Abstract
Purpose
Antineoplastic RNAse proteins, also known as Amphibinases, have been shown effective against various solid tumors but were found selectively neurotoxic to Purkinje cells in the cerebellum. This work describes the use of a waxy biodegradable poly(ricinoleic-co-sebacic acid) for the local controlled delivery of cytotoxic amphibinases in the parietal lobe of the brain in an attempt to overcome cerebellar neuronal toxicity while affecting glioma cells.
Methods
Amphibinase analogues were encapsulated in poly(ricinoleic-co-sebacic acid) formulations using mix-melt technology and loaded onto surgical foam. In-vitro release was monitored by BCA colorimetry and by RNAse specific bioactivity. The implants were inserted into rat brains bearing 9L glioma to assess toxicity and efficacy.
Results
The various formulations showed extended linear release for several weeks with minimal burst effect. Best in-vivo efficacy was obtained with ACC7201 containing implants, resulting in the extension of the median survival from 13 to 18 days with 13% long-term survivors.
Conclusion
Antineoplastic proteins were released from a p(SA-RA) polyanhydride implants in a controlled manner, providing efficacy against 9L glioma, while evading neurotoxicity in the cerebellum. The controlled release of Amphibinases forms the potential for a new therapy against brain tumors.
Keywords: RNAse, Amphibinase, Ranpirnase, polymer, glioma
Introduction
The standard protocol for the treatment of glioblastomas calls primarily for surgical debulking followed by either administration of Gliadel or oral temozolomide in combination with radiation therapy (1, 2). Local drug delivery in the brain at the site of the tumor is an approach that has been well studied and approved by the US FDA and other regulatory agencies worldwide, showing the feasibility and enhanced efficacy of a variety of therapeutic agents when released from a biodegradable polyanhydride wafer implanted in the parietal lobe of the brain (3–9). In this work, the controlled local delivery of anti-neoplastic RNAses as a potential therapy against brain cancer is investigated.
RNAses derived from oocytes of Northern Leopard frogs (Rana pipiens), have been shown effective against a variety of cancers (10). A number of these proteins, generally known as Amphibinases, have been investigated in preclinical and clinical studies (11–14), the best known among them being Ranpirnase, also referred to as Onconase®. Ranpirnase shows cytostatic or cytotoxic activity in-vitro in many different cell lines, being resistant to the mammalian ribonuclease inhibitor (RI) (14). In-vivo inhibition of tumor growth has been reported in various animal models, including multidrug resistant tumors. The exact pathway by which Onconase exerts its cytotoxic or cytostatic effects in neoplastic cells is still unknown. As ATP is required for the uptake, Ranpirnase is suggested to be actively transported into the cell by receptor mediated endocytosis. Once in the cytosol, tRNA is degraded, following G1-phase cell cycle arrest and subsequent apoptosis(14). There is some evidence that the cytotoxic action of Ranpirnase occurs by influencing the expression of cyclin dependent kinases. Other reports point to the cell’s dependency on Ca2+-activated potassium channels to counter Ranpirnase toxicity(14). Though cytotoxic effects of Ranpirnase and related RNAses have always been associated with their catalytic activity in cleaving RNA in general (15), a recent study suggests the cytotoxic effects could be the result of a more specific action in the cleavage of siRNA.(16). In the study it was suggested that the catalytic activity was likely to occur within the RNA-induced silencing complex (RISC) of the RNA interference (RNAi) mechanism. The IC50 values for cytotoxicity of Ranpirnase towards a number of different tumor cell-lines varied in the range from 10−6 M to 10−8 M (10,14).
When administered systemically, Amphibinases have shown only very few toxic side effects including spermatogenetic cytotoxicity and embryo toxicity (17).
Unlike their high tolerance when used systemically, various Amphibinases, and in particular Ranpirnase, have displayed significant neurotoxicity when injected intracranially (18, 19). The toxicity was shown to be dose-dependent and aimed specifically at Purkinje cells in the cerebellum with a reported IC50 concentration of 4.0 10−7 M,. Loss of Purkinje neurons lead to symptoms, generally known as the Gordon phenomenon, including ataxia, muscular rigidity, paralysis, and tremor that may eventually lead to death (19). Albeit their narrow therapeutic window, we hypothesized that the potential anti-tumor effects of Amphibinases in the brain could still be investigated, if the compounds were released in a controlled manner from a biodegradable depot, implanted in the frontal lobe, somewhat removed from the cerebellum. The hypothesis is based on the assumption that a slow continuous release of the active compound creates locally a diffusion-based concentration gradient, high enough in the frontal lobe to have a therapeutic effect and well below the threshold of toxicity in the cerebellum. In order to achieve this envisioned concentration gradient, the local delivery system must show a constant rate of release to maintain a therapeutic level, without any burst release that would cause concentrations in the cerebellum to reach toxic IC50 values.
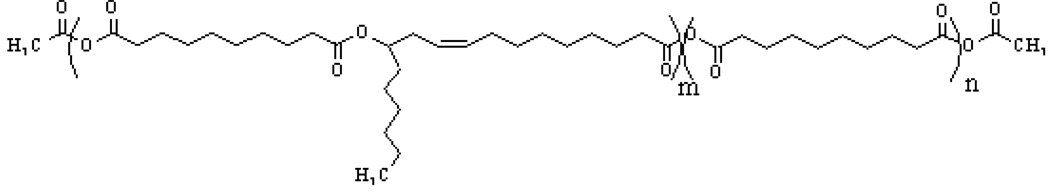
In addition to linear release characteristics and biocompatibility with brain tissue, the delivery system must be suitable for proteins without losing bioactivity during manufacturing. Several polyanhydrides based on co-polymers of sebacic acid with either 1,3-bis(p-carboxyphenoxy) propane (CPP) or with fatty acid dimers (FAD) were found to be biocompatible and have been used successfully to deliver specific chemotherapeutic agents in the brain (1, 2, 20, 21). However, incorporation and release of proteins from these polymers resulted in short release times and loss of activity due to heat and use of incompatible solvents. In this work a member of a newly discovered class of waxy poly(ester-anhydrides) has been used for making an implantable device for the release of Amphibinases. The implants were made under very mild conditions, requiring only mixing at room temperature for drug incorporation without need for additional solvents. The polyesteranhydride is based on sebacic acid (SA) and ricinoleic acid (RA) at a monomer weight ratio of 3:7. The polymer is pasty at room temperature and gels upon contact with water (22–28). Its chemical structure is depicted in Fig 1.
Fig. 1.
Structure of poly(sebacic acid-co-ricinoleic acid anhydride) (poly(SA-RA) 3:7)
The objective of this study was to examine the use of this polymeric implantable system for safe and effective delivery of amphibinases for treating brain tumors. Three amphibinases-Ranpirnase, Ranpirnase conjugated with epithelial growth factor (ACC7201), and Amphinase-were formulated in the biodegradable implant and examined for their in-vitro release, biocompatibility and efficacy in rat brains bearing a 9L glioma tumor model.
