137.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagasaka S, et al. Time course analysis of gene expression over 24 hoirs in Fe-deficient barley roots. Plant Mol Biol. 2008 doi: 10.1007/s11103-008-9443-0. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, et al. Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J. 2006;48:85–97. doi: 10.1111/j.1365-313X.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M, et al. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol Biol. 2008;66:609–617. doi: 10.1007/s11103-008-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SA, Guerinot ML. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007;581:2273–2280. doi: 10.1016/j.febslet.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 25.Salt DE, Baxter I, Lahner B. Ionomics and the study of the plant ionome. Annu Rev Plant Biol. 2008;59:709–33. doi: 10.1146/annurev.arplant.59.032607.092942. [DOI] [PubMed] [Google Scholar]
- 26.Ishimaru Y, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ Plant J. 2006;45:335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- 27.Ishimaru Y, et al. Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc Natl Acad Scie USA. 2007;104:7373–8. doi: 10.1073/pnas.0610555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SA, et al. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science. 2006;314:1295–98. doi: 10.1126/science.1132563. [DOI] [PubMed] [Google Scholar]
- 29.Punshon T, Guerinot ML, Lanzirotti A. Using synchrotron x-ray fluorescence microprobes to study metal(loid) homeostasis in plants. Ann Bot. 2009 doi: 10.1093/aob/mcn264. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domaille DW, Que EL, Chang CJ. Synthetic fluorescent sensors for studying the cell biology of metals. Nat Chem Biol. 2008;4:168–75. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 31.Sinclair SA, Sherson SM, Jarvis R, Camakaris J, Cobbett CS. The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol. 2007;174:39–45. doi: 10.1111/j.1469-8137.2007.02030.x. [DOI] [PubMed] [Google Scholar]
- 32.Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vert G, et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varotto C, et al. The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J. 2002;31:589–599. doi: 10.1046/j.1365-313x.2002.01381.x. [DOI] [PubMed] [Google Scholar]
- 35.Henriques R, et al. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol Biol. 2002;50:587–597. doi: 10.1023/a:1019942200164. [DOI] [PubMed] [Google Scholar]
- 36.Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14:1347–1357. doi: 10.1105/tpc.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerkeb L, et al. Iron-induced turnover of the Arabidopsis IRT1 metal transporter requires lysine residues. Plant Physiol. 2008;146:1964–73. doi: 10.1104/pp.107.113282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta. 2006;1763:595–608. doi: 10.1016/j.bbamcr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Bughio N, Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S. Cloning an iron-regulated metal transporter from rice. J Exp Bot. 2002;53:1677–82. doi: 10.1093/jxb/erf004. [DOI] [PubMed] [Google Scholar]
- 40.Cheng L, et al. Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiol. 2007;145:1647–57. doi: 10.1104/pp.107.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with broad specificity. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- 42.Lee J. PhD thesis. Dartmouth College; 2009. Zinc homeostasis in Arabidopsis: The role of ZIP metal transporters. [Google Scholar]
- 43.Grotz N, et al. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci U S A. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parson B. Dartmouth College. 2005. [Google Scholar]
- 45.Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Van Montagu M. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J Biol Chem. 1995;270:28479–86. doi: 10.1074/jbc.270.47.28479. [DOI] [PubMed] [Google Scholar]
- 46.Sancenón V, Puig S, Mira H, Thiele DJ, Penarrubia L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol. 2003;51:577–87. doi: 10.1023/a:1022345507112. [DOI] [PubMed] [Google Scholar]
- 47.Sancenon V, et al. The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J Biol Chem. 2004;279:15348–15355. doi: 10.1074/jbc.M313321200. [DOI] [PubMed] [Google Scholar]
- 48.Wintz H, et al. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem. 2003;278:47644–53. doi: 10.1074/jbc.M309338200. [DOI] [PubMed] [Google Scholar]
- 49.Curie C, et al. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- 50.Schaaf G, et al. ZmYS1 functions as a proton-coupled symporter for phytosiderophore-and nicotianamine-chelated metals. J Biol Chem. 2004;279:9091–9096. doi: 10.1074/jbc.M311799200. [DOI] [PubMed] [Google Scholar]
- 51.Inoue H, et al. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem. 2008 doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- 52.Curie C, et al. Metal movement within the plant: contribution of nictotianamine and yellow stripe 1-like transporters. Ann Bot. 2008 doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durrett TP, Gassmann W, Rogers EE. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007;144:197–205. doi: 10.1104/pp.107.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green LS, Rogers EE. FRD3 controls iron localization in Arabidopsis thaliana. Plant Physiol. 2004;136:2523–2531. doi: 10.1104/pp.104.045633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers EE, Guerinot ML. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell. 2002;14:1787–1799. doi: 10.1105/tpc.001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 2009;149:297–305. doi: 10.1104/pp.108.128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain D, et al. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. 2004;16:1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becher M, Talke IN, Krall L, Kramer U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 2004;37:251–68. doi: 10.1046/j.1365-313x.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- 59.Weber M, Harada E, Vess C, Roepenack-Lahaye E, Clemens S. Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J. 2004;37:269–81. doi: 10.1046/j.1365-313x.2003.01960.x. [DOI] [PubMed] [Google Scholar]
