Abstract
Background
The objective of this study was to investigate changes in active and passive biomechanical properties of the calf muscle-tendon unit induced by controlled ankle stretching in stroke survivors.
Methods
Ten stroke survivors with ankle spasticity/contracture and ten healthy control subjects received intervention of 60-min ankle stretching. Joint biomechanical properties including resistance torque, stiffness and index of hysteresis were evaluated pre- and post-intervention. Achilles tendon length was measured using ultrasonography. The force output of the triceps surae muscles was characterized via the torque-angle relationship, by stimulating the calf muscles at a controlled intensity across different ankle positions.
Findings
Compared to healthy controls, the ankle position corresponding to the peak torque of the stroke survivors was shifted towards plantar flexion (P<0.001). Stroke survivors showed significantly higher resistance torques and joint stiffness (P<0.05), and these higher resistances were reduced significantly after the stretching intervention, especially in dorsiflexion (P = 0.013). Stretching significantly improved the force output of the impaired calf muscles in stroke survivors under matched stimulations (P<0.05). Ankle range of motion was also increased by stretching (P<0.001).
Interpretation
At the joint level, repeated stretching loosened the ankle joint with increased passive joint range of motion and decreased joint stiffness. At the muscle-tendon level, repeated stretching improved calf muscle force output, which might be associated with decreased muscle fascicle stiffness, increased fascicle length and shortening of the Achilles tendon. The study provided evidence of improvement in muscle tendon properties through stretching intervention.
Keywords: Stroke, Ultrasound, Muscle tendon, Spasticity, Triceps surae muscles
INTRODUCTION
Ankle joint mechanical properties are important in daily ambulation and in functional activities. Deterioration in ankle joint motion, such as reduced range of motion and tight heel cords could result in clumsy gait patterns with excessive energy cost (Perry, 1992; Winters et al., 1987). Passive ankle stretching has been used in both clinical settings and the fields of sports and its effects on characteristics, such as stretch reflex, maximum voluntary contraction, joint stiffness and gait, have been documented (Avela et al., 1999; Bohannon and Larkin, 1985; Cornwell et al., 2002; Kubo et al., 2001; Mahieu et al., 2007; Selles et al., 2005; Zhang et al., 2002). In the clinic, therapists use passive stretching to maintain and/or improve the range of motion and to reduce contracture (Bohannon and Larkin, 1985; Dombovy et al., 1986; Moseley, 1997). For instance, reduced ankle joint stiffness has been shown after either static or cyclic stretching (Bressel and McNair, 2002; Roy et al., 1998; Svantesson and Sunnerhagen, 1997; Svantesson et al., 2000).
Muscle is a dynamic organ capable of significant remodeling and adapting to external loading environments such as tension and length changes. An animal study on cat’s soleus has shown that the number of sarcomeres in series increased with muscle immobilized in lengthened position while the number dropped when set at shortened position (Tabary et al., 1972). In addition, the sarcomere length was altered due to the change in sarcomere number in immobilized muscle. A study on mice showed that when adult muscle was set in lengthened position more sarcomeres will be added and result in shorter sarcomere length (Williams and Goldspink, 1978). Passive stretching may also affect biomechanical properties of muscles and tendons (Zhao et al., 2010). The positive impact of passive stretching on muscle fascicles has been attributed to a chain of protein-protein interactions leading ultimately to myofibrillogenesis (De Deyne, 2001). The application of stretch to treat and even prevent contractures is supported by previous animal studies (Harvey and Herbert, 2002) and human studies and it is suggested that a minimum of six hours stretching per day be sufficient to prevent progressive soleus contracture (Tardieu et al., 1988). Animal studies on immobilized mouse soleus muscle suggested a shorter intervention period (i.e. 15 min of stretch per day) to fight against the degraded muscle adaptation (Williams, 1988; Williams, 1990). In addition, passive stretching may affect biomechanical properties of tendon such as increasing its stiffness (de Almeida et al., 2009a; Zhao et al., 2010). Continuous passive motion and prolonged stretching have each been reported to be effective in reducing passive ankle joint stiffness (Bressel and McNair, 2002; McNair et al., 2001). Motorized devices have been used for quantitative evaluations as well as controlled stretching interventions (Yeh et al., 2004; Zhang et al., 2002). Specifically, stretching under intelligent control has been used effectively in treating ankle contracture and/or spasticity in stroke survivors, and this intervention has reduced ankle joint resistance torque, stiffness, and increased range of motion (Selles et al., 2005; Zhang et al., 2002). So far, most of the studies focused on mechanical properties of the ankle joint and did not examine the underlying changes of active and passive muscle-tendon properties which are closely related to changes at the joint level.
The objective of this study was to investigate in vivo the biomechanical changes of the calf muscle-tendon unit induced by passive ankle stretching in stroke survivors. The patients were treated using an ankle stretching device with intelligent control (Zhang et al., 2002), and the characteristics of muscle-tendon were evaluated pre- and post-intervention using the ankle stretching device and ultrasonography. Electrical stimulation was used to activate the calf muscles and establish the torque-angle relationship pre-and post-intervention. Stretching-induced changes were characterized in terms of both the muscle-tendon and ankle joint biomechanical properties. Healthy control subjects were also evaluated similarly without intervention to quantify in vivo biomechanical changes of the calf muscle-tendon unit post stroke.
