Abstract
Selective serotonin re-uptake inhibitors (SSRIs), which are used commonly to treat anxiety disorders, have characteristic anxiogenic effects following acute administration. Treatment with anxiolytic benzodiazepines (BZs) may reduce these effects, although little is known about potential drug interactions. Our study evaluated acute anxiogenic–like effects of SSRIs, alone and combined with a BZ. Adult male BALB/c mice received fluoxetine (3.0–30.0 mg/kg, i.p.) or citalopram (3.0–30.0 mg/kg, i.p.) alone or in combination with diazepam (0.3–10.0 mg/kg, i.p.), after which they were evaluated with the light/dark and open-field tests for anxiogenesis/anxiolysis. In addition, release of the stress hormone corticosterone was assessed following combined SSRI/BZ administration. In the light/dark and open-field tests, acute SSRIs produced a behavioral profile consistent with anxiogenesis, while diazepam produced an anxiolytic-like profile. Pre-treatment with diazepam (0.3–10 mg/kg) reversed the effects of an anxiogenic-like dose of an SSRI (18 mg/kg fluoxetine, 30 mg/kg citalopram) in both light/dark and open-field tests. Diazepam, fluoxetine or citalopram, and their combination all significantly increased plasma corticosterone levels to the same degree. These findings suggest that a BZ-type drug can attenuate acute anxiogenic-like effects of an SSRI via a mechanism independent of corticosterone regulation.
Keywords: SSRI, benzodiazepine, anxiety, corticosterone, mouse, light/dark, open field
1. Introduction
In patients with anxiety disorders, chronic treatment with selective serotonin reuptake inhibitors (SSRIs) can induce anxiolytic effects comparable to benzodiazepines (BZs), but lack the motor-impairing, amnestic, and abuse-related side effects associated with BZ-type anxiolytics (Baldwin et al., 2005; Bruce et al., 2003; Laux, 1992; Nutt, 2005). The anxiolytic effects of SSRIs emerge only after chronic treatment, and upon acute administration, these drugs often paradoxically increase symptoms of anxiety for some individuals (Bagdy et al., 2001; Nutt, 2005). The acute anxiogenic effect, combined with the relatively long therapeutic lag, may contribute to lack of compliance associated with SSRI treatment for anxiety disorders (cf. Nutt, 2005).
Consistent with the clinical literature, acute administration of SSRIs induce anxiogenic-like effects in preclinical models. For example, the SSRI fluoxetine decreases time spent in open arms of the elevated plus-maze in rats and mice (Kurt et al., 2000; Silva et al., 1999; Silva and Brandao, 2000), social interaction in rats (Bagdy et al., 2001), novel exploration by mice (Belzung et al., 2001), and time spent in the lit chamber of the light/dark test in mice (Artaiz et al., 1998). Acute administration of the SSRI citalopram decreases time spent in the open arms in the elevated plus-maze (Griebel et al., 1994); as well as spent in the lit chamber during the light/dark test in rodents, and increases fearful reactions in response to novel stimuli (Griebel et al., 1994; Sanchez and Meier, 1997). These findings are consistent with observations of acute anxiogenic effects of SSRIs in human patients; however, it is worth noting that in some pre clinical models, acute administration of SSRIs is also associated with anxiolytic effects. For example, SSRIs have shown effects consistent with anxiolysis after acute administration in the four-plate test (e.g., Hascoët et al., 2000).
In order to reduce the impact of the acute anxiogenic effects in clinical use, BZs are often co-prescribed with SSRIs, which some claim also results in faster onset of anxiolytic efficacy of the SSRI (Nutt, 2005). In fact, neurobiological studies suggest adaptations in both serotonergic and GABAergic systems (the target system for BZs) in anxiety disorders (for review, see Nikolaus et al. 2010). The concurrent use of BZs and SSRIs in the treatment of anxiety disorders has shown an increasing trend in recent years (Benitez et al., 2008; Bruce et al., 2003; Kaplan and DuPont, 2005). Despite this trend, limited quantitative data exist addressing the behavioral effects of acute treatment with SSRI/BZ combinations. Clinical studies have suggested that SSRI/BZ combinations can result in improved efficacy measures for treating psychiatric disorders (e.g., depression, Smith et al., 1998). In addition to the lack of data on efficacy, relatively little research is available about the potential side effects of SSRI/BZ combinations. Most studies report very few adverse events resulting from combined SSRI/BZ treatments (e.g., Smith et al., 1998); however, a meta-analysis on driving performance found that even anti-depressants considered to be non-sedating could result in driving impairment when combined with a BZ (Ramaekers, 2003).
Hypothalamic-pituitary-adrenal (HPA) axis activation and stress hormone release may play a role in SSRI-induced anxiogenesis. The serotonergic system has a well-documented role in HPA axis regulation (for review, see Carrasco and Van de Kar, 2003). Acute SSRI treatment has been shown to increase corticosterone (CORT) levels in rodents (Hesketh et al., 2005; Moncek et al., 2003; Sanchez and Kreilgaard, 2004). When tested in the presence of a stressor, acute treatment with citalopram resulted in enhanced CORT release in a rat restraint stressor paradigm (Hesketh et al., 2005). In contrast, BZ-type drugs can attenuate adrenocorticotropin hormone (ACTH), CORT or cortisol levels, particularly following exposure to an anxiogenic stressor (Carrasco and Van de Kar, 2003; Fries et al., 2006; Kalin et al., 1987; Kalman et al., 1997; Pivac and Pericic, 1993; Pomara et al., 2005). Paradoxically, BZs can also increase ACTH and CORT levels (e.g., McElroy et al., 1987; Mikkelsen et al., 2005). Therefore, blunted or reversed HPA axis activation is one potential mechanism by which BZs may attenuate acute SSRI-induced anxiogenesis, although the interaction between these two drugs might be complex, given the potential for BZs to enhance HPA activity.