Materials and Methods
Materials
Poly(sebacic acid-co-ricinoleic acid) in a ratio of 3:7 (poly(SA-RA)3:7) was synthesized as previously described (22, 23), with a molecular weight of Mw = 6000Da and Mn = 4000; Tm = 25–30 °C, PI=1.5. The Amphibinases, Ranpirnase, Amphinase (Mw of both are 11.8 kDa), and ACC7201, a conjugate of Ranpirnase with epithelial growth factor (EGF) at a ratio of 2:1 (MW = 30.1 kDa), were provided by Alfacell Inc, NJ. Tetraglycol was purchased from Aldrich-Sigma (St. Louis, MO) and solvents were obtained from Fisher Scientific (Pittsburgh, PA). Total Protein Assay was purchased from Pierce, Rockford, IL; RNAse Alert Kit from Ambion, Austin, TX. Gelfoam gelatin sponges, size 4, were purchased from Upjohn Co.(Kalamazoo, MI).
Thermal analysis of the polyanhydrides was performed on a Mettler TA 4000-DSC differential scanning calorimeter, calibrated with zinc and indium standards, at a heating rate of 10°C/min. Molecular weights of the polymers were estimated on a gel permeation chromatography (GPC) system consisting of an isocratic HPLC pump (Waters, MA), Waters 2410 Refractive Index Detector, Rheodyne (Coatati, CA) injection valve with a 20-µL loop and Breeze software (Waters, MA). Samples were eluted with CHCl3 through a linear Styragel HR2 column (Waters, 7.8 × 300 mm, 10 µm pore size) at a flow rate of 1 mL/min. The molecular weights were determined relative to polystyrene standards (Polyscience, Warrington, PA) with a molecular weight range of 1100 to 7920 Da.
In-Vitro Cytotoxicity
9L glioma cells were obtained from the Brain Tumor Research Center (San Francisco, CA). 9L cells were seeded in 96 well-plates and maintained in DMEM medium with 10% fetal bovine serum. For assay, cells were seeded at 3000 cells/well and incubated for 24 hours at 37°C. Ranpirnase, ACC7201 or Amphinase at various concentrations were added to the wells (n = 10) and the plate was returned to the incubator. After 72 hours, cytotoxicity was measured using the thiazolyl blue tetrazolium (MTT) assay. MTT at 5 g/mL was added to wells followed by incubation for 2 hours. The medium was removed and 50 µL of DMSO were added and plates were scanned in 96-well plate reader at λ=570 nm.
Polymer Formulation
In a typical formulation, containing 4% protein w/w polymer, poly(SA-RA) 3:7 (187.5 mg) was melted by placing the polymer in a an oven set at 70 °C. The molten polymer was then left at room temperature to cool to an ambient temperature and mixed with a protein suspension (7.5 mg) in tetraglycol (195 µL).
The pasty protein-polymer formulation was embedded in Surgical Gelfoam at a ratio of 30 mg polymer formulation per 1 mg of gelfoam: a pre-weighted piece of gelfoam (4 mg), measuring approximately 2×2×10 cm, was impregnated by placing it directly in the molten polymer-protein formulation until fully saturated. The gelfoam was placed on dry ice to harden, weighed again, and 4 pieces were cut, weighing 31 mg (30 mg polymer formulation with 1 mg of gelfoam). For formulations containing less than 2% of the bioactive protein w/w polymer, BSA was added to keep the total protein load at 2% w/w. The protein mixtures were prepared by lyophilization of aqueous solutions containing the bioactive compound and BSA at the required ratios. For formulations containing higher protein loadings the ratio between polymer and tetraglycol was kept constant as more protein was mixed in.
In-Vitro Release
Pieces of gelfoam loaded with the Amphibinase protein/polyanhydride formulation of 30 mg were suspended in PBS (1 mL) and placed at 37 °C. At specified time intervals, the PBS release medium was removed and replaced with fresh medium. Levels of released protein in the PBS were measured by the copper reduction-dependent Total Protein Assay or by determining specific RNAse activity with the RNAse Alert Kit. The following modification of the protocol of the RNAse Alert Kit was made to enable quantitative measurements: 25 µL of labeled RNA (2.5-fold excess over that required by the manufacturer’s instructions) was added to the reaction mixture of the RNAse sample (80 µL) and RNAse Alert Lab Test buffer (10 µL). Leaving the plate at room temperature the samples were measured within 2 minutes of adding the labeled RNA, using a fluorescence 96-well plate reader (Perkin-Elmer, Shelton, CT) equipped with Fl Winlab 4.00.02 software. Samples were always analyzed alongside calibration samples in triplicate. The plate was read multiple times during a period of several hours following the enzymatic reaction. However, it was found that data collected within 2 minutes after addition of the labeled RNA appeared most reliable based on the calibration curve. Using internal standards, no further kinetic analysis was done.
In additional experiments release medium, collected at specific time points, from the incubation of a polymeric formulation containing either polymer only, polymer with tetraglycol, or a formulation with Ranpirnase (2% w/w polyanhydride), was incubated with 9L cells. After 72 hours the cell viability was measured using a MTT test as described above.
In-Vivo Safety Studies
Blank polymer formulations and formulations with increasing protein content (w/w polymer) were prepared as described and kept on dry ice before implantation. The implantation was conducted according to the method as previously reported (29). Briefly, female Fischer 344 rats, weighing 150–200 g (purchased from Charles River, Wilmington, MA) were anesthetized with an intraperitoneal injection (i.p.) (3 mL/kg) of a mixture of ketamine hydrochloride, 25 mg/mL, xylazine, 2.5 mg/mL, and 14.25% ethyl alcohol in 0.9% NaCl. After anesthetization, the head was shaved and sterilized with povidone-iodine and a burr hole was created. The formulation was then implanted into the brain cavity. After hemostasis was obtained, the scalp incision was closed with surgical staples and buprenorphine was injected intraperitoneally (0.3 mL/kg) for pain medication. The rats were kept in standard facilities with free access to food and water. After surgery, each rat was examined daily for general appearance, behavioral changes, and neurological deficits. Upon death, the brain was harvested and fixed in 10% formalin. A cross-section, including the cerebellum and cerebral hemisphere containing the implant, was prepared and stained with hematoxylin and eosin (H&E).
Intracranial Efficacy
The rodent 9L gliosarcoma was excised from the flanks of carrier rats and cut into 1-mm3 pieces, which were then implanted into the brain similar to the polymer implant procedure as described above (29). In the group receiving simultaneous implantation of tumor and therapeutic protein-loaded polymer, the piece tumor tissue was implanted first and then the polymeric formulation was inserted adjacent to it. Various polymer concentrations (w/w) were examined. In additional experiments the polymeric formulation was implanted 5 days after implantation of the tumor piece.
Postmortem, the brains were removed and placed into 10% formalin. Cross-section slides of the site of implantation as well as of the cerebellum were stained with H&E. Additionally, samples of brain tissue with or without 9L tumor were stained for EGFR expression with EGFR-specific antibody (30).