METHODS
Subjects
Ten stroke survivors (2 females and 8 males; age: 55.2±9.9 year; body mass: 81.9±11.2 kg; height: 174.1±11.2 cm; shank length: 38.6±1.4 cm; stroke duration: 107.3±40.0 months; affected side (left/right): 3/7; walking aids [ankle foot orthoses (AFOs)] (w./w.o.): 8/2) and ten age-matched healthy subjects (3 female and 7 male; age: 53.4±18.1 year; body mass: 70.0±14.5 kg; height: 170.0±9.0 cm; shank length: 38.3±1.9 cm) without any neurological disorder participated in the study. All subjects gave informed consent, which was approved by the Institution Review Board. Muscle tone of the plantar flexors in stroke survivors was estimated using the Modified Ashworth Score (2.57±0.58).
Experimental Setup
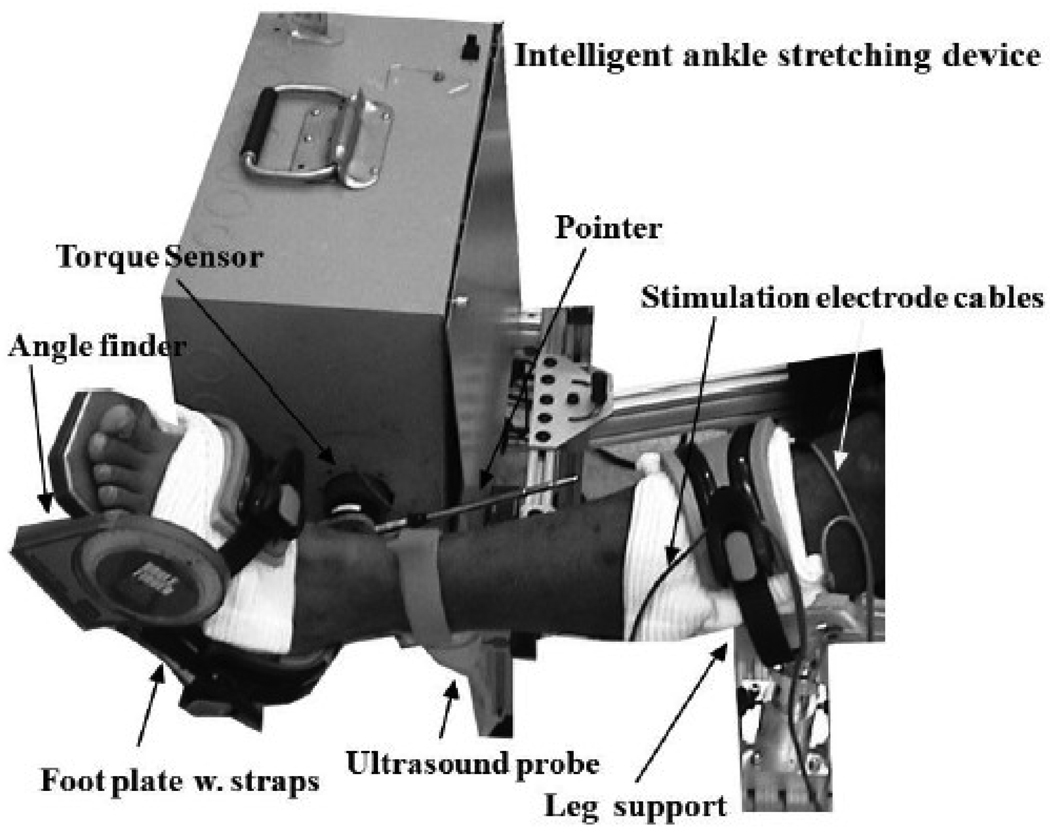
An ankle stretching device under intelligent control (the stretching velocity was inversely proportional to the joint resistance torque) (Fig. 1) was used for intervention and functional evaluation (Zhang et al., 2002). While the subject was comfortably seated, the leg was strapped to a leg support with the knee flexed to 30°. The foot was secured to a footplate at the dorsal side and at the heel using adjustable straps. The footplate was fixed to the motor shaft, and a torque sensor was aligned with the motor shaft to measure ankle torque. The footplate was adjusted to align the ankle flexion axis with the motor shaft. A telescoping pointer was used to align the long axis of the leg with the device and determine the 0° ankle dorsiflexion (Fig. 1). The ankle stretching device was clamped to the chair to avoid movement of the device during stretching. A Compex™ programmable electrical stimulator (Compex®, CA, USA) was used to activate the calf muscles, the gastrocnemius and soleus simultaneously. Paired self-adhesive electrode pads (bipolar configuration), connected to the stimulator, were used to apply the electrical stimulation and induce one contraction each second (1 Hz). Both mechanical stops and electric limit switches were used to restrict the motor ROM within a physiologic range. In addition, a handheld stop switch was used for the subject to terminate the motor movement at anytime during the experiment. The subject was asked to relax and not to react to the stretch during the stretching intervention.
Figure 1.
Experimental setup.
