Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmogenic disease occurring in patients with a structurally normal heart: the disease is characterized by life threatening arrhythmias elicited by stress and emotion. In 2001 the ryanodine receptor was identified as the gene that is linked to CPVT; shortly after, cardiac calsequestrin was implicated in the recessive form of the same disease. It became clear that abnormalities in intracellular Ca2+ regulation could profoundly disrupt the electrophysiological properties of the heart. In this article we will discuss the molecular basis of the disease and the pathophysiological mechanisms that are impacting clinical diagnosis and management of affected individuals. As of today, the interaction between basic scientists and clinicians to understand CPVT and identify new therapeutic strategies is one of the most compelling examples of the importance of translational research in cardiology.
Keywords: Arrhythmias, genetics, ryanodine receptor, triggered activity, calcium regulation
Introduction
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) is an inherited arrhythmogenic disease associated with cardiac arrest in the pediatric population. The disease was first described as a novel clinical entity by Coumel et al. (1) in 1978 and then in a follow-up study in 1995 (2). With the advancements of molecular genetics the discovery of the genetic substrate of the disease (3,4) showed that CPVT results from inherited abnormalities of intracellular Ca2+ regulation caused by dominant mutations in the RYR2 gene, encoding the cardiac Ca2+ release channel (ryanodine receptor isoform 2, RyR2) (3) and by recessive mutations in the CASQ2 gene, encoding the cardiac calsequestrin isoform 2 (CASQ2) (5).
The discovery of the molecular substrate of CPVT has fuelled basic science studies to characterize RYR2 and CASQ2 mutations in vitro and in vivo leading to important advancements in the understanding of intracellular Ca2+ regulation and its relevance to arrhythmogenesis.
In this article we will provide an overview of the developments that have occurred in the ten years following the discovery of the first RYR2 mutations and the involvement of mutant RyR2 in CPVT patients. We will start with the physiology of Ca2+ storage and release in the sarcoplasmic reticulum (SR) and its relevance to rhythm maintenance. We will then discuss how mutations in RyR2 disrupt the Ca2+ handling system leading to cardiac arrhythmias. Finally, we will address how the understanding of the pathophysiology of the disease is leading to novel therapeutic strategies.
1. Fundamental Mechanisms of Intracellular Ca2+ Handling
Understanding of the physiology of Ca2+ handling in cardiac cells is critical for understanding how CPVT mutations alter intracellular Ca2+ regulation in the heart. Excitation contraction (EC) coupling in cardiac muscle, is mediated by a mechanism known as Ca2+-induced Ca2+ release (CICR) (6). During an action potential, the voltage-dependent L-type Ca2+ channel in the transverse T-tubular membrane is activated, resulting in a small influx of external Ca2+ into the cytosol. This Ca2+ binds to the cytosolic Ca2+ sensor in RyR2 located in the sarcoplasmic reticulum (SR) and opens the channel, leading to a large release of Ca2+ from the SR, the major intracellular Ca2+ store containing 1–1.5 mM free Ca2+ (7). The Ca2+ released then binds to troponin C, causing a cascade of conformational changes in the myofilaments and ultimately muscle contraction. During the relaxation phase, SR Ca2+ release is terminated, and the released Ca2+ is recycled back to the SR by the SR Ca2+-ATPase (SERCA) or extruded from the cell by the Na+/Ca2+ exchanger (NCX), effectively lowering the cytosolic Ca2+ concentration and allowing the dissociation of Ca2+ from the myofilaments and muscle relaxation (8) (Figure 1)
Figure. 1. Ca2+ induced Ca2+ release (CICR), store-overload-induced Ca2+ release (SOICR) and triggered arrhythmia.
The left part of the diagram (in blue) depicts the mechanism of CICR, in which an action potential activates the voltage-dependent L-type Ca2+ channel, leading to a small Ca2+ influx. This Ca2+ entry opens the RyR2 channel in the sarcoplasmic reticulum (SR), resulting in SR Ca2+ release and muscle contraction. The right part of the diagram (in red) denotes the mechanism of SOICR, in which spontaneous SR Ca2+ release or Ca2+ spillover occurs under conditions of SR Ca2+ overload caused, for example, by stress via the beta-adrenergic receptor (b-AR)/protein kinase A (PKA)/phospholamban (PLB) signaling pathway. SOICR can activate the Na+/Ca2+ exchanger (Na/CaX), which, in turn, can lead to delayed afterdepolarizations (DADs) and triggered activities. (Illustration Credit: Cosmocyte/Ben Smith).
It has long been known that SR Ca2+ release can also occur in the absence of cellular depolarization through a mechanism referred to as spontaneous Ca2+ release (9) that is facilitated by the presence of SR Ca2+ overload (10–12). Spontaneous SR Ca2+ release can also occur as a result of spontaneous membrane depolarization (13–15). To distinguish spontaneous Ca2+ release in the form of Ca2+ waves from depolarization-initiated Ca2+ release and considering its dependence on the size of the SR Ca2+ store, we have proposed that it be designated as Store Overload Induced Ca2+ Release (SOICR) (16,17).
A number of conditions, including beta-adrenergic stimulation, digitalis toxicity, elevated extracellular Ca2+, and fast pacing can lead to SR Ca2+ overload and subsequently SOICR in cardiac cells (10,18). For instance, the release of catecholamines leads to the activation of beta-adrenergic receptors and adenylate cyclase and to an increase of cAMP. The cAMP-dependent protein kinase A (PKA) is then activated, leading to the phosphorylation of a number of protein targets, including the L-type Ca2+ channel and phospholamban (PLN), an inhibitor of SERCA. Phosphorylation of the L-type Ca2+ channel by PKA increases Ca2+ influx, whereas phosphorylation of PLN by PKA relieves its inhibition on SERCA and consequently increases SR Ca2+ uptake. Excessive beta-adrenergic stimulation would, therefore, lead to augmented Ca2+ influx and SR Ca2+ uptake, resulting in SR Ca2+ overload and subsequently SOICR. In the case of digitalis toxicity, cardioglycosides such as ouabain or digoxin, inhibit the activity of Na+/K+ ATPase, resulting in the accumulation of intracellular Na+, which, in turn, inhibits the activity of the NCX. The decrease in NCX activity reduces Ca2+ extrusion from the cell, leading to more Ca2+ being recycled back to the SR, SR Ca2+ overload and SOICR. Similarly, elevated external Ca2+ or fast pacing will increase Ca2+ influx and SR Ca2+ loading and thus the propensity for SOICR (8). Overall, there are at least three major components of the progression to SR Ca2+ overload: (1) increased Ca2+ influx, (2) increased SR Ca2+ uptake and (3) reduced Ca2+ extrusion (Figure 1).
2. Electrophysiological Consequences of SOICR: Delayed Afterdepolarizations (DADs), Early Afterdepolarizations (EADs), and Triggered Arrhythmias
It has long been recognized that SOICR can alter membrane potential. The large increase in cytosolic Ca2+ as a result of SOICR in the form of Ca2+ waves can activate NCX. Since NCX is electrogenic, activation of NCX will generate a transient inward current. This inward current can depolarize the surface membrane after the action potential is ended, and thus produce delayed afterdepolarizations (DADs) (10–12). If the amplitude of DADs reaches the threshold for Na+ channel activation, DADs can trigger an action potential, which can lead to triggered arrhythmias (19–21). Recently, the mechanisms initiating early afterdepolarizations (EADs) have been reassessed (22). It was suggested that NCX may also be implicated in the generation of EADs (23). SOICR in the form of Ca2+ waves can also trigger intracellular Ca2+ alternans and sudden changes in action potential duration (24).