Results
Formulation: Preparation and Analysis
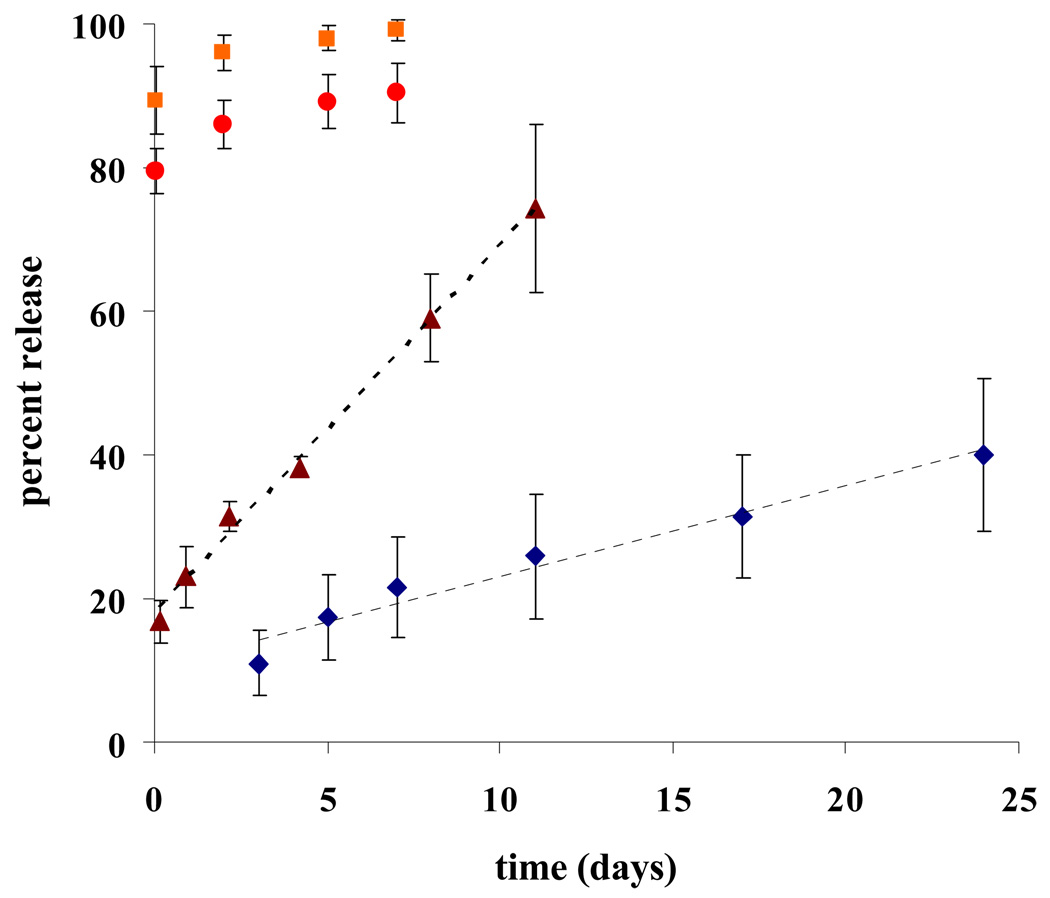
Release of Ranpirnase, ACC7201 and Amphinase from Poly(SA-RA) 3:7 formulations have been measured using a BCA total protein assay or an RNAse specific activity assay. Typical release profiles are shown in Figs 2 and 3. Controlled release of RNAses was followed using a BCA total protein assay. Figure 2 shows continuous release profiles of Ranpirnase and Amphinase from poly(SA-RA) 3:7. From formulations with initial protein loadings up to 4% w/w protein, the controlled release did not show any significant burst effects in the first 24 hours. At 2% w/w protein loading, 40% of the total amount of protein was released from the polyanhydride over a period of 25 days. In the first 24 hours a very modest release of 11% was observed. The release profile was found to be linear (r2 = 0.964). At 4% w/w loaded formulation with Amphinase, an initial release of 17% of the total loading was observed. Over 11 days, 74% of the total protein content was released in a linear fashion (R2 = 0.995). By increasing the loading of protein a faster release rate was observed. In formulations containing 10% or 20% Amphinase, almost 80% of the total protein content was released within the first 24 hours. The remaining protein was released in a controlled manner over a period of several days.
Fig. 2.
Protein release from poly(RA:SA)7:3. Polymer. (187.5 mg) was melted and cooled to ambient temperature. Protein was dispersed in Tetraglycol and mixed with the molten polymer. Surgical gelfoam was saturated with the formulation and put on dry ice to harden. Pieces of 30 mg were cut, weighed and suspended in PBS at 37°C for controlled release measurements. Release was analysed with Total Protein Assay (Pierce).
 2% Ranpirnase w/w polymer;
2% Ranpirnase w/w polymer;  Amphinase in ratio 4%,
Amphinase in ratio 4%,  10%, and
10%, and  20% w/w polymer.
20% w/w polymer.
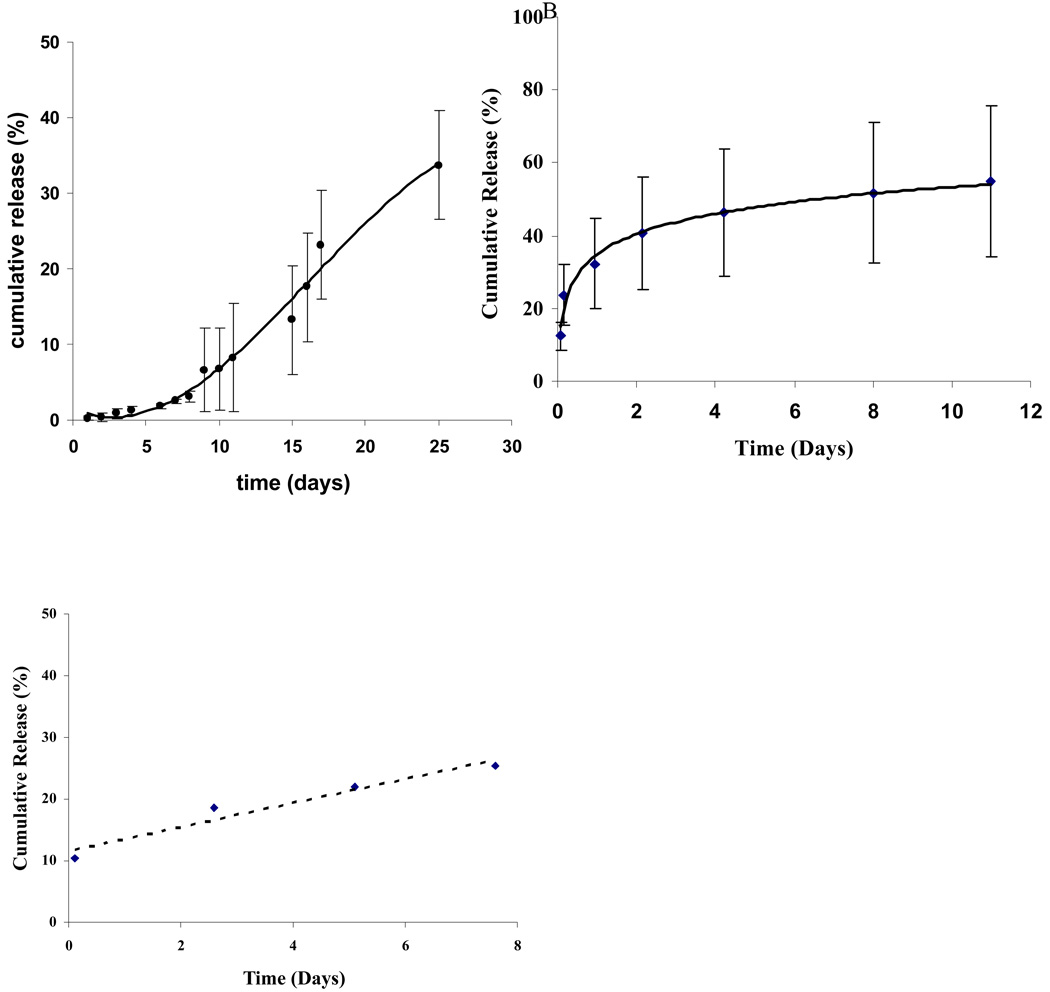
Fig. 3.