Protocol
The length of the lower leg and height of foot (distance from the bottom of foot to the lateral malleolus) were measured in order to properly adjust the setup. The skin was cleaned before attaching the electrode pads. The motor points of the gastrocnemius and soleus muscles were determined by palpation of the tendon/muscle while carefully searching for the most sensitive point for stimulation-induced contraction. The amplitude of stimulation was adjusted for each muscle and each subject, due to subject-dependent thresholds, in order to gain a measurable level of torque (>0.1 Nm) about the ankle joint. Each stimulation period lasted 30 seconds and there were approximately 30 stimulation-induced contractions per trial. Electrical stimulation was applied at various ankle positions with an increment of 10 degrees covering the entire passive range of motion (40° plantar flexion to 30° dorsiflexion for control; 30° plantar flexion to 20° dorsiflexion for stroke) right before the stretching intervention and repeated after the intervention. Subjects were asked to relax as electrical stimulation was applied. The electrodes were kept at the same spots throughout the test to maintain consistent stimulation.
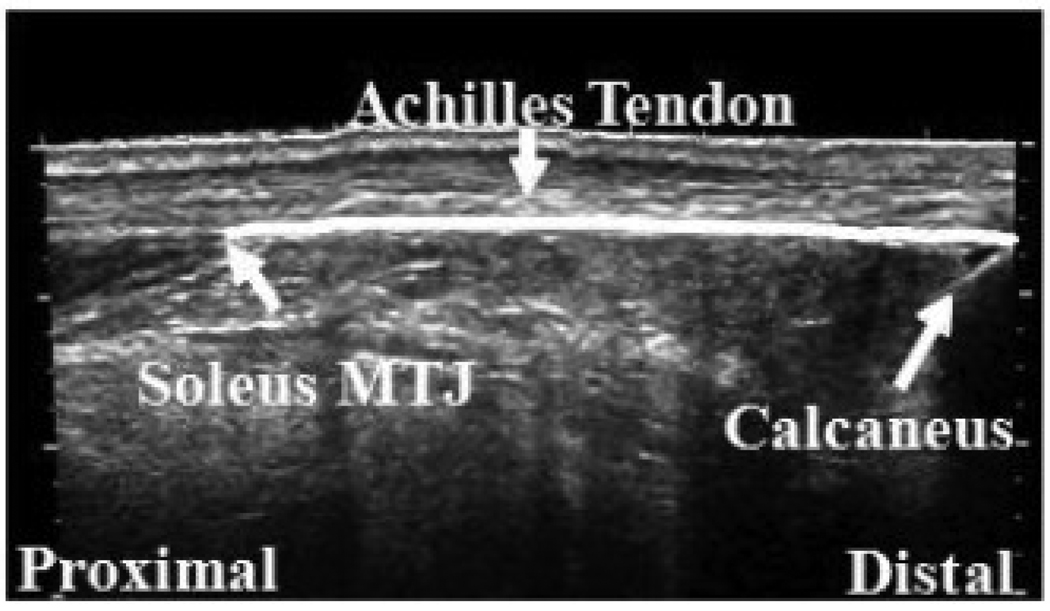
At the beginning of the test, the subject was asked to relax and the resting torque and position were recorded as the initial baseline. The length of the Achilles tendon (AT), from the insertion to the calcaneus notch to the soleus muscle-tendon junction (MTJ), was measured using a GE ultrasound machine (GE LOGIQ-9, Waukesha, WI, USA) operating in the LogiqView mode (Figure 2) with ankle at the neutral position (90° between the leg and the foot). The probe applied with ultrasound transmission gel was placed perpendicular to the skin and moved smoothly along the middle line of the AT. During the movement along the length of the tendon, the probe was kept perpendicular to the skin and moderate pressure was applied to maintain proper contact between the probe and the skin without compressing the muscle/tendon considerably.
Figure 2.
Measurement of the Achilles tendon length using ultrasonography.
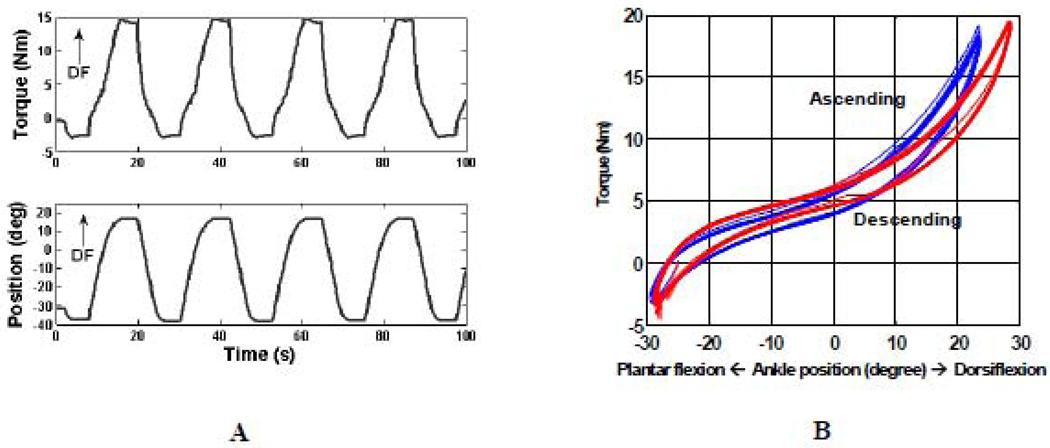
The intelligent stretching device was driven by a servomotor controlled by a digital signal processor (Zhang et al., 2002). Briefly, the stretching velocity was inversely proportional to the joint resistance torque, with the control adjusted at 2000Hz. Torque limits in both plantar- and dorsi-flexion were preset before the stretching intervention. Once the predefined peak resistance torque was reached, the joint was held at the extreme position for a preset period of time to allow for stress relaxation (Figure 3).