Interestingly, both the magnitude and the rate of spontaneous SR Ca2+ release events are critical for the determination of whether triggered activity could occur (25). It has been estimated that a total SR Ca2+ release of 50–60 mmol/L cytosol, or 50–70% of the SR Ca2+ load, is required to produce DADs with an amplitude that is sufficient to trigger an action potential (21). In addition to Ca2+ waves, spontaneous SR Ca2+ release can occur in the form of brief, localized Ca2+ transients (Ca2+ sparks) (26) or invisible Ca2+ leak through `rogue' RyRs (27). These small visible or invisible SR Ca2+ leaks as a result of `leaky' RyR2 channels by themselves are unlikely to generate DADs with amplitudes that are high enough to produce an action potential or triggered activity. It is the Ca2+ overload-induced spontaneous SR Ca2+ release in the form of Ca2+ waves (SOICR) that is capable of producing DAD-mediated triggered arrhythmias.
3. Mechanisms of Store Overload Induced Ca2+ Release
Spontaneous SR Ca2+ release occurs when the SR Ca2+ content reaches a critical load (28), suggesting that luminal Ca2+ concentration is the most plausible trigger for spontaneous SR Ca2+ release during SR Ca2+ overload. However, the evidence that Ca2+ release increases in a steep and nonlinear fashion with increasing SR luminal Ca2+ concentration (29) suggests that Ca2+ release is not a passive flow driven by Ca2+ gradient, rather it is an active process regulated by more complex and perhaps multiple mechanisms (30).
It has been proposed that luminal Ca2+ activates RyR2 by passing through the open channel and acting on the cytosolic Ca2+ activation site of the channel. This is known as the “feed-through” hypothesis (31,32). However, the finding that single RyR2 channels are still activated by luminal Ca2+ under conditions where luminal-to-cytosolic Ca2+ flux is absent does not support the feed-through hypothesis (33,34). As a consequence it was suggested that the luminal Ca2+ is sensed by a luminal Ca2+ activation site distinct from the cytosolic Ca2+ activation site (33). Recently, it has been proposed that luminal-to-cytosolic Ca2+ flux may be required for a full activation of the RyR2 by luminal Ca2+ (35). At present, most evidence supports the view that activation of RyR2 by luminal Ca2+ is mediated by a luminal Ca2+ sensor that is distinct from the cytosolic Ca2+ sensor, yet the molecular identity of this proposed luminal Ca2+ sensor is still undefined.
4. Cytosolic Ca2+ vs Luminal Ca2+ in the Regulation of RyR2
Activation of RyR2 by cytosolic Ca2+ underlies the physiological release of Ca2+ from SR (Ca2+induced-Ca2+ release or CICR); activation of RyR2 by luminal Ca2+ during SR Ca2+ overload leads to spontaneous Ca2+ release (store-overload induced-Ca2+ release or SOICR). Despite the fact that RyR2 mediates CICR, moderate modulation of RyR2 does not have a sustained impact on CICR. This phenomenon, often referred to as “SR auto-regulation”, is thought to be the result of RyR2 regulation by SR luminal Ca2+ (36). Moderate changes in RyR2 activity are compensated for by the SR Ca2+ content. For instance, increasing RyR2 activity with low concentrations of caffeine would result in an increase in Ca2+ release and a decrease in SR Ca2+ content, which, in turn, would reduce the channel activity via the luminal Ca2+ regulatory mechanism (36). While modulation of RyR2 to some extent may not have a sustained impact on CICR, it can significantly influence the properties of SOICR. For example, increasing RyR2 activity with low concentrations of caffeine lowers the critical SR Ca2+ concentration at which SOICR occurs and increases its frequency. Conversely, inhibiting RyR2 activity by tetracaine increases the threshold for SOICR and lowers its frequency (37). As a consequence, even small alterations of RyR2 will have a significant impact on SOICR and thus on the occurrence of DADs and triggered arrhythmias (Figure 1).
5. Role of Calsequestrin in SOICR
Gyorke et al. (38,39) have provided data supporting the view that CASQ2 is the luminal Ca2+ sensor responsible for regulation of RyR2 by luminal Ca2+, and for the initiation and termination of SOICR. The authors proposed that CASQ2 monomers inhibit RyR2 at low SR Ca2+ concentrations by binding to the complex formed by triadin, junctin and RyR2 (39). At high SR Ca2+ concentrations, however, CASQ2 monomers assemble into polymers and dissociate from the RyR2 channel complex, thus relieving the inhibition of RyR2, leading to channel activation and spontaneous Ca2+ release (38).
Knollmann et al. (40) have provided data that is inconsistent with Gyorke's theory by showing instead that cardiac myocytes from CASQ2-null mice still display a steep and nonlinear relationship between SR Ca2+ release and SR Ca2+ load, indicating that the RyR2 channel can sense luminal Ca2+ in the absence of CASQ2. Furthermore, CASQ2-null cardiac myocytes exhibit normal intracellular Ca2+ handling at low SR Ca2+ concentrations, indicating that the removal of CASQ2 does not lead to a marked activation of RyR2 at low SR Ca2+ concentrations. Finally, CASQ2-null cardiac myocytes show spontaneous Ca2+ waves at high SR Ca2+ load, indicating that in the absence of CASQ2, spontaneous SR Ca2+ release can be still initiated and terminated (40). Taken together, these observations demonstrate that CASQ2 may modulate SR Ca2+ release, but is not essential for luminal Ca2+ regulation of RyR2 or for the initiation and termination of spontaneous SR Ca2+ release in cardiac myocytes.
Since purified native and recombinant RyRs remain sensitive to luminal Ca2+ (32,41–43), it is likely that the luminal Ca2+ sensor lies within the primary structure of RyR2, and is potentially regulated by a number of factors and proteins associated with the Ca2+ release complex, including CASQ2, triadin, junctin, FKBP12.6, calmodulin, kinases, or other RyR2 associated proteins.
6. Linking Intracellular Ca2+ Handling with CPVT
The diagnosis of CPVT is most commonly established in apparently healthy pediatric patients suddenly and unexpectedly manifesting stress- or emotion-induced syncopal episodes. Occasionally sudden cardiac death is the first clinical event in an otherwise healthy young individual (44–46). Unless diagnosed and treated the disease is lethal: in our series of patients, up to 30% of misdiagnosed and affected individuals died suddenly before the fourth decade of life.
The diagnosis of CPVT is challenging considering that cardiologic evaluation is often completely normal and patients show unremarkable echocardiogram and electrocardiogram. In the absence of a family history indicative of a genetic arrhythmogenic condition, CPVT can be suspected only during exercise stress testing when patients develop exercise-related ventricular tachycardia. During exercise stress test, CPVT patients show isolated premature beats at the beginning of exercise with a progressive worsening of the complexity of ventricular arrhythmias in response to an increased workload. Typically, when the heart rate reaches 90 to 110 bpm, runs of non-sustained or sustained VT appear and they may degenerate into sustained VT and VF unless exercise is promptly terminated. The morphology of VT is often the hallmark of the disease: the so-called bidirectional VT (1,3) which is characterized by a 180° beat-to-beat rotation of the axis of the QRS complexes on the frontal plane (Figure 2). Interestingly bidirectional VT is also the typical morphology of ventricular tachycardia in the setting of digitalis intoxication thus suggesting that the two arrhythmogenic conditions may share similar electrophysiological bases.