Protein bioactivity from controlled release.
Controlled release measured by specific RNAse activity with the RNAse alert kit. Ranpirnase in ratio of 2% w/w polymer (A), Amphinase in ratio of 4% w/w polymer (B) ACC7201 in ratio of 0.5% w/w polymer (C).
Controlled release of Ranpirnase, ACC7201 and Amphinase have also been established on the basis of RNAse specific activity. The release of Ranpirnase and a short term release of EGF-bound Ranpirnase (ACC7201) in a formulation with BSA in poly(SA-RA) 3:7 implants was found to occur in a similar time-controlled linear manner, see Fig. 3. Amphinase and ACC7201 containing formulations showed initial releases in the first 24 hours of 10 to 20% (Fig. 3B,C). The investigated protein loadings were based on formulations that were tested for toxicity and efficacy as well.
In formulations where initial RNAse concentrations were lower than 2% w/w, BSA was used to maintain an initial total protein concentration in the formulation of 2% w/w. Therefore, release of RNAses from those formulations that also contained BSA could only be determined using the specific RNAse activity assay.
In-Vitro Cytotoxicity
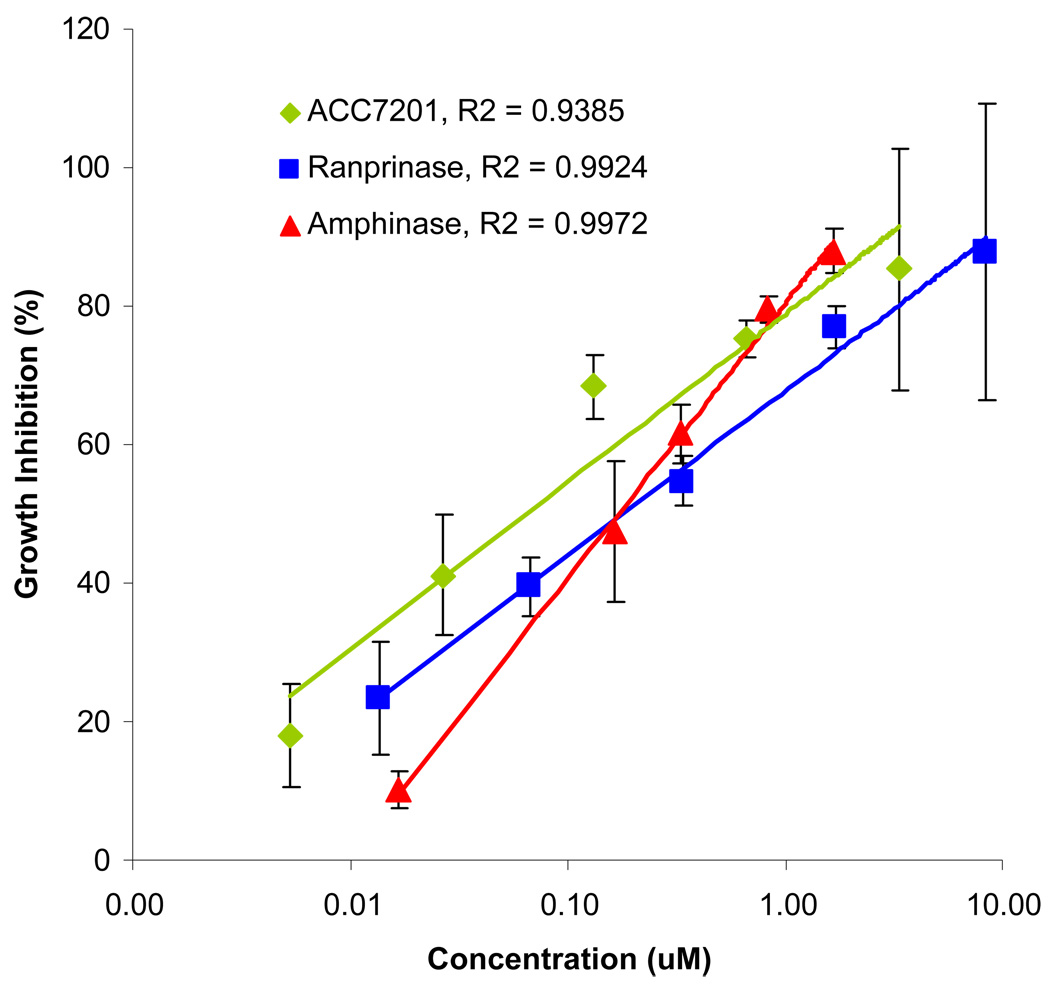
Antineoplastic activity of the various amphibinases has been tested in-vitro on a 9L glioma brain tumor cell line. All amphibinases showed a potent cytotoxic effect at sub-micromolar concentrations. As shown in Fig 4, linear correlations were found when plotting the cell viability versus log(concentration). Concentrations yielding 50%-growth inhibition, the IC50 values, were calculated for the different compounds and presented in table 1.
Fig. 4.
In-vitro cell toxicity.
9L cells were seeded in 96-well plate at 3000 cells per well and incubated for 24 hours. The cells were then treated with compounds for 72 hours and assayed by MTT for growth inhibition. A. Ranpirnase and ACC7201, comparison of cytotoxic effects on 9L glioma cells in-vitro. B. Amphinase cytotoxic effect in-vitro on 9L glioma cells. C. Dose-response correlations.
Table 1.
IC50 values of amphibinases on 9L cells as established using the MTT test.
| Compound | IC50 (nM) |
|---|---|
| Ranpirnase | 180 |
| ACC7201 | 64 |
| Amphinase | 172 |
When release medium, collected from the incubation of a polymeric formulation containing Ranpirnase (2% w/w polyanhydride) for a 12-hour period, was incubated with 9L cells, MTT test showed a 92% growth inhibition. In contrast, release medium obtained from incubating a control polymeric formulation resulted in about 5% growth inhibition for p(SA-RA) only. When tetraethylenglycol was added to the formulation 44.5% growth inhibition was found. Incubation of media of subsequent release periods, however, resulted in high growth inhibitions for all formulations, including polymer alone and for control formulations containing polymer and tetraethylenglycol. In all release media the pH turned acidic after 12 hours as indicated by the media turning yellow.