Figure 3.
A. Typical data from a stretching trial (only the first 100s are shown). B. Torque-angle relation (hysteresis loop) of two short stretching sessions (3rd [in blue] and 12th session [in red] for the same subject). The passive ROM, slope of the loading phase of the hysteresis loop curve (quasi-static stiffness), joint viscosity (the area enclosed by the hysteresis loop) were derived.
The maximum stretching velocity was set at 12°/s to avoid inducing reflex responses (Muraoka et al., 2005; Wiegner and Watts, 1986). The peak resistance torque was adjusted for each trial. Typical stretching parameters were 15- 20 Nm peak resistance torque in dorsiflexion, 3 Nm peak resistance torque in plantar flexion (since we were primarily stretching the stiff plantar flexors), and a 5-second holding period at the extreme positions. These values were chosen based on the stretching torques used by experienced physical therapists during manual stretching and on feedback from subjects during stretching. The peak resistance torque in dorsiflexion was started at a relatively low level during the first intervention session and gradually increased until the subjects had a feeling of comfortable stretching (typically from 3rd short session); from that point the stretching protocol was kept constant throughout the rest of the intervention. In total, there were twelve intervention sessions in sequence, each lasted 5 min with a 30 second break in between. In total, around 120 stretching cycles were run and lasted about 1 hour.
Data analysis
Joint biomechanical signal analysis
The torque and position signals were digitally filtered using a 4th-order zero-lag low pass Butterworth filter at 5 Hz. The peak torque points were identified and the relative torque change between the peak point and the proceeding baseline (i.e. due to passive resistance) was calculated during each stimulation to remove the contribution of passive resistance across tested ankle range. Though the intensity of the electrical stimulation was kept constant across different joint positions for each subject, the magnitude of the induced torque might fluctuate due to shift of the baseline torque. For further analysis, outliers of the peak torque signals, defined as those that are more than 1.5 times the interquartile range away from the 25th or 75th percentile (Zhang et al., 1998), were excluded.
The torque-angle relationship during the back-and-forth stretching intervention was plotted to establish the hysteresis loops. Index of hysteresis was calculated as the ratio of area within the loop to the area below the ascending limb and presented in percentage (%) (Kubo et al., 2001). Each hysteresis loop was further divided into an ascending limb (dorsiflexion) and a descending limb (plantar flexion) (Figure 3B). The data collected in the 3rd and last short sessions were treated pre- and post-intervention evaluation respectively. The multiple ascending and descending limbs were pooled and averaged (n≈12), respectively. As shown in figure 3B the loading curves across cycles were very consistent with little deviation. The consistency across loading cycles was evaluated using Intraclass correlation coefficients (ICCs) (Shrout and Fleiss, 1979) and the averaged ICCs for ascending and descending limbs across subjects were 0.9995 and 0.9998 respectively pre-intervention and 0.9996 and 0.9999 respectively post-intervention (ICCs ranged from 0.9972 to 0.9999). The following biomechanical properties of the ankle were calculated based on the average ascending limbs of the torque-angle curve: passive resistance torque at prescribed positions (especially in the dorsiflexion, e.g. neutral position, 5° and 20°), quasi-static stiffness and index of hysteresis related to the viscoelastic properties of the joint. The ankle positions (AP) corresponding to the peak torque values were identified through curve fitting the Otten’s model (Otten, 1987). Since the optimal ankle position was not known, the non-normalized version of the model was used:
where θ is the ankle dorsiflexion angle, α is the ankle dorsiflexion angle corresponding to the maximal value of ankle joint torque T, ω determines the curve width, β determines the skewness of the curve and ρ determines the roundness (Otten, 1987). For further analysis the torque angle curve was normalized with respect to the peak torque value for individual subject.
Analysis of ultrasound image
Ultrasound images were used to measure the length of the Achilles tendon. The saved image files were loaded in ImageJ (NIH) for further analysis. Three measurements were done and the average value was used for further analysis. The measurements were consistent across trials with standard deviations ranged from 0.2 to 3.6 mm.
Statistical analysis
ANOVA with repeated measure was used to analyze the response variables including the resistance torque, stiffness, index of hysteresis and Achilles tendon length, with the subject population (stroke and control), ankle joint positions (three levels: neutral position, 5° and 20° dorsiflexion) as the factors involved. The significant level was set at 0.05 and adjustments were made if a violation of sphericity was found. All statistics were done in SPSS (SPSS Inc., Chicago, USA).
RESULTS
Biomechanical properties of ankle joint and calf muscle-tendon post stroke pre-intervention
Compared to the healthy controls, stroke survivors had significantly higher resistance torques at comparable dorsiflexion positions (P<0.05). For instance, the averaged resistance torque of the ascending limb (loading phase) at 20° dorsiflexion was 7.1±0.5 Nm and 11.9±3.2 Nm (mean±SD) for the control and stroke groups, respectively. Similarly, a significantly higher ankle joint stiffness was observed in stroke survivors than that in controls. For instance, the stiffness of the ascending (loading) limb at 20° dorsiflexion was 0.29±0.04 Nm/° and 0.6±0.13 Nm/° for control and stroke groups, respectively.