Figure 2.
Example of typical bidirectional Ventricular tachycardia in a CPVT patient during exercise stress test
Bidirectional VT is considered the diagnostic marker of CPVT, however, not all patients with the disease manifest this form of arrhythmias. Based on our patient population, we were able to identify different phenotypic manifestations of CPVT among patients with RyR2 mutations: 1) patients presenting reproducible bidirectional VT and polymorphic VT at exercise stress testing; 2) patients presenting only with polymorphic VT; 3) survivors of cardiac arrest and lacking induction of arrhythmias during exercise stress test (3). The latter group is intriguing and it is arguable whether they should be considered part of the CPVT phenotype. A subset of these patients, referred for genetic testing with the diagnosis of Idiopathic VF (IVF), carry RyR2 mutations. It will be of interest to determine whether the impact of these IVF RyR2 mutations differ from that of the typical CPVT RyR2 mutations.
Molecular screening of the RYR2 and CASQ2 genes importantly contributes to diagnosis in patients with less typical phenotypic manifestations. Functional characterization in vitro as well as knock-in mouse models carrying clinical mutations also provide important information that helps us to understand how abnormalities in RyR2 and CASQ2 disrupt the physiology of intracellular Ca2+ regulation, leading to arrhythmic storms.
7. RyR2 Mutations Linked to CPVT
The RyR2 channel is a homotetramer with each subunit containing a large cytosolic domain formed by the first ~4300 N-terminal residues and a smaller transmembrane (TM) domain formed by the last ~500 C-terminal residues. The TM domain of RyR2 encompasses the channel pore, while the cytosolic domain contains binding sites for a number of channel regulators. In such a large and complex protein, it is challenging to investigate the topology of mutations in an attempt to derive structure-function information.
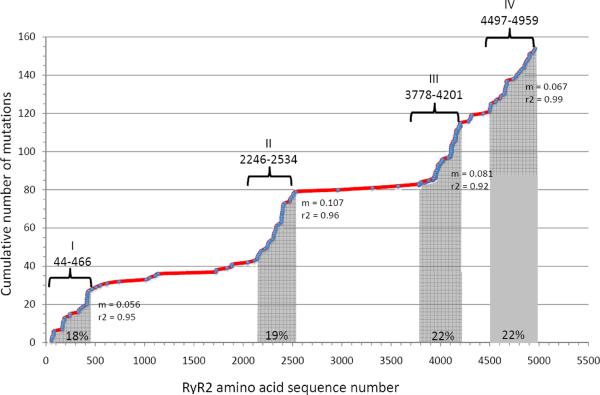
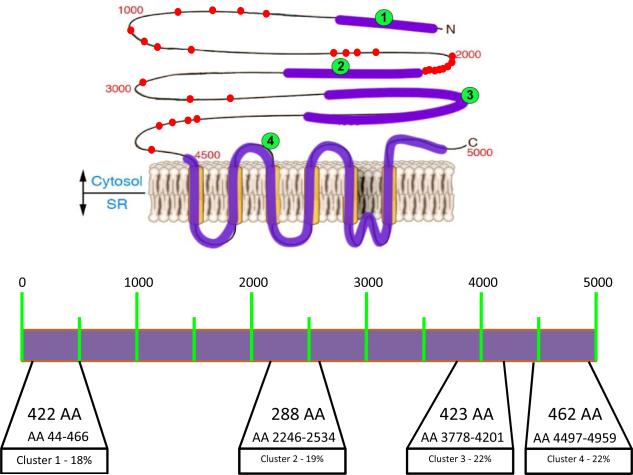
Mutations in genes encoding the voltage dependent ion channels that cause several clinical diseases, are often distributed across the entire coding region of the genes of interest. RyR2 mutations appear to be preferentially located in four regions (Figure 3). The term “domains” indicates those regions, according to this classification, domain I includes amino acids 77 to 466, domain II amino acids 2246–2534 , domain III amino acids 3778–4201 and domain IV amino acids 4497–4959 (Figure 4). These regions are highly conserved in RyR across species and are superimposable (except for region III) to the localization of RyR1 mutations associated with central core disease and malignant hyperthermia (47).
Figure 3.
A plot of the accumulation of cardiac ryanodine receptor mutations (RyR2) reported in CPVT and IVF against amino acids sequence of the human RyR2 protein. A total of 152 mutations are reported in the plot (source www.fsm.it/cardmoc as of December 01, 2010). Each dot represents a mutation. Gray-shaded areas represent the mutations clusters; the number in the gray area is the relative percentage of mutations occurring that cluster. The linear correlation slope (m), which is an index of mutation density, is reported for each cluster. Amino acid numbers (human RyR2 protein sequence) for cluster boundaries are reported above the line together with the cluster ordinal numbering.
Figure 4.
Cartoon (upper panel) and schematic representation (lower panel) of the RyR2 protein. Clusters with frequent mutations are depicted with their location along RyR2 amino acid (AA) sequence. Percentage of known (published) mutation for each cluster is also reported (lower panel). Mutation outside the canonical clusters are depicted as dots (upper panel). Each dot represents a unique mutation.
Mutations in RyR2 are not uniformly distributed across the four domains: mutations in domains III and IV collectively account for 46% of all mutations reported, followed by mutations located in domain II (21%) and by mutations in the N terminus (18%). It has been suggested that only a small number of mutations are positioned in less conserved regions outside the above mentioned domains: Medeiros Domingo et al. (48) reported that only 10% of mutations are outside domains I-IV. At variance with this estimation, analysis of our CPVT cohort by direct ORF sequencing indicates that 24% of RyR2 mutations identified in CPVT patients are located outside the four canonical domains.
Most mutations identified in RyR2 are single nucleotide replacements (also called “point mutations”) leading to an amino acid substitution: this is at variance with the case for other channelopathies where mutations such as truncations, deletions and insertions are more common. Premature stop codons, frameshifts, and out-of-frame insertions or deletions have not been identified in CPVT patients screened for mutation on the RYR2 gene. The lack of identification of loss of function mutations in RyR2 may be the consequence of the fact that the screening of RyR2 is usually targeted to patients with the CPVT phenotype and structurally intact heart. Therefore, it is possible that loss of function mutations result in different diseases possibly including structural abnormalities of the heart consistent with a cardiomyopathic phenotype. Some authors (49–52) have suggested that RYR2 mutations may cause arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2).
Small in-frame duplications or in-frame insertions (48,53) are present in patients with the CPVT phenotype, suggesting that their functional consequences are similar to that of point mutations, rendering the channel more prone to spontaneous SR Ca2+ release during adrenergic stimulation. We have identified 82 RYR2 mutations in our CPVT probands: 79 were point mutations and 3 (3.6%) were small deletions/insertions (one in frame insertion and two in frame deletions). Carriers of insertion/deletions had clinical manifestations consistent with CPVT and no structural abnormalities. Bhuiyan et al (54) using multiplex ligation-dependent probe amplification (MLPA) analysis, identified a large deletion encompassing part of intron 2, exon 3, and part of intron 3 associated with a complex clinical phenotype that included progressive AV block and SAN dysfunction, AF, atrial standstill, and depressed LV function to dilated cardiomyopathy (DCM). However, other reports of large deletions in the same region failed to confirm the association with DCM: large exon 3 deletions were reported by Marjamaa A et al. (55) in 2 apparently unrelated patients. One of these patients showed no major structural abnormalities, while the other patient showed increased trabeculation of the left ventricle, suggestive of non-compaction cardiomyopathy. Medeiros-Domingo et al. (48) reported a large 3.6 Kb exon 3 deletion, but the phenotype of the patient was not reported. Large deletions encompassing exon 3 seem to be relatively frequent, possibly because the region presents Alu repeats that may predispose genomic rearrangements (56). Whether these cause distinguishing phenotypes in addition to CPVT is still undefined.