This study evaluated the anxiogenic–like effects of acute SSRI administration alone and in combination with the reference BZ, diazepam, in BALB/c mice, a strain previously shown to be sensitive to anxiogenic-like and anxiolytic-like effects of several classes of drugs (Belzung et al., 2001; Bouwknecht and Paylor, 2002; Dulawa et al., 2004; Griebel et al., 2000; Piappert and Pilz, 2002; Roy et al., 2001). Two SSRIs, fluoxetine and citalopram, were chosen for comparison in these studies due to their differing selectivity for the SERT and interaction with cytochrome P450 enzyme metabolism. Fluoxetine is approximately 100 times more selective for SERT versus the norepinephrine transporter and the dopamine transporter (Nutt et al., 1999), whereas citalopram is approximately 10,000 times more selective for SERT compared to the norepinephrine transporter (Koch et al., 2002). In addition, SSRIs may have significant action at 5-hydroxytryptamine2C (5-HT2C) receptors, which has been shown particularly for fluoxetine (Ni and Miledi, 1997; Sakhner and Singewald, 2006). Metabolism of both SSRIs and BZs involves isozymes of the cytochrome P450 (CYP450) enzyme family. Fluoxetine binds to CYP2D6, an enzyme associated with BZ metabolism (Tanaka, 1998, Tanaka and Hisawa, 1999). Citalopram and its metabolites, however, do not interact with the enzymes associated with BZ metabolism (Sproule et al., 1997). Finally, we assessed changes in CORT following an SSRI/BZ combination in order to evaluate HPA axis activity as a potential mechanism of action underlying interactions between BZs and SSRIs.
2. Methods
2.1 Animals
All experiments and housing were approved by the University of Massachusetts-Amherst Institutional Animal Care and Use Committee and adhered to NIH guidelines for the care and use of animals. Adult (2–3 months of age) male, BALB/c mice, weighing ~20–30 g, were used in all experiments (Charles River Laboratories, Wilmington, MA). Mice were housed four per cage in Plexiglas cages under a 14-hour light/10-hour dark schedule. Food and water were freely available throughout the studies. In an effort to minimize the number of animals used, individual animals were tested in both open field and light/dark tests. Animals received a total of two sets of injections (one before each behavioral test) and were allowed to recover for at least 48 hours between experiments. All experiments were conducted during the light phase of the light-dark cycle.
For CORT measures, a separate cohort of adult male, BALB/c mice, weighing ~20–30 g, was used (Charles River Laboratories). Mice were socially housed (4–5 per cage) in Plexiglas cages under a 14-hour light/10-hour dark schedule. Food and water were freely available throughout the studies. To mimic the experience of the animals used in behavioral testing, mice in the CORT study were handling- and injection-naïve prior to removal from the colony room for testing. It should be noted that while this approach controlled for potential handling and injection effects on the behavioral findings, it also resulted in moderately elevated CORT values due to the animals’ responses to those same handling and injection procedures. Therefore, while these effects may play a part in the behavioral and physiological effects seen, they were controlled across all treatment conditions.
2.2. Drugs
All drugs were administered via intraperitoneal (i.p.) injection, 30 min prior to behavioral testing at 1.0 ml/kg injection volume. For experimental procedures requiring two injections, injections were administered bilaterally to minimize local injection volume. Fluoxetine (3.0–30.0 mg/kg; Tocris Biosciences, USA) and citalopram (3.0–30.0 mg/kg; Tocris Biosciences, USA) were dissolved in sterile saline. Diazepam (0.3–10.0 mg/kg; Sigma Biochemicals and Reagents, USA) was dissolved in 50% propylene glycol, 50% sterile water.
2.3. Behavioral Testing
All behavioral measures were collected following a 30-min drug pre-treatment period in a behavior testing room adjacent to the animal colony rooms.
Light/dark testing was conducted using Med Associates activity arenas (St. Albans, VT; 27.3 × 27.3 × 20.3 cm, ENV 515) equipped with infrared sources and sensors. Each arena was divided into two equally illuminated and darkened halves. A dark, opaque Plexiglas insert with lid was placed in the left half of each arena to create the darkened half. Testing arenas were placed inside sound-attenuating chambers, equipped with lights for constant illumination (~15–20 lux, 2–5 watt incandescent light bulb positioned 30.5 cm above the testing area to light the open half) and fans to provide ventilation and white noise. At the start of the test, animals were placed in the lit half of the arena, oriented randomly and allowed to move freely during the 10-min test session. Animals had access to the dark half of the arena through a small opening at floor level in the middle of the dark insert. Analysis of time spent in the light and dark areas was performed using Med Associates Activity Monitor software. Dependent measures included the percent of time spent in the lit half of the arena and distance traveled (cm).