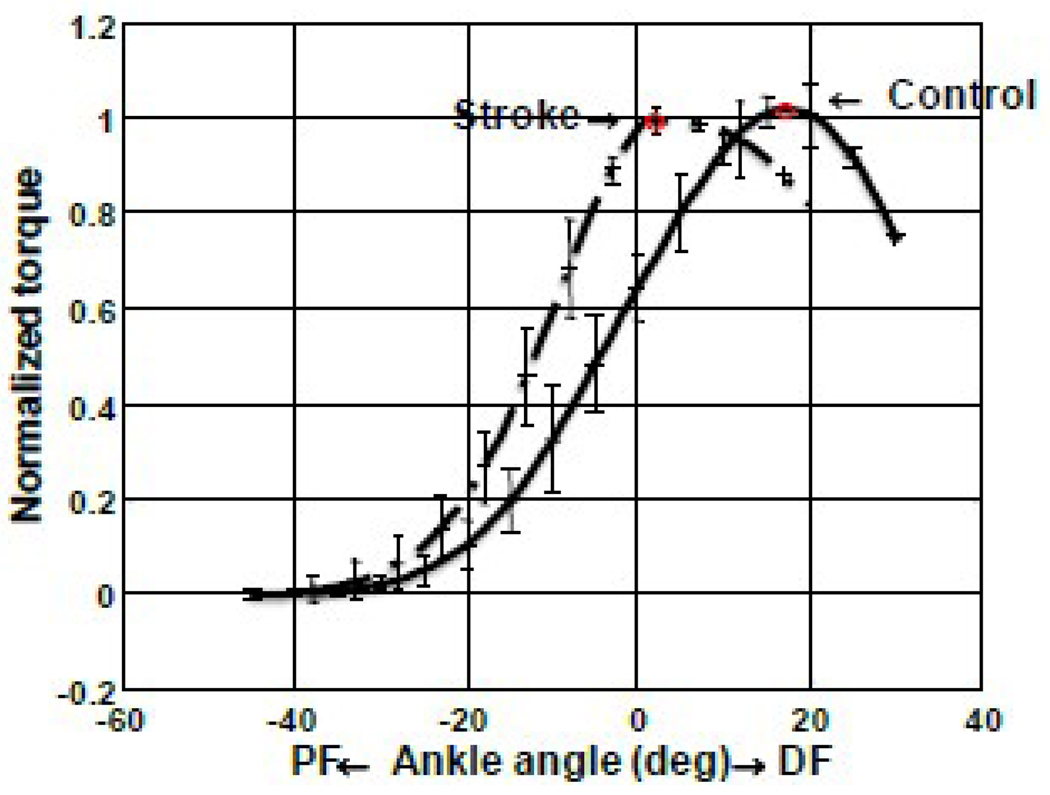
The force output of the triceps surae muscles was characterized by the torque-angle relationship under matched stimulations. In general, the curve of the stroke survivors was shifted towards plantar flexion compared to that of healthy control. The mean and standard deviation of ankle position (AP, corresponding to the peak torque) were 13.41±5.23° and 1.25±1.41° plantar flexion for control and stroke (before stretching) groups respectively and the difference was statistically significant (p<0.001) (Figure 4).
Figure 4.
Torque-angle relation of the Control and Stroke groups with the torque values normalized to the corresponding peak values.
The Achilles tendon length was 57.6±14.9 mm and 61.8±19.2 mm for the stroke and control groups, respectively, and there is no significant difference between them (P=0.609). Similar results of no significant differences were obtained if the AT lengths were normalized to shank length (the shank lengths are 38.6±1.4 cm and 38.3±1.9 cm for stroke and control respectively).
Biomechanical changes of joint and muscle-tendon induced by repeated ankle stretching
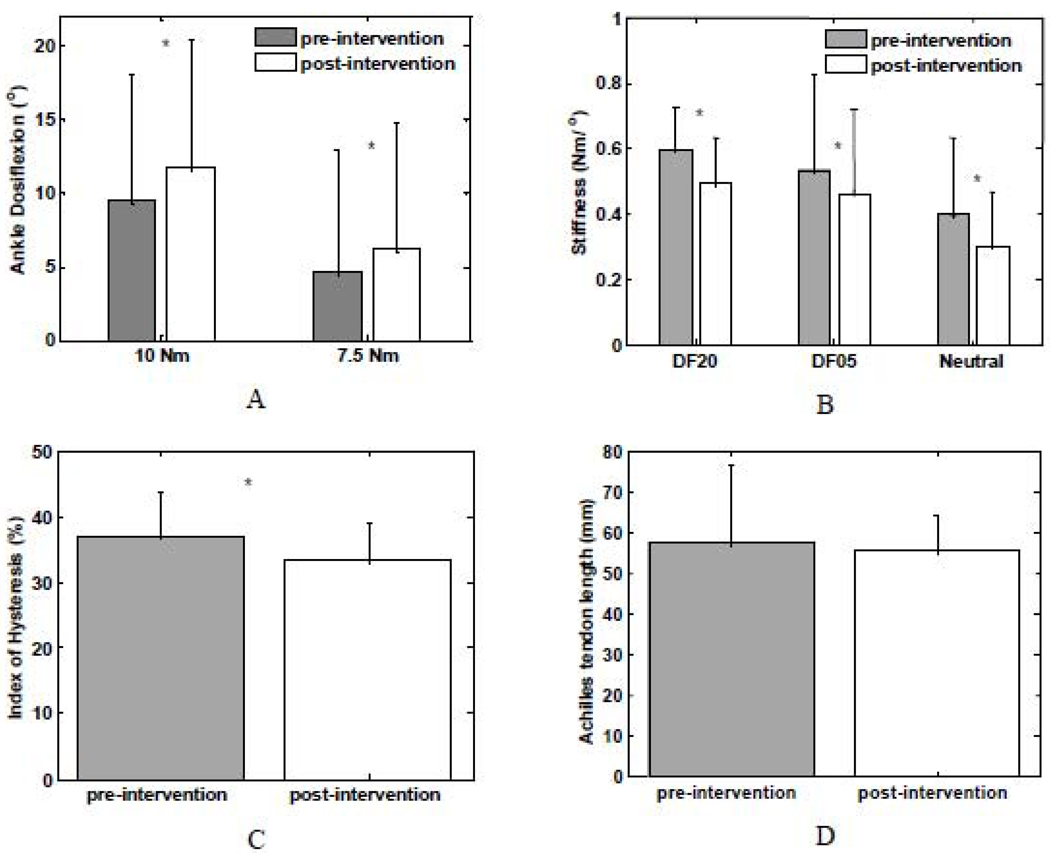
For stroke survivors, in general, the ankle joint stiffness was reduced after the stretching intervention and the ankle joint stiffness were reduced at comparable ankle dorsiflexion positions (Figure 5A and B). For instance, the resistance torque of the ascending limb at 20° dorsiflexion was 11.9±3.2 Nm and 10.5±3.3 Nm (mean±SD) pre- and post-intervention, respectively (P=0.009).