8. Functional Consequences of RyR2 Mutations
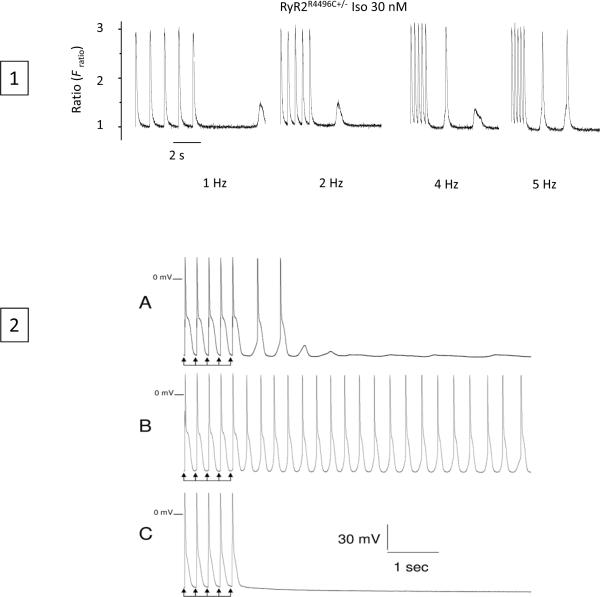
Delayed afterdepolarization and triggered activity has been shown in knock-in models of CPVT (57) (Figure 5). A key and fundamental question is what aspects of RyR2 function are impaired by these disease-causing RyR2 mutations. Considering the important role of luminal Ca2+ in triggering SOICR and DADs, Jiang et al. (16,17) directly determined the impact of a number of CPVT RyR2 mutations on the sensitivity of single RyR2 channels to luminal Ca2+ activation in the near absence of cytosolic Ca2+ using single channel recordings in planar lipid bilayers. In these studies, a spectrum of RyR2 mutations were used, extending from the N- to the C-terminus, with small phenotypic differences among carriers: L433P and R176Q/T2504M located in the N-terminal region of the channel; mutations S2246L and R2474S located in the central region; and Q4201R, N4104K, R4496C, I4867M, N4895D, and V4653F (58) located in the C-terminal region. All of these mutations showed a consistent behavior characterized by an enhanced response of the channel to luminal Ca2+ activation. Interestingly, [3H]ryanodine binding or single channel studies revealed that most of the CPVT RyR2 mutations tested did not markedly affect the response of the RyR2 channel to cytosolic Ca2+. Exceptions were N4104K and V4653F, which also significantly increased the activation of [3H]ryanodine binding by low concentrations of cytosolic Ca2+. The R4496C mutation (3) also increases the cytosolic Ca2+ activation of [3H]ryanodine binding (59). These observations indicate that CPVT RyR2 mutations preferentially sensitize the channel to luminal Ca2+ activation, while only a few CPVT RyR2 mutations sensitize the channel to both cytosolic and luminal Ca2+ activation.
Figure 5.
Ca2+ transients (1) and action potential (2) in R4496C knock-in mouse. 1) Ca2+ transients at stimulation rates from 1Hz to 5Hz during isoproterenol superfusion; last five stimulated transients of each train followed by diastolic pause are shown. Spontaneous diastolic releases occurs with increasing frequency at faster rates where triggered activations are also evident. 2) Action potential recording show DAD and triggered beats (a); triggered activity is greatly enhanced by isoproterenol (30nM) (B) and completely suppressed by ryanodine (10uM)
In agreement with the role of luminal Ca2+ activation of RyR2 in SOICR, CPVT RyR2 mutations that increase the response of the channel to luminal Ca2+ also enhance the propensity for SOICR in HEK293 cells by reducing the threshold luminal Ca2+ level at which SOICR is triggered. Enhanced SOICR has also been observed in cardiomyocytes isolated from various knock-in mice harboring CPVT RyR2 mutations, R176Q (60), R4496C (59,61), or R2474S (62). The same manifestation of CPVT RyR2 mutations in both cardiomyocytes and in HEK293 cells, which lack a number of cardiac specific proteins, including CASQ2, suggests that SOICR is not unique to cardiac cells, and is primarily determined by the intrinsic properties of the RyR2 channel. Together, these observations support the view that the luminal Ca2+ sensor of RyR2 is located within its primary sequence and is responsible for the initiation and termination of SOICR.
Although CPVT RyR2 mutations alter the sensitivity of the channel to Ca2+ activation, they apparently have little or no impact on excitation-contraction (EC) coupling, as patients with CPVT RyR2 mutations do not show arrhythmias in unstimulated conditions (63). This is likely attributable to the unique auto-regulatory property of SR Ca2+ release. The SR Ca2+ content may compensate for the defective luminal or cytosolic Ca2+ activation of RyR2. This is because an enhanced sensitivity to luminal or cytosolic Ca2+ activation would lead to increased SR Ca2+ release and a reduction in SR Ca2+ content, which, in turn, would decrease luminal Ca2+ activation of RyR2. Hence, due to the auto-regulation of SR Ca2+ release, altered Ca2+ activation of RyR2 would not have a sustained impact on EC coupling under normal conditions (64). However, under conditions of SR Ca2+ overload, SR auto-regulation becomes ineffective, resulting in SOICR and, consequently, in DADs and triggered arrhythmias (Figure 6).
Figure 6. A unifying theory for CPVT?
The SOICR thresholds and free luminal Ca2+ levels in normal SR (B) and abnormal SR associated with CASQ2 mutations (A) or RyR2 mutations (C) in the resting state (top panels) and under the conditions of SR Ca2+ overload (bottom panels) are shown. The normal SOICR threshold is depicted as a dashed red bar, whereas the mutation-lowered SOICR threshold is depicted as a solid red bar. The blue area represents the free SR luminal Ca2+ concentration, while the yellow area represents the increased free SR luminal Ca2+ level during a sudden increase in SR Ca2+ loading. The RyR2 channel complex is depicted as a black structure and the Ca2+-CASQ2 complexes are shown as pink diamond structures. Mutations in RyR2 lower the SOICR threshold (C), while mutations in CASQ2 reduce the level of CASQ2 protein and/or Ca2+ buffering capability (A). The R33Q CASQ2 mutation or a reduction in the CASQ2 protein level has also been shown to lower the SOICR threshold (A). During SR Ca2+ overload, the free luminal Ca2+ level is more likely to exceed the RyR2 mutation-lowered SOICR threshold (panel C, bottom) or the normal or reduced SOICR threshold in the absence or lack of SR Ca2+ buffering as a result of CASQ2 mutations (panel A, bottom), leading to SOICR that can produce DADs and triggered arrhythmias (adapted from MacLennan, D. H., and Chen, S. R. W. (2009) J. Physiol. 587:3113-3115). (Illustration Credit: Cosmocyte/Ben Smith).