Open field behavioral testing was conducted using the apparatus described above, with the dark Plexiglas insert removed. The open field test consisted of a 60-min test session in which an individual mouse was placed in the center of the testing arena inside the sound-attenuating chambers and allowed to move freely. After each session, activity analysis was conducted using Med Associates Behavior Monitor software to calculate the time spent in the center of the arena (~18.25×18.25 cm2, manufacturer specifications), the periphery of the arena (27×27 cm2 around the central area), and distance traveled (cm).
2.4. Corticosterone Measures
Time- and drug combination- response functions for the glucocorticoid hormone corticosterone were determined following drug administration. For all experiments; following injection (i.p.), animals were returned to home cages for the duration of the pre-treatment time. After the pre-treatment interval, animals were rapidly decapitated and trunk blood collected. Blood was centrifuged (1000 rpm, 15 min) and separated, and plasma was stored at −70°C until assay.
To determine the time of peak corticosterone response following SSRI administration in the time-response experiment, trunk blood samples were collected from groups of animals that received i.p. injections of 30 mg/kg CIT and were sacrificed at 0, 15, 30, 45, 60 min post-injection, . To determine drug combination-response, vehicle, diazepam (10.0 mg/kg), citalopram (30.0 mg/kg) and a diazepam/citalopram combination were administered (i.p) to groups of animals (n=5/group) and trunk blood collected at the optimal time point established in the time-reponse experiment.
CORT levels were determined using commercial radioimmunoassay kits (MP Biomedicals, Orangeburg, NY) designed for direct assay of samples without the need for prior extraction or purification (for additional methods, see Massoco and Palermo-Neto, 1999). CORT levels were obtained by averaging samples in triplicate and interpolating concentrations (ng/ml) from the standard curve. Average intra-assay and inter-assay coefficients were 5.2% and 4.0%, respectively. To control for the circadian rhythm of CORT release, all samples were collected between 10 and 11 am, approximately 5–6 h after light onset in the colony room. This time point was chosen based on the previous finding that the nadir of CORT release in BALB/c mice occurs 4–6 h after lights on (Oishi et al., 2006). Thus, the relatively low levels of CORT at this point in the circadian cycle minimized the possibility of a ceiling effect of elevated CORT following SSRI administration.
2.5. Statistical Analysis
Behavioral data were analyzed by a one-way analysis of variance (ANOVA) using SigmaPlot v. 11.0 software. Subsequent comparisons between control and treatment groups were conducted using the Holm-Sidak test. Alpha levels were constrained to p≤0.05 for all tests. CORT concentrations were interpolated from the standard curve using nonlinear regression analysis (Prism v.4). Data were then analyzed by a one-way ANOVA. Subsequent comparisons between control and treatment groups were conducted using the Holm-Sidak test.
3. Results
3.1. Tests with fluoxetine alone
Fluoxetine (figure 1A) dose-dependently decreased time spent in the lit compartment of the light/dark test [F (3, 39)=3.603 p<0.05], and multiple comparison tests revealed that the 30 mg/kg dose resulted in significantly less time spent in the lit compartment relative to control. A similar behavioral pattern was evident in the open field test (figure 1B). Fluoxetine significantly decreased time spent in the center of the testing arena [F (3, 39)=5.46, p<0.05], with multiple comparison tests showing that the 10 and 30 mg/kg groups were significantly different from control. Together, these data are consistent with an acute anxiogenic-like effect for these doses of fluoxetine in both the light/dark and open field tests.
Figure 1.
Effects of acute fluoxetine treatments on measures of anxiogenic-like behavior (light/dark test, panel A; open field test, panel B) and locomotor activity in male BALB/c mice. Data are mean ± SEM of behavioral measures following acute fluoxetine treatment (3–30 mg/kg i.p.) to separate groups of mice (N=10 per group). For the light/dark test, anxiogenic-like behavior was concluded if mice showed a significant decrease in time spent in the “aversive” lit side of the apparatus. For the open field test, anxiogenic-like behavior was concluded if mice spent significantly less time exploring the center of the apparatus. Locomotor activity was measured as distance traveled during a 10-min session (light/dark test, panel C) or a 60-min session (open field test, panel D). Note that *p<.05 vs. control, Holm-Sidak test.
Fluoxetine dose-dependently decreased distance traveled in the light/dark [figure 1C; F(3,39)=7.59, p<0.05] and open field tests [figure 1D, F(3,39)= 5.43, p<0.05], with significant reductions associated the highest dose in both tests (30 mg/kg; p<.05).
3.2. Tests with citalopram alone
Citalopram decreased time spent in the lit compartment of the light/dark test [figure 2A; F (3, 65)=4.337 p<0.05]. Multiple comparison tests revealed that this effect was not consistent with respect to dose. Time spent in the lit compartment with 3.0 and 30 mg/kg doses was below control level, and with the 10 mg/kg, not different from control level (all p<0.05). In the open field test, however, citalopram did not produce any significant effects at the doses tested. These findings suggest that although citalopram induced acute axiogenic-like effects similar to fluoxetine, the magnitude of effect for citalopram may be less than that of fluoxetine.
Figure 2.
Effects of acute citalopram treatments on measures of anxiogenic-like behavior (light/dark test, panel A; open field test, panel B) and locomotor activity in male BALB/c mice. Sample sizes were n=16 (control), n=8 (3 mg/kg dose), n=10 (10 mg/kg), and n=16 (30 mg/kg). Other details as in Figure 1.