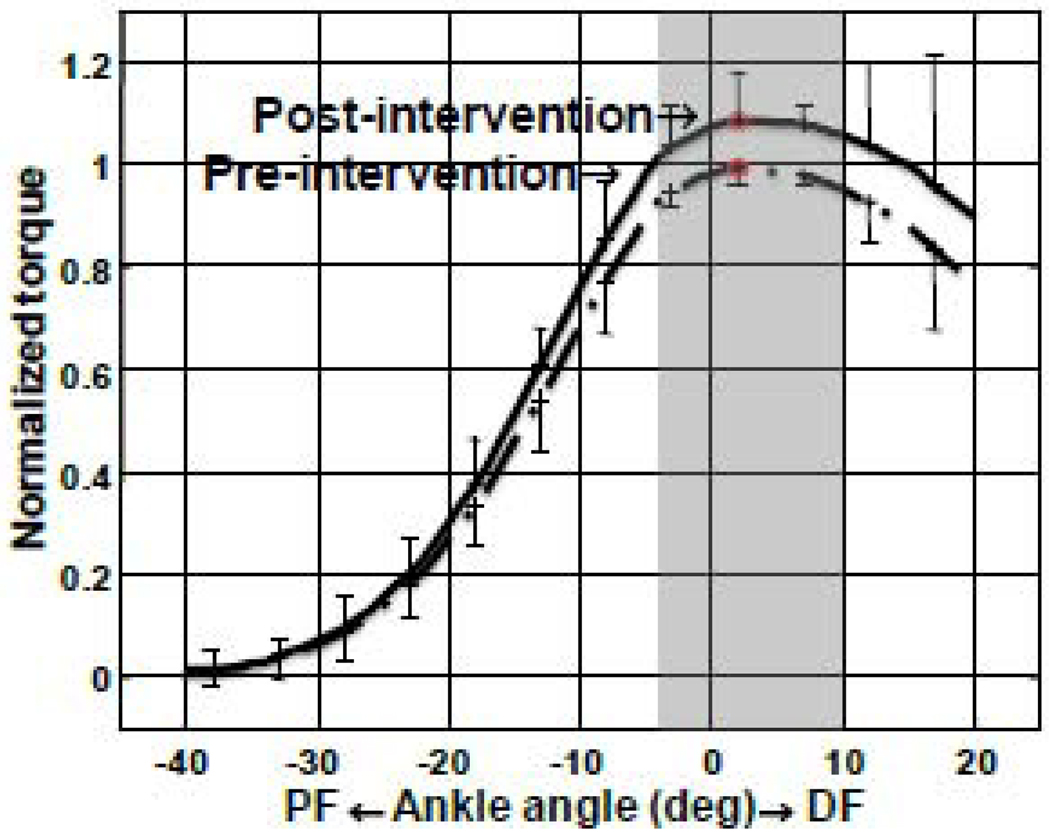
Figure 5.
Normalized torque-angle relationship of stroke survivors (pre-intervention v.s. postintervention). Significant differences were highlighted by the shaded area (p<0.05).
Similarly, a significantly reduction in ankle joint stiffness was also observed after the stretching intervention for stroke survivors. For instance, the stiffness of the ascending limb at 20° dorsiflexion was 0.60±0.13 Nm/° and 0.49±0.14 Nm/° (mean±SD) pre- and post-intervention, respectively (P = 0.013). In addition, the index of hysteresis was also reduced significantly post-intervention (P = 0.002) and the index of hysteresis was 0.37±0.07 and 0.33±0.06 pre- and post-intervention, respectively (Figure 5C).
There was no significant change in the Achilles tendon length after the repeated ankle stretching. For instance, the Achilles tendon length of stroke survivors were 57.6±14.9 and 55.5±8.6 mm (P =.39) pre- and post- intervention, respectively. Similarly, in healthy controls, the Achilles tendon lengths were 61.8±19.2 and 60.9±21.3 mm pre- and post- intervention, respectively (P=0.62).