- 60.Hanikenne M, et al. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–5. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- 61.Milner MJ, Kocian LV. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot. 2008;102:3–13. doi: 10.1093/aob/mcn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kramer U. The dilemma of controlling heavy metal accumulation in plants. New Phytol. 2009;181:3–5. doi: 10.1111/j.1469-8137.2008.02699.x. [DOI] [PubMed] [Google Scholar]
- 63.Kramer U, Talke IN, Hanikenne M. Transition metal transport. FEBS Lett. 2007;581:2263–72. doi: 10.1016/j.febslet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Andres-Colas N, et al. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006;45:225–36. doi: 10.1111/j.1365-313X.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi Y, et al. Amino acid polymorphisms in strictly conserved domains of a P-type ATPase HMA5 are involved int he mechanism of copper tolerance variation in Arabidopsis. Plant Physiol. 2008;148:969–980. doi: 10.1104/pp.108.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Wiren N, et al. Nicotianamine chelates both Fe(III) and Fe(II). Implications for metal transport in plants. Plant Physiol. 1999;119:1107–1114. doi: 10.1104/pp.119.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Jean M, Schikora A, Mari S, Briat JF, Curie C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. Plant J. 2005;44:769–82. doi: 10.1111/j.1365-313X.2005.02569.x. [DOI] [PubMed] [Google Scholar]
- 68.Waters BM, et al. Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 2006;141:1446–58. doi: 10.1104/pp.106.082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DiDonato RJ, Roberts L, Sanderson T, Eisley R, Walker E. Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 2004;39:403–14. doi: 10.1111/j.1365-313X.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- 70.Schaaf G, et al. A putative function for the Arabidopsis Fe-phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol. 2005;46:762–74. doi: 10.1093/pcp/pci081. [DOI] [PubMed] [Google Scholar]
- 71.Koike S, et al. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004;39:415–24. doi: 10.1111/j.1365-313X.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- 72.Stacey MG, et al. The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiol. 2008;146:589–601. doi: 10.1104/pp.107.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331–41. [PubMed] [Google Scholar]
- 74.Myouga F, et al. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell. 2008;20:3148–3162. doi: 10.1105/tpc.108.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohu CM, Pilon M. Regulation of superoxide dismutase expression by copper availability. Physiol Plant. 2007;129:747–755. [Google Scholar]
- 76.Yamasaki H, et al. Regulation of copper homeostasis by micro-RNA in Arabidopsis. J Biol Chem. 2007;282:16369–16378. doi: 10.1074/jbc.M700138200. [DOI] [PubMed] [Google Scholar]
- 77.Abdel-Ghany SE, Pilon M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem. 2008;283:15932–45. doi: 10.1074/jbc.M801406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikana iT. SQUAMOSA. Promoter Binding Protein-Like7 Is a Central Regulator for Copper Homeostasis in Arabidopsis. Plant Cell. 2009 doi: 10.1105/tpc.108.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eriksson M, et al. Genetic dissection of nutritional copper signaling in Chlamydomonas distinguishes regulatory and target genes. Genetics. 2004;168:795–807. doi: 10.1534/genetics.104.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duy D, et al. PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell. 2007;19:986–1006. doi: 10.1105/tpc.106.047407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teng YS, et al. Tic21 is an essential translocon component for protein translocation across the chloroplast inner envelope membrane. Plant Cell. 2006;18:2247–57. doi: 10.1105/tpc.106.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeong J, et al. Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions. Proc Natl Acad Sci USA. 2008;105:10619–10624. doi: 10.1073/pnas.0708367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heazlewood JL, et al. Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell. 2004;16:241–56. doi: 10.1105/tpc.016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdel-Ghany SE, Muller-Moule P, Niyogi KK, Pilon M, Shikanai T. Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell. 2005;17:1233–51. doi: 10.1105/tpc.104.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shikanai T, Muller-Moule P, Munekage Y, Niyogi KK, Pilon M. PAA1, a P-type ATPase of Arabidopsis, functions in copper transport in chloroplasts. Plant Cell. 2003;15:1333–46. doi: 10.1105/tpc.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seigneurin-Berny D, et al. HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J Biol Chem. 2006;281:2882–2892. doi: 10.1074/jbc.M508333200. [DOI] [PubMed] [Google Scholar]