In both the light/dark and open field tests, citalopram had little effect on distance traveled. An intermediate dose of citalopram (10 mg/kg) increased distance traveled in the light/dark test (figure 2C; 10 mg/kg vs. control, p<0.05). No significant effect was observed for any dose tested in the open field test (figure 2D).
3.3. Tests with diazepam alone
In the light/dark test, diazepam significantly increased time in light [Figure 3A, F (3, 47)=3.394, p<0.05] at 1.0 and 3.0 mg/kg doses (p<.05). In the open field test, 10 mg/kg diazepam produced a non-significant increase in time spent in the center of the open field arena (figure 3B; p=0.07).
Figure 3.
Effects of acute diazepam treatments on measures of anxiolytic-like behavior (light/dark test, panel A; open field test, panel B) and locomotor activity in male BALB/c mice. Data are mean ± SEM of behavioral measures following acute diazepam treatment (0.3–10 mg/kg i.p.) to separate groups of mice. The sample sizes were n=14 (control), n=10 (0.3 mg/kg), n=13 (1.0 mg/kg), and n=14 (3.0 mg/kg). For the light/dark test, anxiolytic-like behavior was concluded if mice showed a significant increase in time spent in the “aversive” lit side of the apparatus. For the open field test, anxiolytic-like behavior was concluded if mice spent significantly more time exploring the center of the apparatus. Locomotor activity was measured as distance traveled during a 10-min session (light/dark test, panel C) or a 60-min session (open field test, panel D). Note that *p<.05 vs. control, Holm-Sidak test.
In the light/dark tests, diazepam had no effect on locomotor activity over the dose range tested (figure 3C, ANOVA and multiple comparison tests, p’s>0.05). In the open field test, diazepam significantly decreased distance traveled [figure 3D; F(3,47)= 6.77, p<0.05] at the highest dose tested (10 mg/kg; p<.05).
3.4. Effects of diazepam-fluoxetine combinations
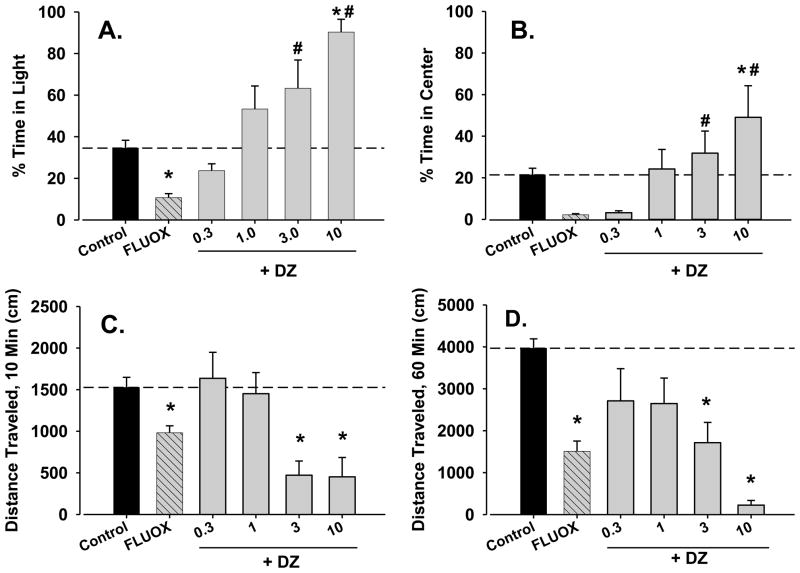
The results of combined fluoxetine and diazepam treatment are shown in figure 4. For this experiment, a representative 18 mg/kg dose of fluoxetine was selected to minimize severe locomotor activity suppression that was anticipated with a combination of diazepam and the highest fluoxetine dose (30 mg/kg). The 18 mg/kg dose of fluoxetine significantly decreased time in light in the light/dark test (figure 4A, compare bars above “control” and “FLUOX”).
Figure 4.
Reversal of anxiogenic-like and locomotor-suppressant effects of fluoxetine (FLUOX) by diazepam (DZ) in male BALB/c mice. Data are mean ± SEM of behavioral measures following acute fluoxetine treatment (18 mg/kg, i.p.), alone and combined with several doses of diazepam (0.3–10 mg/kg, i.p.) to separate groups of mice. Sample sizes were n=8 (control), n=8 (fluoxetine alone), n=6 (0.3 mg/kg diazepam + fluoxetine), n=8 (1.0 mg/kg diazepam + fluoxetine; 3.0 mg/kg diazepam + fluoxetine; 10 mg/kg diazepam + fluoxetine). Note that *p<.05 vs. control and #p<0.05 vs. fluoxetine alone, Holm-Sidak test.
As can be seen in figure 4A, treatment with diazepam significantly altered fluoxetine’s effects in the light/dark test [F(5,38)=4.89, p<0.05]. Specifically, the addition of diazepam attenuated (figure 4A, compare 0.3 and 1.0 mg/kg diazepam combined with fluoxetine vs. fluoxetine alone), and then reversed (3 and 10 mg/kg diazepam plus fluoxetine) the decrease in time in light induced by 18 mg/kg fluoxetine. Stated another way, the 3.0 and 10 mg/kg doses of diazepam combined with an anxiogenic-like dose of fluoxetine still engendered effects consistent with anxiolysis.