9. How RyR2 Mutations Alter the Sensitivity of the Channel to Luminal or Cytosolic Ca2+ Activation?
In the previous section, we have presented robust evidence supporting the view that CPVT RyR2 mutations alter the sensitivity of the channel to luminal and/or cytosolic Ca2+ activation, leading to enhanced spontaneous Ca2+ release during SR Ca2+ overload (SOICR). What remains unclear today is how CPVT RyR2 mutations exert these effects. Two mechanisms have been proposed and will be presented below: Domain unzipping and FKBP12.6 unbinding.
Domain Unzipping
It has been proposed that the N-terminal domain in RyR2 interacts with the central domain, and that CPVT RyR2 mutations in the N-terminal and central domains weaken this interaction (domain unzipping) (65,66). These domain interactions are believed to be involved in the stabilization of the closed state of the channel. Hence, domain unzipping as a result of mutations would destabilize the closed state of the channel, rendering the channel more sensitive to stimuli, such as luminal or cytosolic Ca2+.
Evidence for unzipping of the interaction between the N-terminal and central domains raises the question of whether “domain unzipping” is present in other regions of RyR2 in which CPVT mutations are located. In support of this view are data showing that multiple, large conformational changes in the RyR2 structure occur during channel gating. Therefore, it is reasonable to hypothesize that mutations located throughout the molecule could alter physiologically important conformational changes, resulting in channel dysfunction. Recently investigators reported the three dimensional structures of fragments of the N-terminal region of RyR1 and RyR2 that contain a number of disease-linked RyR1 or RyR2 mutations and showed that most of them are located on the surface of domains and within domain interfaces where they could disrupt domain-domain interactions (67–69)
FKBP12.6 unbinding
Another mechanism by which mutations may alter the sensitivity of the channel to Ca2+ activation is the disruption of critical protein-protein interactions. In this regard, it has been proposed that RyR2 mutations may specifically impair the interaction between RyR2 and the 12.6 kDa FK506 binding protein (FKBP12.6) (70). FKBP12.6 is thought to play an important role in stabilizing the RyR2 channel and dissociation of FKBP12.6 from RyR2 as a result of phosphorylation of RyR2 by PKA during beta-adrenergic stimulation has been shown to increase the sensitivity of the channel to cytosolic Ca2+ activation (71). In the hypothesis proposed by Wehrens et al. (70) RyR2 mutations may impair FKBP12.6 binding to RyR2 making the channel leaky. A number of studies have been carried out to determine the impact of CPVT RyR2 mutations on RyR2-FKBP12.6 interaction. Wehrens et al. (70) showed that CPVT RyR2 mutations, S2246L and P2328S located in the central region, and Q4201R, R4496C, and V4653F located in the C-terminal region, reduced the affinity of FKBP12.6 binding to RyR2. Based on these observations they proposed that reduced FKBP12.6 binding affinity is a common defect of CPVT RyR2 mutations. Subsequently, Wehrens et al. (70,72) showed that the R2474S mutation also decreases the affinity of FKBP12.6 binding.
These findings concerning causative alterations in the binding of FKBP12.6 to RyR2 have not been confirmed in more recent studies. For example, Tiso et al. (73) showed that the same R2474S mutation was found to increase the affinity of FKBP12.6 binding. Moreover, Liu et al. (57), studying the R4496C knock-in mouse model, showed that a compound that promotes the binding of FKBP12.6 to RyR2, K201, did not alter arrhythmogenesis in this mouse model. These findings suggest that either FKBP12.6 binding is not critical for arrhythmogenesis in CPVT or that it is critical only for selected mutations. Studies by George et al. (16,74) and Jiang et al. (16,74) then demonstrated that CPVT RyR2 mutations had no effect on FKBP12.6 binding. Recent data from Bers and coworkers demonstrated that PKA is not involved in the dissociation of FKBP12.6 from RyR2, thus questioning any link between RyR2 phosphorylation and FKBP12.6 dissociation (75). Therefore, whether CPVT RyR2 mutations alter the affinity of FKBP12.6 binding (16), and the question of whether the dissociation of FKBP12.6 affects cytosolic Ca2+ activation of RyR2 or increases the propensity for ventricular arrhythmias (76) are still highly controversial. Although it may be possible that selected mutations alter FKBP12.6 binding to RyR2, an increasing body of evidence clearly demonstrates that alterations in FKBP12.6-RyR2 interaction are unlikely to be the common cause of CPVT associated with RyR2 mutations.
10. Functional and Structural Consequences of CASQ2 Mutations
Mutations in the gene encoding the cardiac calsequestrin isoform 2 (CASQ2), mapping to chromosom 1p13.3-p11, causes an autosomal recessive form of CPVT (CPVT2). The first homozygous CASQ2 mutation (D307H) was identified in a large consanguineous Bedouin family and reported by Lahat et al (5) in 2001. As expected for a recessive disease, CPVT2 is much less common than the autosomal dominant form of the disease. As a consequence, eight years after the original description of the first CASQ2 mutations, only a few additional variants have been identified. As of today 12 CPVT-associated mutations and 3 non synonymous polymorphisms (cSNP) are known (http://www.fsm.it/cardmoc/). Among the 12 CPVT CASQ2 mutations, 4 are nonsense mutations that will lead to the expression of a truncated protein (77,78). Among the remaining 8 missense mutations, R33Q and D307H have been shown to reduce the level of CASQ2 protein to 5% and 45%, respectively (79,80). Hence, both the nonsense and some missense mutations lead to reduced CASQ2 protein levels and consequently reduced SR Ca2+ buffering capacity. The R33Q, K206N, L167H, and D307H CASQ2 missense mutations have also been shown to alter the Ca2+ binding capacity and/or the Ca2+ dependent polymerization of CASQ2 (79,81–84). Since the CASQ2 polymer is responsible for high capacity Ca2+ binding, such alterations will also lead to a reduction in SR Ca2+ buffering capacity. The molecular defects of other missense mutations (Y55C, P308L, E177Q,and F189L) have not been characterized (85–87)
The Y55C and P308L are compound heterozygous CASQ2 mutations associated with CPVT (85). Nevertheless, these observations clearly demonstrate that reduced SR Ca2+ buffering capacity is a common consequence of CPVT CASQ2 mutations. Reduced SR Ca2+ buffering will result in a fast recovery of SR free Ca2+ after each Ca2+ release and a potentially higher level of SR free Ca2+ during a sudden increase in SR Ca2+ loading, both of which will increase the propensity for SOICR and thus DADs and triggered activity (Figure 6).
Some CASQ2 mutations may alter the interactions between CASQ2 and the RyR2 channel complex, thus affecting the response of RyR2 to elevating luminal Ca2+. For example, unlike the CASQ2 wt, the R33Q CASQ2 mutant has been shown to be unable to effectively inhibit the RyR2 channel at low SR Ca2+ concentrations (88,89). Cardiomyocytes over-expressing the R33Q mutant showed a reduced threshold SR Ca2+ level at which spontaneous Ca2+ release occurs. These observations have led to the proposal that the R33Q mutation may alter the interaction of CASQ2 with the RyR2 channel complex and affect the response of the channel to luminal Ca2+ (89). However, it is important to point out that CASQ2 null cardiomyocytes exhibit no abnormal SR Ca2+ release at low SR Ca2+ concentrations, and that CASQ2 null mice show no ventricular arrhythmias at rest (40). Therefore, the significance of CASQ2's inhibitory effect on RyR2 at low SR Ca2+ concentrations is unclear.