The force output, represented by the torque-angle relationship under matched stimulations, was improved significantly in stroke survivors, especially around the AP after the repeated ankle stretching (Figure 6). The normalized torque at AP was 1.10±.07 post intervention indicating a significant increase of 10% in force-generation capacity compared to that pre intervention (P = 0.017).
Figure 6.
Ankle dorsiflexion position at specified resistance torques (A) and joint passive stiffness (B) at the specified ankle positions and index of hysteresis pre- and post-intervention (C) as well as Achilles tendon length (D) for stroke survivors pre- and post-intervention (group average with standard deviation; * P<0.05).
DISCUSSION
The current study showed the ankle position corresponding to the peak torque shifted towards plantar flexion, higher resistance torque and higher joint stiffness post stroke, and stretching-induced improvements in ankle ROM and force output of the impaired calf muscles in stroke survivors. The pathological consequences post stroke have been studied extensively and the biomechanical changes post stroke as characterized in the current study were consistent with previous studies which emphasized the reduced range of motion and increased joint stiffness (Chung et al., 2004; Vattanasilp et al., 2000). The results of intervention agreed well with previous studies that passive stretching reduces the stiffness of ankle joint (Selles et al., 2005; Zhang et al., 2002). Specifically, using ultrasonography and electrical stimulation, we showed the effects of passive ankle stretching on calf muscle and tendon biomechanical properties post stroke.
Improvement in force output could be attributed to neural and/or biomechanical factors (or equivalently, muscle tendon properties). In the present study electrical stimulation with constant intensity was applied across various joint conditions, therefore the improvement in torque magnitude after repeated passive stretching could be attributed to a change in potentiation and/or the threshold of motor point. However, previous studies showed that passive stretching decreased the reflex sensitivity (Avela et al., 1999) and motor neuron excitability (Tsai et al., 2001). Therefore it is possible that the improvement was resulted from changes in fascicular biomechanical properties. Biomechanically, loosening of stiff muscle fascicles tendon and/or aponeuroses may facilitate force generation among fascicles and increase their overall force output. The muscle fascicles may work together more effectively with higher force generation after stretching.
In addition, the torque-angle relationship of stroke survivors showed a shifted pattern compared to healthy controls and was skewed more to the direction of plantar flexion, which agreed well with previous results (Gao and Zhang, 2008). The shifted pattern suggested that the lengths of plantar flexor muscles in stroke survivors were relatively shorter compared to those of healthy controls. In a recent study we showed that muscle fascicle lengths of spastic plantar flexors were shorter than those of controls (Gao et al., 2009), which might contribute to the muscle weakness post stroke. Hypothetically, it was possible that muscle fascicles were elongated after the stretching, which made the fascicles contract more effectively on a more favorable part of the muscle fascicle force-length curve to produce higher force.
Previous studies suggested that the change in mechanical properties of tendon depended on the stretching protocol. Mahieu et al. showed that stiffness of Achilles tendon were not changed under static stretching but would be decreased under ballistic stretching (Mahieu et al., 2007). Repeated ankle stretching could reduce the tightness of ankle joint and alter mechanical properties of the Achilles tendon. However, even the fact the ankle joint was loosened as a whole unit, and the stiffness of the joint was reduced, the stiffness of the Achilles tendon might still be either increased or decreased. A recent study showed that the Achilles tendon was more compliant in the impaired side than in the unimpaired side in stroke survivors (Zhao et al., 2009). Anatomically, tendon and aponeurosis are aligned in series and it has been shown that the contribution of GM tendon to the elongation of muscle tendon unit is more than that of aponeurosis in able-bodied subjects (De Monte et al., 2006). The current study did not show significant AT length change post intervention and relaxation of the calf muscle tendon unit might be attributed to potential mechanical changes in aponeurosis such as decrease in stiffness (Kubo et al., 2001). On the other hand, although no significant Achilles tendon shortening was observed after a single-session stretching in adult stroke in this study, significant shortening of the Achilles tendon was observed after six-week passive stretching combined with active movement training in a related study on children with cerebral palsy (Zhao et al., 2010). In an animal study, passive stretching led to altered synthesis of extracellular matrix and increased elasticity in tendon (De Almeida et al., 2009b).
Besides the changes of joint mechanical properties, repeated ankle stretching could alter the properties at the muscle fascicle level. It has been reported that stretching of muscles could be a stimulus for normal postnatal increase in sarcomere numbers (Williams and Goldspink, 1978). Spastic muscles have relatively shorter muscle fascicles compared to that in healthy control (Gao et al., 2009) and the response to the stretching stimulus might result in elongation of muscle fascicles, which may be accompanied by an increase of the sarcomere length and/or increment of sarcomere numbers in series. A study on sarcomere response in single muscle fiber of the Rana temporaria semitendinosus muscle showed stretching with fused tetanic contractions increased the length of sarcomere and there was force enhancement after stretch at the same sarcomere length during isometric tetani (Edman et al., 1978). Muscle fascicle or sarcomere length was not quantified in the current study and further study on muscle structural properties pre and post intervention needs to be conducted to corroborate this.