Fluoxetine induced a strong trend toward decreasing time in center in the open-field test (figure 4B, p=0.06). Interestingly, the addition of diazepam dose-dependently increased time in center [F(5,38)=5.11, p<0.05]. As with the light/dark test, anxiolytic-like effects of fluoxetine-diazepam combined were revealed when fluoxetine was combined with the highest dose of diazepam tested (10 mg/kg, p<0.05).
A similarly complex pattern of effects was observed with locomotor activity in the light/dark test [Figure 4C, F(5,38)=8.34, p<0.05]. Thus, 18 mg/kg of fluoxetine significantly decreased mean distance traveled, but this effect was reversed by the two lowest doses of diazepam (0.3 and 1.0 mg/kg). Interestingly, both 3.0 and 10 mg/kg of diazepam combined with fluoxetine suppressed mean distance traveled compared with control—the dose of 3.0 mg/kg of diazepam did not alter distance traveled with tested alone (compare Figure 4C with Figure 2C).
In the open field test, a similar pattern of locomotor activity was observed [figure 4D, F(5,38)=4.97, p<0.05]. That is, fluoxetine alone significantly decreased locomotor activity, and doses of diazepam first attenuated fluoxetine-induced decreases in locomotor activity, and then resulted in attenuation of locomotor activity compared with control for the two highest doses of diazepam (3 and 10 mg/kg).
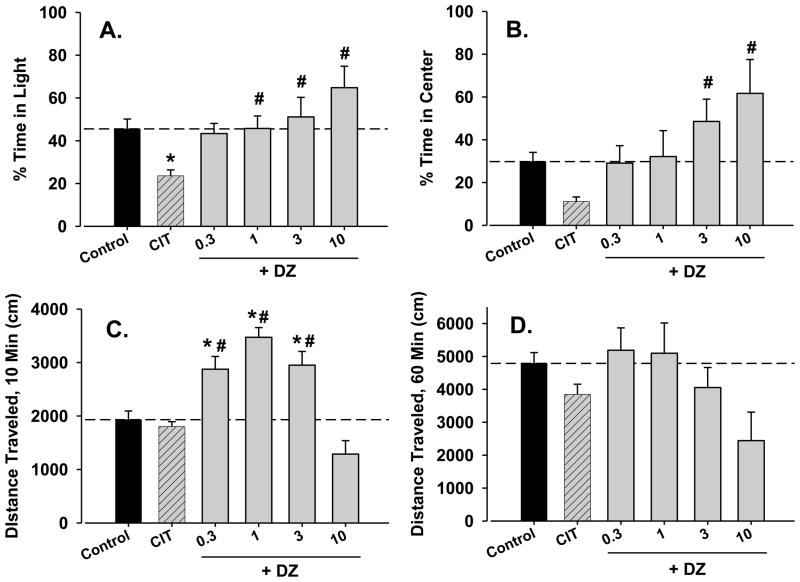
3.5. Effects of diazepam-citalopram combinations
Similar to fluoxetine-diazepam combinations, the addition of diazepam attenuated the decrease in time in light induced by 30 mg/kg citalopram [figure 5A, F(5,38)=5.54, p<0.05]. In contrast to fluoxetine-diazepam combinations, diazepam reversed the effects of citalopram but did not induce anxiolytic-like effects at the higher doses. A very similar pattern of results was obtained with the open field test [figure 5B, F(5,38)=5.01, p<0.05], although for this test, the effects of citalopram alone approached, but did not achieve statistical significance.
Figure 5.
Reversal of the anxiogenic-like effects of citalopram (CIT) and increased locomotor activity by combination with diazepam (DZ) in male BALB/c mice. Data are mean ± SEM of behavioral measures following acute citalopram treatment (30 mg/kg, i.p.), alone and combined with several doses of diazepam (0.3–10 mg/kg, i.p.) to separate groups of mice. Sample sizes were n=6 (control), n=7 (citalopram alone, 0.3 mg/kg diazepam + citalopram), and n=8 (1.0 mg/kg diazepam + citalopram, 3.0 mg/kg diazepam + citalopram). Note that *p<.05 vs. control and #p<0.05 vs. citalopram alone, Holm-Sidak test.
In the light/dark test, locomotor activity changes as a result of citalopram-diazepam combinations were strikingly different from those observed with fluoxetine-diazepam combinations [figure 5C, F(5,38)=7.44, p<0.05]. As shown in figure 2C, citalopram did not alter locomotor activity when tested alone. However, the addition of 0.3, 1, 3 mg/kg diazepam to 30 mg/kg citalopram in the light/dark test significantly increased distance traveled (figure 5C, p’s<0.05). No significant changes in locomotor activity were seen at the doses tested in the open field test (figure 5D).
3.6. Effects on corticosterone levels
As shown in figure 6A, administration of 18 mg/kg fluoxetine, 10 mg/kg diazepam, and the combination of these doses resulted in significantly increased levels of CORT compared with the saline control [F(3,20)=13.51, p<0.05]. The combination of fluoxetine and diazepam was not significantly different from either drug alone. Similarly, administration of 30 mg/kg citalopram, 10 mg/kg diazepam, and the combination of these doses resulted in significantly increased levels of CORT compared with the saline control [figure 6B, F(3,16)=20.350, p<0.05], and the combination of citalopram and diazepam was not significantly different from either drug alone. These results suggest that both SSRIs and BZs may activate the HPA axis in our model, regardless of the behavioral response characterized as anxiogenic versus anxiolytic, and no interaction between diazepam and citalopram was evident.