The level of CASQ2 has also been suggested to be important in modulating the activity of RyR2. Chopra et al. (90) showed that heterozygous CASQ2-null cardiac cells displayed more SR Ca2+ leak than the wild type control cells at the same free SR Ca2+ concentration. This observation led to the conclusion that a modest reduction in CASQ2 protein level (~25%) can reduce the threshold for spontaneous SR Ca2+ leak. However, Kubalova et al. (91) have demonstrated that increasing (~3.5 fold) or decreasing (to 30% of control) the level of CASQ2 protein has no effect on the threshold SR Ca2+ level at which spontaneous Ca2+ waves (SOICR) occurs. Changing the level of CASQ2 protein, however, markedly alter the dynamics of SR Ca2+ recovery after SR Ca2+ release. These observations indicate that CASQ2 via its Ca2+ buffering function mainly affects the dynamics of SR Ca2+ recovery or the frequency of SOICR, but not the threshold for SOICR. In this regard, it will be important to determine whether a reduction (25%) of CASQ2 in heterozygous CASQ2-null cardiac cells alters the dynamics and amplitude of free SR Ca2+ concentrations during spontaneous Ca2+ waves.
The clinical phenotype of patients affected by the recessive variant of CPVT is virtually identical to the autosomal dominant form except that carriers of homozygous CASQ2 mutation have more severe manifestations than carriers of heterozygous RyR2 mutations. In analogy with humans, mice with homozygous CPVT CASQ2 mutations display phenotypes virtually identical to, but more severe than, those observed in mice with heterozygous CPVT RyR2 mutations (40,78–80,92), suggesting that CASQ2-linked CPVT and RyR2-associated CPVT share a common causal mechanism (Figure 5). In other words, DADs induced by spontaneous SR Ca2+ release during Ca2+ overload (SOICR) is likely to be the cause for CASQ2-linked CPVT. An unresolved question is how CPVT CASQ2 mutations lead to abnormal activation of normal RyR2 by elevating luminal Ca2+, thus leading to enhanced SOICR and DADs. Some important clues to this question are emerging. An increasing body of evidence indicates that a reduction in the level of CASQ2 protein and Ca2+ buffering capacity is a common defect of CPVT CASQ2 mutations (93).
11. Therapeutic Approaches to CPVT
Understanding of the basic mechanisms of arrhythmogenesis in CPVT should guide the identification of novel therapeutic strategies. It seems that promising appraoches to suppress CPVT are (1) the prevention of SR Ca2+ overload and 2) the resolution of the defect in SOICR by RyR2 modulation. Agents like beta blockers and calcium inhibitors have been used in experimental as well as clinical settings, primarely act by reducing calcium overload via the reduction of heart rate and L-type channel current, and through the inhibition of phosphorylation of PLB and thus the activation of SERCA.
Inhibition of RyR2 may also be an important determinant of the effectiveness of antyarrhythmic drugs in CPVT. As suggested by recent data discussed below, Flecainide may cause an open state block of the RyR2 channel, which may prevent the arrhythmogenic calcium waves without affecting SR lead-load balance.
As of today the clinical management of CPVT aims at attenuating the arrhythmogenic effect of adrenergic stimulation through the use of beta-blockers.
However already in 2002 we reported (3) that beta-blockers can only confer partial protection from life-threatening arrhythmias among patients compliant to therapy with the maximal tolerated dose of beta-blockers. When patients present recurrence of arrhythmic events on beta-blockers, they are often implanted with an implantable cardioverter defibrillator. However, given the young age of patients, the use of the defibrillator is often challenging and associated with complications such as lead-fracture and infection of the pocket that contains the generator.
Clearly there are now expectations that recent basic science advancements in the understanding of the disease may guide the development of novel therapies. Prompted by the evidence that CPVT is caused by abnormalities in Ca2+ handling, some authors decided to test the efficacy of verapamil in mice and humans. Sumitomo et al (94) observed that in their cohort of CPVT patients that Ca2+ antagonists partially protected patients from arrhythmic events. A few years later Swan et al (95) reported suppression of arrhythmias during exercise stress test with verapamil, yet they did not report the survival benefit of the drug. In our experience, verapamil and diltiazem show a partial improvement of frequency of arrhythmic events but their effect is not sufficient to avoid the need for an implantable defibrillator. The molecular mechanism by which verapamil effectively suppresses CPVT is unclear: in vitro studies have suggested that verapamil may bind to RyR2 and inhibit its channel activity (96). However, whether this mechanism is present at clinically relevant concentrations of the drug, has not been established.
Recently, in vitro data showed that, in analogy with Ca2+ channel blockers, flecainide, a Na+ channel blocker, inhibits single RyR2 channel activity and suppresses spontaneous SR Ca2+ release in cardiac cells (97,98). Interestingly, the drug was also found to suppress ventricular arrhythmias in the CASQ2-knock-out mouse model of CPVT. Whether the antiarrhythmic activity of flecainide is dependent on its RyR2 blockade (95) or on the sodium channel blocking activity that reduces the probability for DADs to reach the threasold for action potential generation (99), is still uncertain. The antiarrhythmic effect of flecainide, however, seems to be confirmed in patients, thus providing an important therapeutic breakthrough.
Animal studies have proposed additional therapeutic strategies that have not been tested in humans. K201(JTV519), a 1,4-benzothiazepine derivative that shares a high degree of structural similarity with the L-type channel blocker diltiazem, was originally discovered on the basis of its ability to protect cardiomyocytes from cell injury and death due to Ca2+ overload induced by epinephrine, caffeine and high external Ca2+(100). K201 is thought to inhibit RyR2 and prevent SR Ca2+ leak by stabilizing FKBP12.6 binding to RyR2. However, recent studies revealed that K201 binds to the central region of RyR2 (2114–2149 aa) (101) and suppresses SR Ca2+ leak and spontaneous Ca2+ waves irrespective of FKBP12.6 association (101,102). Despite its inhibitory action on RyR2 and spontaneous Ca2+ release, K201 was found to be unable to prevent ventricular arrhythmias in the R4496C+/- knock-in mouse model of CPVT (57), although it is able to prevent ventricular arrhythmias in FKBP12.6+/- mice (103,104). Since human studies with K201 or its derivative have not yet been completed, the clinical role of these agents remains to be defined.
An increasing body of evidence indicates that the Ca2+ and calmodulin-dependent protein kinase II (CaMKII) plays an important role in the generation of spontaneous SR Ca2+ waves upon beta-adrenergic stimulation (105). Suppression of the CaMKII pathway may, therefore, represent another effective therapeutic approach for the suppression of Ca2+-mediated arrhythmias. In support of this idea, preliminary evidence shows that KN93, an inhibitor of CaMKII, is able to prevent ventricular arrhythmias in the R4496C+/- mouse model of CPVT (106).
Summary.
Proteins responsible for regulation of intracellular Ca2+ handling have been implicated in genetic diseases. In particular, mutations in the RyR2 gene encoding the cardiac ryanodine receptor isoform 2 and the CASQ2 gene encoding the cardiac calsequestrin isoform 2 cause a clinical condition called Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) that is highly lethal and often manifests in the pediatric population. RyR2 mutations result in an abnormal protein that is prone to spontaneous Ca2+ release from the sarcoplasmic reticulum. Mutations in the cardiac calsequestrin reduce the amount of protein and Ca2+ buffering in the SR. In vitro studies and development of knock-in mice have provided important information that have advanced the field and suggest that Store Overload Induced Ca2+ Release (SOICR) is likely to be the common mechanism for a variety of mutations in these two genes. The field has been particularly productive in bringing advancements to the clinics where new therapies have already been introduced. Advances in our understanding of the regulation of intracellular Ca2+ in health and disease will facilitate the development of novel risk stratification and management scheme to improve survival and quality of life of CPVT patients.