In-Vivo Toxicity Studies
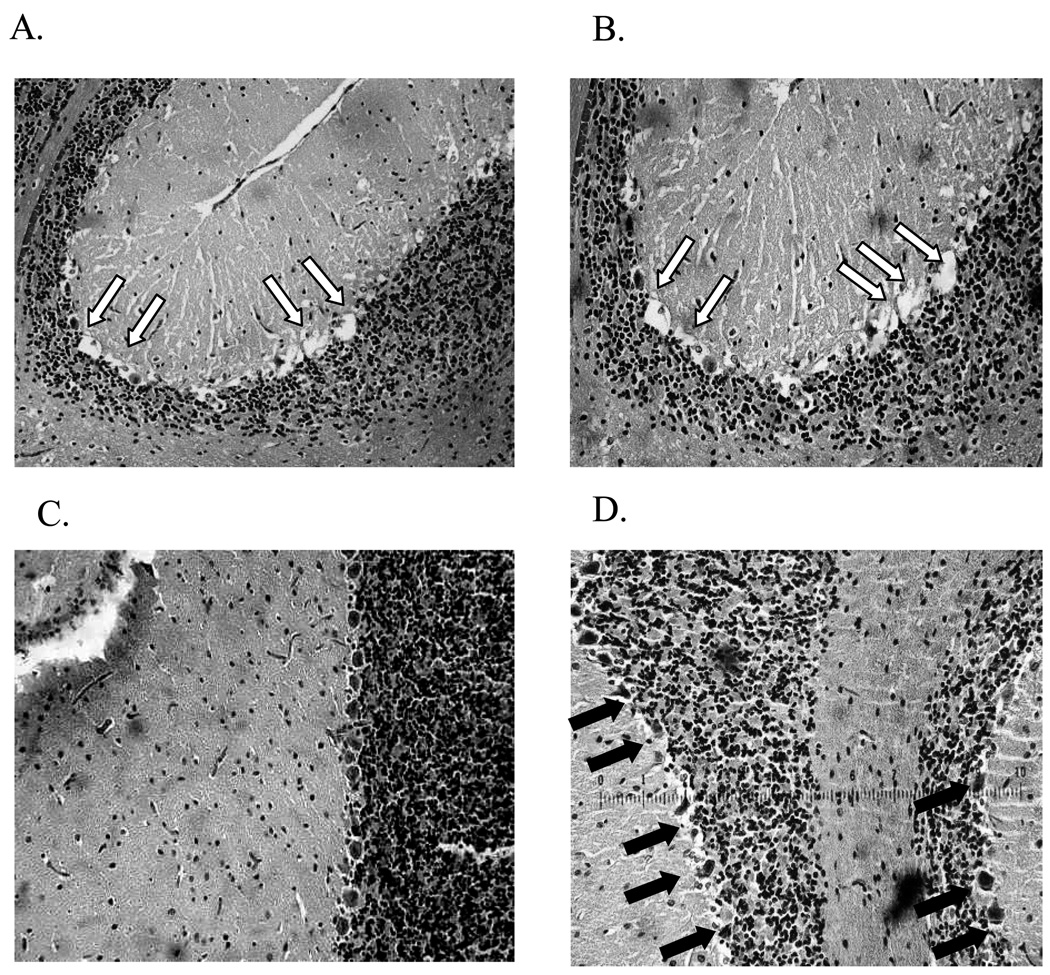
In safety studies conducted in Fisher 344 rats (n=3), pieces of polymer in gelfoam (30 mg) were implanted intracranially. Severe neurotoxicity was observed for polymer formulations containing 5%, 1% and 0.1% Ranpirnase w/w polymer (Fig. 5A). Symptoms of Purkinje neuron toxicity consisted of hind paw paralysis, leading to animal death within 3 days. H&E staining of sagittal cross-sections, including tumor, cortex, and the cerebellum, revealed a significant loss of Purkinje cells along the granular layer in the cerebellum (as indicated by the white arrows in Fig. 5B). The formulation with 0.05% Ranpirnase w/w polymer was found to be the maximally tolerated dose that resulted in reduced loss of Purkinje cells and no animal deaths 30 days after implantation.
Fig. 5.
In-vivo safety study.
Postmortem H&E staining of cerebellum cross-sections, 3 days after polymer implantation (pieces of 30 mg) with A) Ranpirnase 5% w/w and B) ACC7201 conjugate 5% w/w poly(SA-RA)3:7. White arrows indicate Purkinje cell deletion along the granular layer of the cerebellum. C) H&E staining of Purkinje cells in cerebellum upon implantation of Amphinase 10% w/w poly(SA-RA)3:7. D) ACC7201 1% w/w poly(SA-RA) 3:7. Pieces of formulation containing gelfoam were intracranially implanted. Black arrows point to intact Purkinje cells.
Amphinase was tested at various loadings up to 20% w/w. At 10% and 20% w/w protein formulations several deaths were observed. H&E staining of the cerebellar tissue showed neuronal damage, similar in nature to the lesions caused by Ranpirnase. However, the overall amount of observed damage was less in comparison. (Fig.5C). No deaths were observed with 5% w/w formulations up to 30 days after implantation.
Despite some Purkinje cell death, implantations of polymer formulations with ACC7201 in concentrations up to 4% w/w polymer did not result in any mortality. Figure 5D shows an H&E staining of the cerebellum after exposure to ACC7201 from a formulation containing 1% w/w of the protein, indicating limited damage to Purkinje cells.
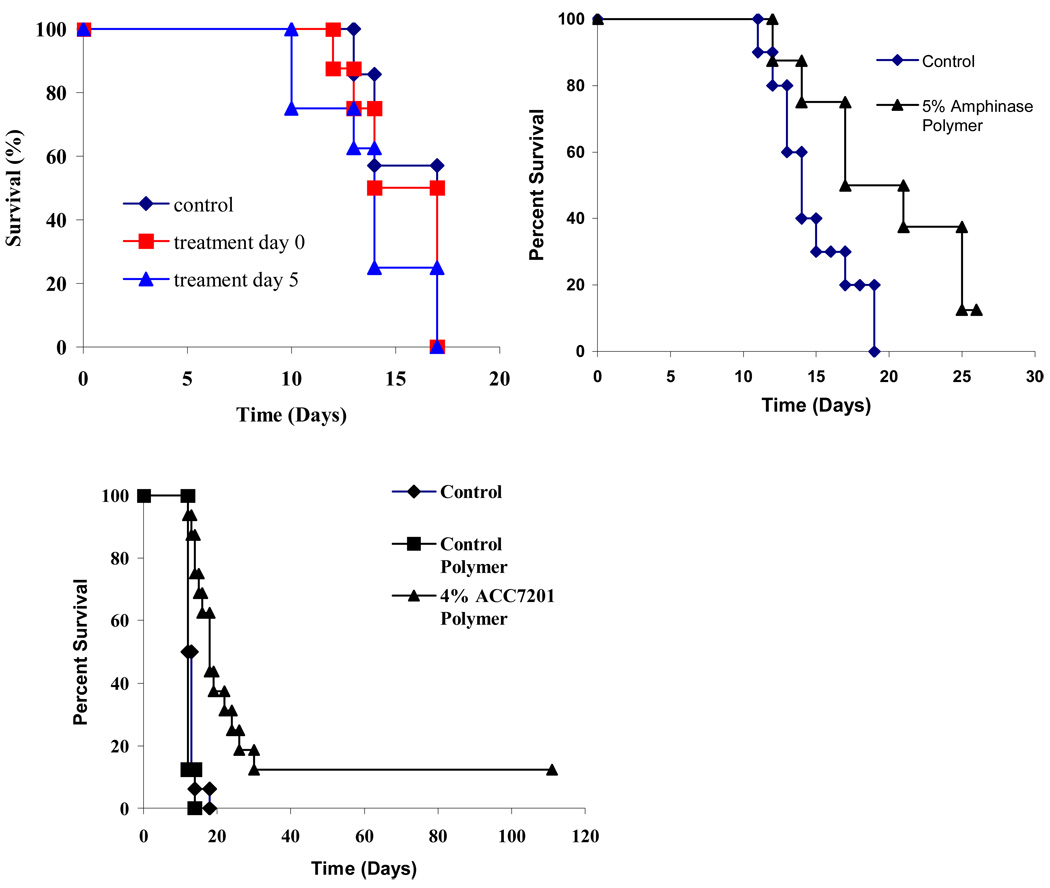
In Vivo Efficacy
For in-vivo experiments, in which rats were challenged with intracranially implanted 9L glioma, treatment with polymer implants containing 0.05% w/w Ranpirnase showed little to no prolongation in median survival, whether implanted directly with the tumor piece or 5 days after tumor implantation (Fig 6A). The control group (n=8) had a median survival of 13 days. Groups treated with Ranpirnase-containing formulations (n=8) had a median survival of 14 days. Postmortem histochemical analysis by H&E staining of the brains from rats that died in efficacy experiments with 0.05% w/w Ranpirnase confirmed that these animals died from 9L glioma. Also, even though Ranpirnase-containing formulations at the maximally tolerated dose of 0.05% w/w polymer were implanted, damage to Purkinje cells was observed.