- 87.Moreno I, et al. AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. J Biol Chem. 2008;283:9633–41. doi: 10.1074/jbc.M800736200. [DOI] [PubMed] [Google Scholar]
- 88.Kushnir S, et al. A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell. 2001;13:89–100. doi: 10.1105/tpc.13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen S, Sanchez-Fernandez R, Lyver ER, Dancis A, Rea PA. Functional characterization of AtATM1, AtATM2 and AtATM3, a subfamily of Arabidopsis half-molecule ABC transporters implicated in iron homeostasis. J Biol Chem. 2007;282:21561–21571. doi: 10.1074/jbc.M702383200. [DOI] [PubMed] [Google Scholar]
- 90.Maxfield AB, Heaton DN, Winge DR. Cox17 is functional when tethered to the mitochondrial inner membrane. J Biol Chem. 2004;279:5072–80. doi: 10.1074/jbc.M311772200. [DOI] [PubMed] [Google Scholar]
- 91.Lanquar V, et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005;24:4041–4051. doi: 10.1038/sj.emboj.7600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003;34:685–95. doi: 10.1046/j.1365-313x.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- 93.Desbrosses-Fonrouge AG, et al. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett. 2005;579:4165–74. doi: 10.1016/j.febslet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 94.Arrivault S, Senger T, Kramer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 2006;46:861–879. doi: 10.1111/j.1365-313X.2006.02746.x. [DOI] [PubMed] [Google Scholar]
- 95.Kobae Y, et al. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol. 2004;45:1749–58. doi: 10.1093/pcp/pci015. [DOI] [PubMed] [Google Scholar]
- 96.Gustin JL, et al. MTP1-dependent Zn sequestration into shoot vacuoles suggest dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 2008 doi: 10.1111/j.1365-313X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- 97.Whiteman SA, Nühse TS, Ashford DA, Sanders D, Maathuis FJM. A proteomic and phosphoproteomic analysis of Oryza sativa plasma membrane and vacuolar membrane. Plant J. 2008;56:146–156. doi: 10.1111/j.1365-313X.2008.03578.x. [DOI] [PubMed] [Google Scholar]
- 98.Halliwell B, Gutteridge JMC. Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 99.Hintze KJ, Theil EC. Cellular regulation and molecular interactions of the ferritins. Cell Mol Life Sci. 2006;63:591–600. doi: 10.1007/s00018-005-5285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ravet K, et al. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009;57:400–412. doi: 10.1111/j.1365-313X.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- 101.Carrondo MA. Ferritins, iron uptake and storage from the bacterioferritin viewpoint. EMBO J. 2003;22:1959–68. doi: 10.1093/emboj/cdg215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Busch A, Rimbauld B, Naumann B, Rensch S, Hippler M. Ferritin is required for rapid remodeling of the photosynthetic apparatus and minimizes photo-oxidative stress in response to iron availability in Chlamydomonas reinhardtii. Plant J. 2008;55:201–11. doi: 10.1111/j.1365-313X.2008.03490.x. [DOI] [PubMed] [Google Scholar]
- 103.Long JC, Sommer F, Allen MD, Lu SF, Merchant SS. FER1 and FER2 encoding two ferritin complexes in Chlamydomonas reinhardtii chloroplasts are regulated by iron. Genetics. 2008;179:137–47. doi: 10.1534/genetics.107.083824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petit JM, Briat JF, Lobréaux S. Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem J. 2001;359:575–582. doi: 10.1042/0264-6021:3590575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marchetti A, et al. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature. 2008 doi: 10.1038/nature07539. [DOI] [PubMed] [Google Scholar]
- 106.Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–10. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mira H, Martínez-García F, Peñarrubia L. Evidence for the plant-specific intercellular transport of the Arabidopsis copper chaperone CCH. Plant J. 2001;25:521–528. doi: 10.1046/j.1365-313x.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 108.Abdel-Ghany SE, et al. AtCCS is a functional homolog of the yeast copper chaperone Ccs1/Lys7. FEBS Lett. 2005;579:2307–12. doi: 10.1016/j.febslet.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 109.Attallah CV, Welchen E, Pujol C, Bonnard G, Gonzalez DH. Characterization of Arabidopsis thaliana genes encoding functional homologues of the yeast metal chaperone Cox19p, involved in cytochrome c oxidase biogenesis. Plant Mol Biol. 2007;65:343–55. doi: 10.1007/s11103-007-9224-1. [DOI] [PubMed] [Google Scholar]
- 110.Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochim. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Guo WJ, Meetam M, Goldsbrough PB. Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 2008;146:1697–1706. doi: 10.1104/pp.108.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol. 2008;11:530–5. doi: 10.1016/j.pbi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Tottey S, et al. Protein-folding location can regulate manganese-binding versus copper-or zinc-binding. Nature. 2008;455:1138–42. doi: 10.1038/nature07340. [DOI] [PubMed] [Google Scholar]
- 114.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nature Rev Microbiol. 2009;6:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 115.Pais I, Jones JB. The Handbook of Trace Elements. St. Lucie Press; Boca Raton, FL: 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.