Figure 6.
Effects of fluoxetine (FLUOX, panel A) and citalopram (CIT, panel B), alone and combined with diazepam (DZ), on plasma corticosterone levels in male BALB/c mice. Data are mean ± SEM of plasma corticosterone (ng/ml) response following i.p. injection for n=5 per group (panel A) or n=6 per group (panel B). Note that *p<.05 vs. control, Holm-Sidak test.
4. Discussion
The results of the present study indicate that acute treatment with the SSRIs fluoxetine and citalopram produced primarily anxiogenic-like effects in the light/dark and open field tests in BALB/c mice. In contrast, acute treatment with diazepam, a standard BZ, resulted in anxiolytic-like effects (i.e., an increase in time spent in the lit compartment). The combination of the SSRIs with diazepam resulted in an attenuation of the anxiogenic-like behavior induced by acute SSRI treatment, and in the case of fluoxetine combined with diazepam, the highest dose of the latter resulted in anxiolytic-like effects in both light/dark and open-field tests. The effects of these drug combinations on locomotor activity were complex, with low but not higher doses of diazepam attenuating the locomotor activity-impairing effects of fluoxetine, whereas stimulation of locomotor activity was observed with diazepam-citalopram combinations. A cautionary note about these studies is that the same mice were used in both tests of anxiogenic/anxiolytic-like effects. Initial tests might influence the results of a second test (Holmes et al., 2001); however, the overall pattern of results was similar between tests, suggesting that repeated testing had minimal influence on the results of our studies.
With acute administration, both SSRIs alone induced anxiogenic-like behavior in the light/dark tests, whereas only fluoxetine induced anxiogenic-like effects in the open field test. While it is unclear why fluoxetine and citalopram differed in these two procedures, the preponderance of data was consistent with the observation of acute anxiogenic effects associated with SSRI treatments in humans (Bagdy et al., 2001; Nutt, 2005). Interestingly, the locomotor activity data differed between the two SSRIs, with fluoxetine engendering decreases and citalopram generally without effect except for a significant increase in activity at one dose (10 mg/kg). Both SSRIs are generally considered to be non-sedating (e.g., Ramaekers, 2003), thus, our findings were somewhat surprising. Although we do not know the mechanism responsible for suppression of locomotor activity by SSRIs, one intriguing possibility is raised by reports that some SSRIs increase levels of endogenous neuroactive steroids, some of which bind to GABAA receptors and act as positive allosteric modulators of GABA (Serra et al., 2001) which, in turn, would have profound suppressive effects on locomotor behavior. Regardless, our findings suggest that both light/dark and open field tests in BALB/c mice represent a useful technique for assessing anxiogenic-like effects of serotonergic drugs.
In contrast to SSRIs, the BZs act as positive allosteric modulators of the GABAA receptor, producing anxiolytic and sedative effects via positive modulation of GABA-mediated chloride conductance (see Rudolph and Möhler, 2004 for review). In the present study, diazepam significantly increased time spent in the lit compartment in the light/dark test, but was ineffective in the open field test. Anxiolytic-like behavior (in the light/dark and open field tests) in response to BZ treatment has been well-established in the literature (e.g., Bourin and Hascoet, 2003; Cryan and Holmes, 2005; Prut and Belzung, 2003). Thus, the finding that diazepam was ineffective in the open field test might indicate a difference in sensitivity between the light/dark and open field tests, as suggested previously by Prut and Belzung (2003).
When doses of the SSRIs that induced anxiogenic-like effects were combined with diazepam, a dose-dependent reversal of anxiogenic-like behavior was observed. Moreover, when combined with fluoxetine, higher doses of diazepam induced anxiolytic-like effects in both light/dark and open field tests. Altogether, our findings from two preclinical animal models suggest that diazepam can reverse acute anxiogenic effects of SSRIs. Our results lend support to the practice of using anxiolytic BZs to reduce anxiogenesis associated with the early period of SSRI treatment.
Of particular interest were the interactions between diazepam and the SSRIs in tests for locomotor activity. As noted above, fluoxetine alone attenuated locomotor activity, and the combination with diazepam appeared to enhance this effect (i.e., the dose-response function appeared to be shifted to the left). In contrast, citalopram had predominantly a stimulant-like effect that was significantly enhanced by combination with diazepam—an unexpected finding for which no clear mechanisms are apparent. To the extent that decreases in locomotor activity induced by SSRIs or BZs are predictive of sedative properties of drugs in humans, these findings suggest that diazepam may have a deleterious effect on any sedative-motor properties of fluoxetine, but not citalopram. The mechanism(s) of action underlying these strikingly different effects on locomotor behavior between fluoxetine and citalopram are unclear at present; one possibility might be differences between the two SSRIs in interactions with the 5-HT2C site (Ni and Miledi, 1997; Sakhner and Singewald, 2006).
In an initial attempt at understanding the mechanisms of action underlying the ability of diazepam to reverse anxiogenic effects of SSRIs, we investigated the role of the HPA axis by evaluating plasma levels of the stress hormone, CORT, following diazepam-SSRI treatments. CORT levels were increased in response to an anxiogenic-like dose of both fluoxetine and citalopram. However, these levels also were increased in response to an anxiolytic-like dose of diazepam alone. These results replicate previous findings that acute SSRI or BZ treatment alone can increase stress hormone levels, with BZs often showing biphasic effects (De Souza, 1990; McElroy et al., 1987; Pericic et al., 1984; Vargas et al., 2001).