Fig. 6.
Efficacy of locally delivered Ranpirnase, Amphinase and ACC7201 in the intracranial 9L glioma rodent model.
Rodent 9L gliosarcoma was excised from the flanks of carrier rats and cut into 1-mm3 pieces, which were then implanted into the brain. The piece tumor tissue was implanted first and then the polymeric formulation was inserted adjacent to it. The polymeric formulation was implanted either simultaneously or 5 days after implantation of the tumor piece.
Implants (30 mg) contained A) Ranpirnase 0.05% w/w w/w poly(SA-RA), B) Amphinase 5% w/w poly(SA-RA) 3:7. and C) ACC7201 4% w/w (n=16 per group).
In an efficacy experiment testing 5% w/w Amphinase, a modest increase in overall survival was observed. The median survival was increased from 14 days in the control group to 17 days in the animals treated with Amphinase (Fig 6B).
Median survival increased when animals were treated with an implant containing 4% w/w ACC7201. The median survival shifted from 12 days in the control group to 18 days in the group of animals treated with 4% w/w ACC7201 (n=16, p<0.0001) (Fig 6B): Two out of 16 animals, treated with 4% w/w ACC7201 implants, were long-term survivors (>120 days).
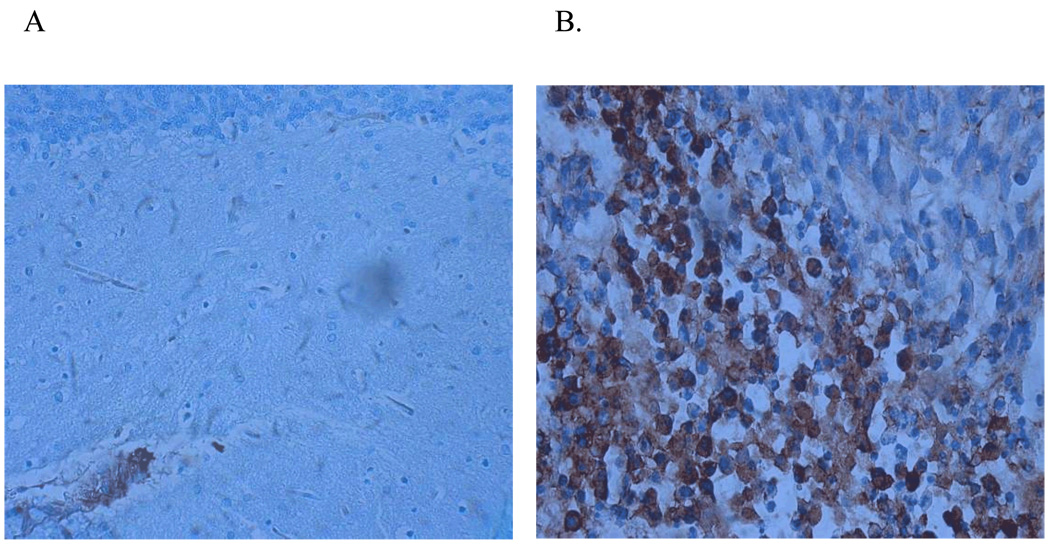
It is assumed that part of the action of ACC2701 against tumor cells is due to targeting based on the EGF portion of the conjugate. EGFR-specific staining of brain tissue samples, with or without implanted 9L tumor, clearly show abundant, albeit inhomogeneous expression of the EGFR receptor in the tumor tissue. Except for local blood vessels, no EGFR expression could be found in Purkinje cells or their surrounding tissues (Fig 7A,B).
Fig. 7.
EGFR expression: cerebellum vs 9L-glioma.
Immunohistochemical staining for Epithelial Growth Factor Receptor (EGFR) in A) cross section of cerebellum; B) cross section of 9L glioma, implanted intracranially in the parietal lobe.
Discussion
Using the novel mixed polyester anhydride comprising sebacic and ricinoleic acids, poly(SA:RA) 3:7, we have shown that the different Amphibinases can be released effectively and in a controlled manner both in-vitro and in-vivo in the brain of rodents. No organic solvents were needed for the preparation of this formulation, because the protein could be dispersed in the polymer while in the molten state. In this manner, loss of bioactivity due to solvent-interface interaction with the protein was prevented. At physiological temperature the polymer formulation was of a waxy consistency. Among several different agents examined for their ability to disperse the protein in the molten polymer, tetraglycol was found to be the most suitable excipient, which kept the protein dispersed while being miscible with the liquid polymer. For making the implants, the optimal ratio of tetraglycol to polymer was 1:1 where a controlled release profile was still maintained.
Looking at values obtained by BCA total protein analysis, we found a general linear trend in the release profiles, where the release rate increased with the total protein loadings up to 4% w/w of the formulation. Higher loading percentages at 10% w/w or 20% w/w resulted in a burst, releasing about 80% of the total protein load within the first 24 hours. The burst was followed by controlled release of only a few days. The linear release rate was shown in formulations with Ranpirnase and with Amphinase. The linear release profiles of the protein is thought to be the result of a number of factors, including diffusion based release, and release based on the degradation of the anhydride bonds in the mixed polyesteranhydride. The BCA assay is known to show some background in the presence of ethyleneoxide-based compounds such as PEG and tetraglycol. Although this has been accounted for using formulations without protein, the values at the onset of the release can still be higher as most of the tetraglycol was found to release in the first 24 hours.