ACKNOWLEDGMENTS
We would like to thank Dr. David MacLennan, University of Toronto, for critical reading of the manuscript, and Dr. Alexander Kraev for help in the preparation of Figure 3.
SOURCES OF FUNDING Original research from the authors' laboratories, described in this review, was supported by research grants from the National Institutes of Health (NIH) and the Canadian Institutes of Health Research (CIHR) to SRWC, and Telethon grant GGP06007, Italian Ministero dell'Università e della Ricerca Scientifica e Tecnologica grants FIRB RBNE01XMP4_006, RBLA035A4X_002, and by Fondation Leducq Research Grant no. 08 CVD 01 Alliance for Calmodulin Kinase II in heart disease to SGP.
LIST OF ABBREVIATIONS
- (CPVT)
Catecholaminergic polymorphic ventricular tachycardia
- (RyR2)
Ryanodine receptor isoform 2
- (CASQ2)
Cardiac calsequestrin isoform 2
- (SR)
Sarcoplasmic reticulum
- (CICR)
Ca2+-induced Ca2+ release
- (EC)
Excitation contraction
- (NCX)
Na+/Ca2+ exchanger
- (SERCA)
SR Ca2+-ATPase
- (SOICR)
Store Overload Induced Ca2+ Release
- (PKA)
Protein kinase A
- (PLN)
Phospholamban
- (DADs)
Delayed after depoarizations
- (TM)
Transmembrane
- (ARVD2)
Arrhythmogenic right ventricular cardiomyopathy type 2
- (DCM)
Dilated cardiomyopathy
Footnotes
DISLOSURES None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coumel P, Fidelle J, Lucet V, Attuel P, Bouvrain Y. Catecholaminergic-induced severe ventricular arrhythmias with Adams-Stokes syndrome in children: report of four cases. Br Heart J. 1978;40:28–37. [Google Scholar]
- 2.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the Cardiac Ryanodine Receptor Gene (hRyR2) Underlie Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 4.Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am.Coll.Cardiol. 1999;34:2035–2042. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 5.Lahat H, Pras E, Olender T, Avidan N, Ben Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum.Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am.J.Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 7.Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 9.Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- 10.Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- 11.Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- 13.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res. 2010;106:659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shtifman A, Paolini C, Lopez JR, Allen PD, Protasi F. Ca2+ influx through alpha1S DHPR may play a role in regulating Ca2+ release from RyR1 in skeletal muscle. Am J Physiol Cell Physiol. 2004;286:C73–78. doi: 10.1152/ajpcell.00194.2003. [DOI] [PubMed] [Google Scholar]
- 15.Tsien RW, Kass RS, Weingart R. Cellular and subcellular mechanisms of cardiac pacemaker oscillations. J Exp Biol. 1979;81:205–215. doi: 10.1242/jeb.81.1.205. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 17.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc.Natl.Acad Sci U.S.A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation? J Cardiovasc Electrophysiol. 1993;4:473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 19.Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–17. [PubMed] [Google Scholar]
- 20.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc.Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 22.Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am.J.Physiol. 1990;258:H1796–H1805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 24.Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol. 2009;297:H997–H1002. doi: 10.1152/ajpheart.00390.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297:H1235–1242. doi: 10.1152/ajpheart.01320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 27.Sobie EA, Guatimosim S, Gomez-Viquez L, Song LS, Hartmann H, Saleet Jafri M, Lederer WJ. The Ca 2+ leak paradox and rogue ryanodine receptors: SR Ca 2+ efflux theory and practice. Prog Biophys Mol Biol. 2006;90:172–185. doi: 10.1016/j.pbiomolbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz ME, Trafford AW, O'Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol. 1997;501(Pt 1):3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovetti R, Cui X, Garfinkel A, Weiss JN, Qu Z. Spark-induced sparks as a mechanism of intracellular calcium alternans in cardiac myocytes. Circ Res. 106:1582–1591. doi: 10.1161/CIRCRESAHA.109.213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathy A, Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys J. 1998;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 34.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys.J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J. 2007;92:3541–3555. doi: 10.1529/biophysj.106.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trafford AW, Sibbring GC, Diaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 37.Eisner DA, Trafford AW, Diaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 38.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 40.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong H, Wang R, Chen W, Zhang L, Chen K, Shimoni Y, Duff HJ, Chen SR. Skeletal and cardiac ryanodine receptors exhibit different responses to Ca2+ overload and luminal ca2+ Biophys J. 2007;92:2757–2770. doi: 10.1529/biophysj.106.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sitsapesan R, Williams AJ. Regulation of current flow through ryanodine receptors by luminal Ca2+ J Membr Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 45.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 46.Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner's cases. Mayo Clin.Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 47.Maclennan DH, Zvaritch E. Mechanistic models for muscle diseases and disorders originating in the sarcoplasmic reticulum. Biochim Biophys Acta. doi: 10.1016/j.bbamcr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JP, Mannens MM, Wilde AA, Ackerman MJ. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009;54:2065–2074. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.d'Amati G, Bagattin A, Bauce B, Rampazzo A, Autore C, Basso C, King K, Romeo MD, Gallo P, Thiene G, Danieli GA, Nava A. Juvenile sudden death in a family with polymorphic ventricular arrhythmias caused by a novel RyR2 gene mutation: evidence of specific morphological substrates. Hum Pathol. 2005;36:761–767. doi: 10.1016/j.humpath.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 50.Milting H, Lukas N, Klauke B, Korfer R, Perrot A, Osterziel KJ, Vogt J, Peters S, Thieleczek R, Varsanyi M. Composite polymorphisms in the ryanodine receptor 2 gene associated with arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Res. 2006;71:496–505. doi: 10.1016/j.cardiores.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Nishio H, Iwata M, Suzuki K. Postmortem molecular screening for cardiac ryanodine receptor type 2 mutations in sudden unexplained death: R420W mutated case with characteristics of status thymico-lymphatics. Circ J. 2006;70:1402–1406. doi: 10.1253/circj.70.1402. [DOI] [PubMed] [Google Scholar]
- 52.Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification on mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum.Mol.Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 53.Tester DJ, Kopplin LJ, Will ML, Ackerman MJ. Spectrum and prevalence of cardiac ryanodine receptor (RyR2) mutations in a cohort of unrelated patients referred explicitly for long QT syndrome genetic testing. Heart Rhythm. 2005;2:1099–1105. doi: 10.1016/j.hrthm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Bhuiyan ZA, van Den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, Van LI, Mannens MM, Wilde AA. Expanding Spectrum of Human RYR2-Related Disease. New Electrocardiographic, Structural, and Genetic Features. Circulation. 2007;116:1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 55.Marjamaa A, Laitinen-Forsblom P, Lahtinen AM, Viitasalo M, Toivonen L, Kontula K, Swan H. Search for cardiac calcium cycling gene mutations in familial ventricular arrhythmias resembling catecholaminergic polymorphic ventricular tachycardia. BMC Med Genet. 2009;10:12. doi: 10.1186/1471-2350-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu W, Zhang F, Lupski JR. Mechanisms for human genomic rearrangements. Pathogenetics. 2008;1:4. doi: 10.1186/1755-8417-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, Imbriani M, Napolitano C, Lai FA, Priori SG. Arrhythmogenesis in Catecholaminergic Polymorphic Ventricular Tachycardia. Insights From a RyR2 R4496C Knock-In Mouse Model. Circulation Research. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 58.Jones PP, Jiang D, Bolstad J, Hunt DJ, Zhang L, Demaurex N, Chen SR. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem J. 2008;412:171–178. doi: 10.1042/BJ20071287. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Velasco M, Rueda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, Priori SG, Richard S, Gomez AM. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009;104:201–209. doi: 10.1161/CIRCRESAHA.108.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc.Natl.Acad Sci U.S.A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, Gronau P, Maier LS, Vos MA, Lai FA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–59. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 62.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohamed U, Napolitano C, Priori SG. Molecular and electrophysiological bases of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2007;18:791–797. doi: 10.1111/j.1540-8167.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 64.Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522(Pt 2):259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikemoto N, Yamamoto T. Postulated role of inter-domain interaction within the ryanodine receptor in Ca(2+) channel regulation. Trends Cardiovasc Med. 2000;10:310–316. doi: 10.1016/s1050-1738(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 66.Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, Uchinoumi H, Okuda S, Oda T, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc Res. 2009;81:536–545. doi: 10.1093/cvr/cvn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amador FJ, Liu S, Ishiyama N, Plevin MJ, Wilson A, MacLennan DH, Ikura M. Crystal structure of type I ryanodine receptor amino-terminal beta-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc Natl Acad Sci USA. 2009;106:11040–11044. doi: 10.1073/pnas.0905186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lobo PA, Van Petegem F. Crystal structures of the N-terminal domains of cardiac and skeletal muscle ryanodine receptors: insights into disease mutations. Structure. 2009;17:1505–1514. doi: 10.1016/j.str.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 468:585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 70.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 71.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 72.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tiso N, Salamon M, Bagattin A, Danieli GA, Argenton F, Bortolussi M. The binding of the RyR2 calcium channel to its gating protein FKBP12.6 is oppositely affected by ARVD2 and VTSIP mutations. Biochem.Biophys.Res Commun. 2002;299:594–598. doi: 10.1016/s0006-291x(02)02689-x. [DOI] [PubMed] [Google Scholar]
- 74.George CH, Higgs GV, Lai FA. Ryanodine Receptor Mutations Associated With Stress-Induced Ventricular Tachycardia Mediate Increased Calcium Release in Stimulated Cardiomyocytes. Circulation Research. 2003;93:531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 75.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 106:1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao J, Tian X, Jones PP, Bolstad J, Kong H, Wang R, Zhang L, Duff HJ, Gillis AM, Fleischer S, Kotlikoff M, Copello JA, Chen SR. Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J Biol Chem. 2007;282:34828–34838. doi: 10.1074/jbc.M707423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med.Genet. 2005;42:863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raffaele di Barletta M, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG. Clinical phenotype and functional characterization of CASQ2 mutations associated with Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation. 2006;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 79.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Bigioggera M, Scelsi M, Villani L, Volpe P, Priori SG. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circulation Research. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 80.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bal NC, Sharon A, Gupta SC, Jena N, Shaikh S, Gyorke S, Periasamy M. The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization. J Biol Chem. 2010;285:17188–17196. doi: 10.1074/jbc.M109.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim E, Youn B, Kemper L, Campbell C, Milting H, Varsanyi M, Kang C. Characterization of human cardiac calsequestrin and its deleterious mutants. J Mol.Biol. 2007;373:1047–1057. doi: 10.1016/j.jmb.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 83.Kirchhefer U, Wehrmeister D, Postma AV, Pohlentz G, Mormann M, Kucerova D, Muller FU, Schmitz W, Schulze-Bahr E, Wilde AA, Neumann J. The human CASQ2 mutation K206N is associated with hyperglycosylation and altered cellular calcium handling. J Mol Cell Cardiol. 2010;49:95–105. doi: 10.1016/j.yjmcc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Valle G, Galla D, Nori A, Priori SG, Gyorke S, De FV, Volpe P. Catecholaminergic polymorphic ventricular tachycardia-related mutations R33Q and L167H alter calcium sensitivity of human cardiac calsequestrin. Biochem.J. 2008;413:291–303. doi: 10.1042/BJ20080163. [DOI] [PubMed] [Google Scholar]
- 85.de la Fuente S, Van Langen IM, Postma AV, Bikker H, Meijer A. A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin Electrophysiol. 2008;31:916–919. doi: 10.1111/j.1540-8159.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 86.Liu QQ, Oberti C, Zhang XQ, Ke T, Zhang T, Scheinman M, Hu DY, Wang QK. A Novel mutation of F189L in CASQ2 in families with catecholaminergic polymorphic ventricular tachycardia. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25:334–337. [PubMed] [Google Scholar]
- 87.Wong CH, Koo SH, She GQ, Chui P, Lee EJ. Genetic variability of RyR2 and CASQ2 genes in an Asian population. Forensic Sci Int. 2009;192:53–55. doi: 10.1016/j.forsciint.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen.Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, Viatchenko-Karpinski S, Bers DM, Williams SC, Volpe P, Gyorke S. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys.J. 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 91.Kubalova Z, Gyorke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, Gyorke S. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dirksen WP, Lacombe VA, Chi M, Kalyanasundaram A, Viatchenko-Karpinski S, Terentyev D, Zhou Z, Vedamoorthyrao S, Li N, Chiamvimonvat N, Carnes CA, Franzini-Armstrong C, Gyorke S, Periasamy M. A mutation in calsequestrin, CASQ2D307H, impairs Sarcoplasmic Reticulum Ca2+ handling and causes complex ventricular arrhythmias in mice. Cardiovasc Res. 2007;75:69–78. doi: 10.1016/j.cardiores.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sumitomo N, Harada K, Nagashima M, Yasuda T, Nakamura Y, Aragaki Y, Saito A, Kurosaki K, Jouo K, Koujiro M, Konishi S, Matsuoka S, Oono T, Hayakawa S, Miura M, Ushinohama H, Shibata T, Niimura I. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swan H, Laitinen P, Kontula K, Toivonen L. Calcium channel antagonism reduces exercise-induced ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia patients with RyR2 mutations. J Cardiovasc Electrophysiol. 2005;16:162–166. doi: 10.1046/j.1540-8167.2005.40516.x. [DOI] [PubMed] [Google Scholar]
- 96.Valdivia HH, Valdivia C, Ma J, Coronado R. Direct binding of verapamil to the ryanodine receptor channel of sarcoplasmic reticulum. Biophys.J. 1990;58:471–481. doi: 10.1016/S0006-3495(90)82392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2009;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu N, Denegri M, Ruan Y, Bachetti T, Seregni M, Napolitano C, Priori SG. Sodium Channel Blockers Prevent Triggered Activity but Not Abnormal Ca2+ Release in a Knock-in Mouse Model With Ryanodine Receptor Mutation R4496C. Circulation. 2010;122:A13707. [Google Scholar]
- 100.Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–438. [Google Scholar]
- 101.Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, Oda T, Kobayashi S, Ikemoto N, Matsuzaki M. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation. 2008;117:762–772. doi: 10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- 102.Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, Shimoni Y, Chen SR. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lehnart SE, Wehrens XHT, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden Death in Familial Polymorphic Ventricular Tachycardia Associated With Calcium Release Channel (Ryanodine Receptor) Leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 104.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 105.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG. Calmodulin kinase II inhibition prevents arrhythmias in RyR2R4496C+/− mice with catecholaminergic polymorphic ventricular tachycardia. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.10.001. in press. [DOI] [PubMed] [Google Scholar]