When fluoxetine or citalopram were combined with diazepam, CORT levels were again increased significantly; however, no further enhancement was evident (i.e., no additive or supra-additive effects were apparent). Because the CORT levels were collected at the approximate circadian time of the behavioral tests, these findings suggest that changes in HPA axis—or at least CORT level regulation—do not underlie the ability of diazepam to attenuate the acute anxiogenic-like effects of SSRIs.
A potentially important factor that may contribute to the mechanism of action of diazepam’s reversal of SSRI effects is pharmacokinetic changes in the activity of hepatic metabolic enzymes. In this regard, fluoxetine inhibits liver CYP2C19 and CYP3A4 isozymes, which are responsible in part for diazepam metabolism (Tanaka, 1998, Tanaka and Hisawa, 1999). By inhibiting metabolism, fluoxetine may increase bioavailability of diazepam, thereby enhancing its behavioral effects. However, citalopram is not known to affect BZ metabolism and the combined effects of citalopram and diazepam were qualitatively similar to those of fluoxetine and diazepam. Interestingly, the ability of diazepam to reverse citalopram’s effects in the anxiolysis tests was somewhat less robust. This modest difference in effectiveness may reflect a pharmacokinetic interaction between fluoxetine and diazepam that did not occur between citalopram and diazepam. Regardless, the interactive effects of the SSRIs with diazepam in the present study likely were not due solely to pharmacokinetic factors.
Altogether, these results suggest that the SSRIs may induce behavioral and locomotor changes to varying degrees following acute administration, even though their mechanism of action is similar. The anxiogenic-like effects of SSRIs can be attenuated or reversed with the addition of a BZ. While HPA axis activation is not supported as a relevant mechanism of action in the current studies, further research into other components of this system is warranted (e.g., CRH, ACTH). Finally, the contributions of specific serotonergic and GABA-ergic receptor subtypes in these SSRI/BZ interactions remain to be evaluated.
Acknowledgments
This research was supported by USPHS grants DA011792 and RR000168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: Mouse strain-dependent outcomes. Beh Neurosci. 2001;115(2):443–54. [PubMed] [Google Scholar]
- Artaiz I, Zazpe A, Del Rio J. Characterization of serotonergic mechanisms involved in the behavioural inhibition induced by 5-hydroxytryptophan in a modified light-dark test in mice. Behav Pharm. 1998;9:103–12. [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharm. 2001;4(4):399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JRT, den Boer JA, Fineberg NA, Knapp M, Scott J, Wittchen HU. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharm. 2005;19(6):567–96. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Barreau S, Calataynd F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12(3):151–62. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Benitez CI, Smith K, Vasile RG, Rende R, Edelen MO, Keller MB. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;6(1):5–13. doi: 10.1097/JGP.0b013e31815aff5c. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Vasile RG, Goisman RM, Salzman RM, Spencer M, Machan JT, et al. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia? Am J Psychiatry. 2003;160:1432–8. doi: 10.1176/appi.ajp.160.8.1432. [DOI] [PubMed] [Google Scholar]
- Cassasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Cyran JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9):775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmology. 2004;29:1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–70. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology. 1994;113:463–70. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Hascoët M, Bourin M, Colombel MC, Fiocco AJ, Baker GB. Anxiolytic-like effects of antidepressants after acute administration in a four-plate test in mice. Pharmacol Biochem Behav. 2000;65:339–344. doi: 10.1016/s0091-3057(99)00191-4. [DOI] [PubMed] [Google Scholar]
- Hesketh S, Jessop DS, Hogg S, Harbuz MS. Differential actions of acute and chronic citalopram on the rodent hypothalamic-pituitary-adrenal axis response to acute restraint stress. J Endocrinol. 2005;185:373–82. doi: 10.1677/joe.1.06074. [DOI] [PubMed] [Google Scholar]
- Holmes A, Iles JP, Mayell SJ, Rodgers RJ. Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res. 2001:159–167. doi: 10.1016/s0166-4328(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Separation distress in infant rhesus monkeys: effects of diazepam and Ro 15-1788. Brain Res. 1987;408:192–8. doi: 10.1016/0006-8993(87)90371-4. [DOI] [PubMed] [Google Scholar]
- Kaplan EM, DuPont RL. Benzodiazepines and anxiety disorders: a review for the practicing physician. Cur Med Res Opinion. 2005;21(6):941–50. doi: 10.1185/030079905X48401. [DOI] [PubMed] [Google Scholar]
- Kim D, Jung J, Song D, Suh H, Huh S, Kim Y. Intracerebroventricular injection-induced increase in plasma corticosterone levels in the mouse: A stress model. J Pharmacol Toxicol Methods. 1998;39:71–3. doi: 10.1016/s1056-8719(97)00105-6. [DOI] [PubMed] [Google Scholar]
- Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FP. R-fluoxetine increases extracellular DA, NE as well as 5-HT in rat prefrontal cortex and hypothalamus: An in vivo microdialysis and receptor binding study. Neuropsychopharmacology. 2002;27(6):949–59. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Kurt M, Arik AC, Celik S. The effects of sertraline and fluoxetine on anxiety in the elevated plus maze in mice. J Clin Physiol Pharmacol. 2000;11(2):173–80. doi: 10.1515/jbcpp.2000.11.2.173. [DOI] [PubMed] [Google Scholar]
- Laux G. The role of non-benzodiazepine agents in the treatment of anxiety and depression. In: Stahl SM, editor. Serotonin 1A receptors and depression and anxiety. New York: Raven Press, Ltd; 1992. [Google Scholar]
- McElroy JF, Miller JM, Meyer JS. Comparison of the effects of chlordiazepoxide and CL 218,872 on serum corticosterone concentrations in rats. Psychopharmacology. 1987;91(4):467–72. doi: 10.1007/BF00216012. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Søderman A, Kiss A, Mirza N. Effects of benzodiazepine receptor agonists on the hypothalamic-pituitary-adrenocortical axis. Eur J Pharmacol. 2005;519:223–230. doi: 10.1016/j.ejphar.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Jezova D. Repeated citalopram treatment but not stress exposure attenuates hypothalamic-pituitary-adrenocortical axis response to acute citalopram injection. Life Sci. 2003;72(12):1353–65. doi: 10.1016/s0024-3205(02)02409-8. [DOI] [PubMed] [Google Scholar]
- Ni YG, Miledi R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac) Proc Natl Acad Sci USA. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders—results from in vivo imaging studies. Rev Neurosci. 2010;21:119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- Nutt D. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectrums. 2005;10(1):49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Forshall S, Bell C, Rich A, Sandford J, Nash J, Argyropoulos S. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur Neuropharm. 1999;9:S81–6. doi: 10.1016/s0924-977x(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Odio MR, Maickel RP. Comparative biochemical responses of rats to different stressful stimuli. Physiol Behav. 1985;34:595–9. doi: 10.1016/0031-9384(85)90054-x. [DOI] [PubMed] [Google Scholar]
- Piappert CF, Pilz PKD. Difference in anxiety and sensitization of the acoustic startle response between the two inbred mouse strains BALB/c and DBA/2N. Genes Brain Behav. 2002;1:178–86. doi: 10.1034/j.1601-183x.2002.10306.x. [DOI] [PubMed] [Google Scholar]
- Pomara N, Willoughby LM, Sidtis JJ, Cooper TB, Greenblatt DJ. Cortisol response to DZ: its relationship to age, dose, duration of treatment, and presence of generalized anxiety disorder. Psychopharmacology. 2005;178:1–8. doi: 10.1007/s00213-004-1974-8. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG. Antidepressants and driver impairment: Empirical evidence from a standard on-the-road test. J Clin Psychiatry. 2003;64:20–29. [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA. 2005;102(3):915–20. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V, Belzung C, Delarue C, Chapillon P. Environmental enrichment in BALB/c mice Effects in classical tests of anxiety and exposure to a predatory odor. Physiol Behav. 2001;74:313–20. doi: 10.1016/s0031-9384(01)00561-3. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Ann Rev Pharmacol Toxicol. 2004;44:475–98. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Salchner P, Singewald N. 5-HT receptor subtypes involved in the anxiogenic-like action and associated Fos response of acute fluoxetine treatment in rats. Psychopharmacology. 2006;185:282–288. doi: 10.1007/s00213-005-0247-5. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Kreilgaard M. R-citalopram inhibits functional and 5-HTP-evoked behavioural responses to the SSRI, escitalopram. Pharmacol Biochem Behav. 2004;77:391–8. doi: 10.1016/j.pbb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression: Are they all alike? Psychopharmacology. 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Muggironi M, Parodo V, Papi G, Sari R, Dazzi L, Spiga F, Purdy RH, Biggio G. Opposite effects of short- versus long-term administration of fluoxetine on the concentrations of neuroactive steroids in rat plasma and brain. Psychopharmacology. 2001;158:48–54. doi: 10.1007/s002130100853. [DOI] [PubMed] [Google Scholar]
- Silva MTA, Alves CRR, Santarem EMM. Anxiogenic-like effect of acute and chronic fluoxetine on rats tested on the elevated plus-maze. Braz J Med Biol Res. 1999;32(2):333–9. doi: 10.1590/s0100-879x1999000300014. [DOI] [PubMed] [Google Scholar]
- Silva RC, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacol Biochem Behav. 2000;65(2):209–16. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Smith WT, Londborg PD, Glaudin V, Painter JR. Short-term augmentation of fluoxetine with clonazepam in the treatment of depression: A double-blind study. Am J Psychiatry. 1998;155:1339–1345. doi: 10.1176/ajp.155.10.1339. [DOI] [PubMed] [Google Scholar]
- Sproule BA, Naranjo CA, Bremner KE, Hassan PC. Selective serotonin reuptake inhibitors and CNS drug interactions. Clin Pharmacokinet. 1997;33:454–71. doi: 10.2165/00003088-199733060-00004. [DOI] [PubMed] [Google Scholar]
- Tanaka E. Clinically important pharmacokinetic drug-drug interactions: role of cytochrome P450 enzymes. J Clin Pharm Ther. 1998;23:403–16. doi: 10.1046/j.1365-2710.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Hisawa S. Clinically significant pharmacokinetic drug interactions with psychoactive drugs: antidepressants and antipsychotics and the cytochrome P450 system. J Clin Pharm Ther. 1999;24:7–16. doi: 10.1046/j.1365-2710.1999.00200.x. [DOI] [PubMed] [Google Scholar]