Ranpirnase and Amphinase are the smallest members of the ribonuclease (RNAse) family. The compact quaternary structure of Ranpirnase and its distinctive fourth disulfide bond bridging the C-terminal cysteine contribute to the unusual thermal stability of the protein: the denaturation temperature was found to be 90 °C, compared to 62.5 °C for RNAse A (14). It has been assumed, based on this extraordinary thermal stability of Amphibinases, and the absence of organic solvents, that no activity would be lost during the processing of the formulations. In order to ascertain this assumption and to mitigate the effect of tetraglycol on the BCA assay, we used a modified RNAse Alert Kit to quantify RNAse based on specific activity. Little difference was observed in the total release of both Ranpirnase and Amphibinase when comparing the values obtained from the BCA total protein assay with those obtained from the RNAse specific activity assay: total release of Ranpirnase found 39% versus Amphinase at 72%. Although the trends in total protein release were found similar, the profiles of the release did not match up as the release curves obtained from RNAse specific activity data were less linear in nature. Specifically differences were found in the onset of the release. Part of these differences can potentially be explained by effects of tetraglycol on the BCA kit that could not be accounted for using blank formulations.
Initial data of short term release of EGF-bound Ranpirnase (ACC7201) from formulations with BSA in poly(SA-RA) 3:7 implants was also found to occur in a time-controlled linear manner (Fig. 3C). This overall profile was similar to the release of Ranpirnase from this polymer formulation. This result may be unexpected given the significant difference in molecular weight of ACC7201 and the addition of BSA to the formulation. About 30% of the ACC7201 was released over 7 days, using the specific RNAse activity assay.
Solutions of the amphibinases in cell medium in different concentrations were tested on cells in order to establish cytotoxic activity against the 9L glioma cell line and assess any dose response correlation. All three tested amphibinases showed potent cytotoxicity against 9L glioma in-vitro Based on the measured dose-response curves, IC50 values were calculated. Ranpirnase and Amphinase displayed similar IC50 values, whereas ACC7201 showed to be 3 times more potent. However, ACC7201, a Ranpirnase conjugate with EGF, contains two active Ranpirnase moieties that have to be accounted for.
We have tried to show cytotoxicity of Ranpirnase, when released from a 2% w/w polymer formulation. A significant difference in growth inhibition was seen when comparing the first 12-hour release media of this formulation with the various control formulations, including controls that contained tetraglycol. In-vitro incubation of release media on 9L cells from of subsequent release periods, however, resulted already in high growth inhibitions for polymer alone. The release media at longer time points must contain ricinoleic acid, a breakdown product of the polyanhydride, which, at higher concentrations works as a surfactant causing cell damage. This assumption is also based on the fact that the pH in the release medium of polymer alone turned acidic after 12 hours, as indicated by the cell medium pH indicator. Therefore no difference could be seen between control and ranpirnase containing formulations in subsequent time points of the controlled release.
In the present study, we found that polymer formulations containing more than 0.1 % Ranpirnase w/w polymer showed severe neurotoxicity in intracranial toxicity studies, conducted in rats, The toxicity expressed itself as what is known as the Gordon effect and was followed by animal death. Post mortem tissue staining showed that the toxicity was accompanied by an almost complete loss of Purkinje cells in the cerebellum. Unlike earlier reported findings (19), the Gordon effect was not reversed once its onset was observed. Intracranially delivered Ranpirnase at a concentration of 0.05% w/w polymer, did not affect animal survival, but partial toxicity to Purkinje cells was still observed since H&E staining of rat brains showed some Purkinje cell deletion (as indicated by the black arrows in Fig. 5D).
Amphinase showed a surprisingly lower specific neurotoxicity in the cerebellum as it may have a lower specific affinity for Purkinje cells. Still, intracranial bolus injections show the occurrence of the Gordon effect, similar to Ranpirnase. One death was observed in safety studies with a 10% w/w Amphinase polymer formulation and, therefore, efficacy studies were conducted with 5% w/w formulations. Based on the in-vitro release results, where it was shown that control of the release was lost in formulations containing 10% w/w protein or more, there is some evidence to suggest that the increase in mortality at higher loadings may be related to an increase in the burst release. The efficacy study showed a modest increase in the median survival (p<0.023, Fig 6). The increase in median survival is thought to be the result of the combination of both its potency on 9L glioma cells in-vitro, being comparable to Ranpirnase, and its lower neurotoxicity enabling the increase of the total loading in the implant.
In-vivo safety studies with ACC7201 in concentrations up to 4% w/w polymer did not result in mortality, although slight toxicity to the cerebellum was observed. Unlike Ranpirnase, a 1% w/w ACC7201 formulation only slightly affected Purkinje cells (fig 5D). This increase in the maximally tolerated dose of ACC7201 was roughly 80 times higher (weight ratio) as compared to non-conjugated Ranpirnase. At higher concentrations of ACC7201 in a polymer formulation or as bolus intracranial injection, neuronal damage, similar to that caused by Ranpirnase, was observed as shown by histochemical staining in Figure 5B. Part of the increase in tolerance for ACC7201 can be attributed to the EGF moiety in the conjugate. Many tumor cell lines over-express endothelial growth factor receptor (EGFR) making it a diagnostic marker in some cases (30). Except for the endothelium of blood vessels EGFR is generally not expressed abundantly in the brain, including the Purkinje cells. Although specific binding of ACC7201 to EGFR has not been verified in the present study, immunohistochemical staining confirmed abundant, though inhomogeneous, expression of EGFR in the 9L-tumor cell-line whereas Purkinje cells only weakly expressed the EGFR cell-surface receptor (Fig 7A and B).
Locally delivering the 4% ACC7201 polymer formulation resulted in a modest but significant increase in survival with 13% long-time survivors in the treatment group (p<0.0001, n=16 per group; Figure 6B). Specific targeting due to the difference in receptor expression may form an important factor in the success of ACC7201 to evade neurotoxicity and increase the median survival in addition to being locally released in a controlled manner.
Conclusion
We have been able to release anti-neoplastic proteins from a p(SA-RA) polyanhydride implant, establishing general linear release trends with low burst effects for formulations up to 4% w/w protein loading. Using mix-melt technology under mild conditions prevented any significant loss in protein activity. From these implants the various Amphibinase analogues were delivered intracranially in a rodent model in a controlled manner, successfully evading neurotoxicity in the cerebellum. Efficacy studies showed a modest but promising increase in median survival in two formulations, containing 5% Amphinase or 4% ACC7201. It can be concluded that Amphibinases provide a novel series of compounds with a potential for new therapies against brain tumors. The aspect of local controlled delivery of the Aphibinases forms an enabling step in the realization of their full potential.
Acknowledgements
We acknowledge Alfacell Inc. for generously providing Ranpirnase. This work was supported in part by NIH NCDDG CA52857
References
- 1.Lesniak MS, Brem H. Targeted Therapy for Brain Tumours. Nat Rev Drug Discov. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 4.Lesniak MS. Novel advances in drug delivery to brain cancer. Technol Cancer Res Treat. 2005;4:417–428. doi: 10.1177/153303460500400409. [DOI] [PubMed] [Google Scholar]
- 5.DiMeco F, Li KW, Tyler BM, Wolf AS, Brem H, Olivi A. Local Delivery of Mitoxantrone for the Treatment of Malignant Brain Tumors in Rats. Neurosurgery. 2002;97:1173–1178. doi: 10.3171/jns.2002.97.5.1173. [DOI] [PubMed] [Google Scholar]
- 6.Frazier JL, Case D, et al. Local Delivery of Minocycline and Systemic BCNU have Synergistic Activity in the Treatment of Intracranial Glioma. J Neuro oncol. 2003;64:203–209. doi: 10.1023/a:1025695423097. [DOI] [PubMed] [Google Scholar]
- 7.Menei P, Capelle L, Guyotat J, Fuentes S, Assaker R, Bataille B, François P, Dorwling-Carter D, Paquis P, Bauchet L, Parker F, Sabatier J, Faisant N, Benoit JP. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: a randomized phase II trial. Neurosurgery. 2005;56(2):242–248. doi: 10.1227/01.neu.0000144982.82068.a2. [DOI] [PubMed] [Google Scholar]
- 8.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 9.Hanes J, Sills AK, Zhao Z, Suh KW, Tyler BM, DiMeco F, Brat DJ, Choti MC, Leong KW, Pardoll DM, Brem H. Controlled local delivery of interleukin-2 by biodegradable polymers protects animals from experimental brain tumors and liver tumors. Pharm Res. 2001;18:899–906. doi: 10.1023/a:1010963307097. [DOI] [PubMed] [Google Scholar]
- 10.Lee I, Lee YH, Mikulski S, Lee J, Covone K, Shogen K. Tumoricidal Effects of Onconase on Various Tumors. J Surg Oncol. 2000;73:164–171. doi: 10.1002/(sici)1096-9098(200003)73:3<164::aid-jso10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Mittelman A, Puccio C, Chun H, et al. A New Anti-Cancer RNase (ONCONASE): Preliminary Results in Patients with Breast Cancer. 21st Meeting of the International Association for Breast Cancer Research; July 1996. [Google Scholar]
- 12.Mikulski SM, Costanzi JJ, Vogelzang NJ, et al. Phase II Trial of a Single Weekly Intravenous Dose of Ranpirnase in Patients with Unresectable Malignant Mesothelioma. J Clin Oncol. 2002;20:274–281. doi: 10.1200/JCO.2002.20.1.274. [DOI] [PubMed] [Google Scholar]
- 13.Halicka HD, Murakami T, Papageorgio CN, et al. Induction of Differentiation of Leukaemic (HL-60) or Prostate Cancer (LNCaP, JCA-1) Cells Potentiates Apoptosis Triggered by Onconase. Cell Prolif. 2000;33:407–417. doi: 10.1046/j.1365-2184.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak SM, Newton DL. Review: Natural and Engineered Cytotoxic Ribonucleases: Therapeutic Potential. Exp Cell Res. 1999;253:325–335. doi: 10.1006/excr.1999.4718. [DOI] [PubMed] [Google Scholar]
- 15.Schulenburg C, Ardelt B, Ardelt W, Arnold U, Shogen K, Ulbrich-Hofmann R, Darzynkiewicz Z. The Interdependence Between Catalytic Activity, Conformational Stability and Cytotoxicity of Onconase. Canc Biol Ther. 2007;6(8):1233–1239. doi: 10.4161/cbt.6.8.4423. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Ardelt B, Ardelt W, Shogen K, Darzynkiewicz Z. The cytotoxic ribonuclease onconase targets RNA interference (siRNA) Cell Cycle. 2008;7(20):3258–3261. doi: 10.4161/cc.7.20.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matousek J, Soucek J, Slavik T, Tomanek M, Lee JE, Raines RT. Comprehensive comparison of the cytotoxic activities of onconase and bovine seminal ribonuclease. Comp Biochem Phys Part C. 2003;136:343–356. doi: 10.1016/j.cca.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Ourednik J, Ourednik V, Redmond DE, et al. A primate model for cerebellar atrophy using selective lesioning of Purkinje cells by onconase. Exper Neurol. 2002;175:423. [Google Scholar]
- 19.Newton DL, Walbridge S, Mikulski SM, et al. Toxicity of an Antitumor Ribonuclease to Purkinje Neurons. J Neuroscience. 1994;14:538–544. doi: 10.1523/JNEUROSCI.14-02-00538.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiMeco F, Li KW, Tyler BM, Wolf AS, Brem H, Olivi A. Local Delivery of Mitoxantrone for the Treatment of Malignant Brain Tumors in Rats. J Neurosurg. 2002;97:1173–1178. doi: 10.3171/jns.2002.97.5.1173. [DOI] [PubMed] [Google Scholar]
- 21.Domb AJ, Nudelman R. Biodegradable Polymers Derived from Natural Fatty-Acids. J Pol Sci Part A-Pol Chem. 1995;33:717–725. [Google Scholar]
- 22.Krasko MY, Shikanov A, Kumar N, Domb AJ. Polyanhydrides with hydrophobic terminals. Pol Adv Technol. 2002;13:960–968. [Google Scholar]
- 23.Krasko MY, Shikanov A, Ezra A, Domb AJ. Poly(ester anhydride)s prepared by the insertion of ricinoleic acid into poly(sebacic acid) J Pol Sci Part A-Pol Chem. 2003;41:1059–1069. [Google Scholar]
- 24.Teomim D, Nyska A, Domb AJ. Ricinoleic acid-based biopolymers. J Biomed Mat Res. 1999;45:258–267. doi: 10.1002/(sici)1097-4636(19990605)45:3<258::aid-jbm14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Shikanov A, Shikanov S, Vaisman B, Golenser J, Domb AJ. Paclitaxel tumor biodistribution and efficacy after intratumoral injection of a biodegradable extended release implant. Int J Pharm. 2008;358(1–2):114–120. doi: 10.1016/j.ijpharm.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Shikanov A, Domb AJ, Weiniger CF. Long acting local anesthetic-polymer formulation to prolong the effect of analgesia. J Cont Rel. 2007;117(1):97–103. doi: 10.1016/j.jconrel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Krasko MY, Domb AJ. Pasty injectable biodegradable polymers derived from natural acids. J Biomed Mat Res Part A. 2007;83A(4):1138–1145. doi: 10.1002/jbm.a.31395. [DOI] [PubMed] [Google Scholar]
- 28.Shikanov A, Domb AJ. Poly(sebacic acid-co-ricinoleic acid) biodegradable injectable in situ gelling polymer. Biomacrom. 2006;7(1):288–296. doi: 10.1021/bm050648+. [DOI] [PubMed] [Google Scholar]
- 29.Frazier JL, Wang PP, Case D, et al. Local Delivery of Minocycline and Systemic BCNU have Synergistic Activity in the Treatment of Intracranial Glioma. J Neuro-Oncol. 2003;64:203–209. doi: 10.1023/a:1025695423097. [DOI] [PubMed] [Google Scholar]
- 30.Tsutsui S, Kataoka A, Ohno S, Murakami S, Kinoshita J, Hachitanda Y. Prognostic and Predictive Value of Epidermal Growth Factor Receptor in Recurrent Breast Cancer. Clin Canc Res. 2002;8:3454–3460. [PubMed] [Google Scholar]