Ankle plantar flexors are essential in locomotion (Winter, 2004) and the lack of push-off significantly reduces the forward thrust and results in reduced swing initiation on the ipsilateral side (Neptune et al., 2001) and reduced swing phase on the contralateral side (Olney and Richards, 1996; Perry, 1992). Although a single session of passive stretching may not improve gait significantly (Maynard et al., 2005), it is possible that improvement in muscle force output induced by repeated stretching will help stroke survivors gain better control of the push-off and swing with improved gait patterns characterized as increased walking speed and more symmetric gait cycles.
Controlled twitch was used in this study to reduce the discomfort induced by strong stimulation. Though under twitch mode it was hard to achieve maximum , under sub-maximum contraction the torque angle relation was similar to those induced by either tetanus or maximum voluntary contraction (Sale et al., 1982). The accuracy and precision using ultrasonography to quantify muscle and tendon properties have been shown in previous studies (Hansen et al., 2006; Magnusson et al., 2001; Zhao et al., 2009). In the current study, an extended-field-of-view technique implemented in GE LOGIQ9 was used. Studies on comparable techniques have shown the measurement accuracy was within 5% (Fornage et al., 2000; Weng et al., 1997). However, accurate quantification of Achilles tendon is challenging due to the complex 3D structure of the tendon and other imaging techniques with better resolution, such as MRI might be used in the future. Surface EMG of calf muscles was registered on selected subject and showed minimum level of muscle activity. Ideally it will be preferred to monitor muscle activity across all tested subjects. The muscle tendon unit property is characterized as viscoelasticity and it creeps (i.e. change in strain) under constant loading. In current study, the ankle was held at preset torque for about 5 seconds. A recent study shows relative changes in ankle position in the time window of 30 seconds and the change in position is predominant in the first 5 seconds followed by the second 5 seconds (Ryan et al., 2010). Creep might affect the results and result in inconsistency across loading cycles, however, there is a fairly good consistency across loading cycles as characterized as high ICCs in the current study.
In summary, the main findings of this study include: 1) compared to healthy control, stroke survivors have significantly shifted torque-angle relationship towards plantar flexion; 2) repeated ankle stretching could reduce the tightness of the ankle joint and decrease ankle joint stiffness and increase ROM; 3) repeated ankle stretching could also alter the properties of the muscle-tendon, with improved muscle force output and possibly shortening of the Achilles tendon associated with relaxation and possibly lengthening of muscle fascicles.
The study provided evidences of improvement in muscle tendon properties through a stretching intervention. Clinically, long term effects need to be investigated with repeated stretching done over multiple intervention sessions.
Acknowledgments
The authors would like to acknowledge the supports of the National Institutes of Health and National Institute on Disability and Rehabilitation Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Avela J, Kyrolainen H, Komi PV, Rama D. Reduced reflex sensitivity persists several days after long-lasting stretch-shortening cycle exercise. J Appl Physiol. 1999;86:1292–1300. doi: 10.1152/jappl.1999.86.4.1292. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Larkin PA. Passive ankle dorsiflexion increases in patients after a regimen of tilt table-wedge board standing. A clinical report. Phys Ther. 1985;65:1676–1678. doi: 10.1093/ptj/65.11.1676. [DOI] [PubMed] [Google Scholar]
- Bressel E, McNair PJ. The effect of prolonged static and cyclic stretching on ankle joint stiffness, torque relaxation, and gait in people with stroke. Phys Ther. 2002;82:880–887. [PubMed] [Google Scholar]
- Chung SG, van Rey E, Bai Z, Roth EJ, Zhang L-Q. Biomechanic changes in passive properties of hemiplegic ankles with spastic hypertonia. Arch Phys Med Rehabil. 2004;85:1638–1646. doi: 10.1016/j.apmr.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Cornwell A, Nelson AG, Sidaway B. Acute effects of stretching on the neuromechanical properties of the triceps surae muscle complex. Eur J Appl Physiol. 2002;86:428–434. doi: 10.1007/s00421-001-0565-1. [DOI] [PubMed] [Google Scholar]
- de Almeida F, Tomiosso T, Nakagaki W, Gomes L, Matiello-Rosa S, Pimentel E. Effects of Passive Stretching on the Biochemical and Biomechanical Properties of Calcaneal Tendon of Rats. Connective Tissue Research. 2009a;50:279–284. [PubMed] [Google Scholar]
- De Almeida FM, Tomiosso TC, Nakagaki WR, Gomes L, Matiello-Rosa SMG, Pimentel ER. Effects of Passive Stretching on the Biochemical and Biomechanical Properties of Calcaneal Tendon of Rats. Connective Tissue Research. 2009b;50:279–284. [PubMed] [Google Scholar]
- De Deyne PG. Application of passive stretch and its implications for muscle fibers. Phys Ther. 2001;81:819–827. doi: 10.1093/ptj/81.2.819. [DOI] [PubMed] [Google Scholar]
- De Monte G, Arampatzis A, Stogiannari C, Karamanidis K. In vivo motion transmission in the inactive gastrocnemius medialis muscle-tendon unit during ankle and knee joint rotation. J Electromyogr Kinesiol. 2006;16:413–422. doi: 10.1016/j.jelekin.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Dombovy ML, Sandok BA, Basford JR. Rehabilitation for stroke: a review. Stroke. 1986;17:363–369. doi: 10.1161/01.str.17.3.363. [DOI] [PubMed] [Google Scholar]
- Edman KA, Elzinga G, Noble MI. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol. 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornage BD, Atkinson EN, Nock LF, Jones PH. US with extended field of view: phantom-tested accuracy of distance measurements. Radiology. 2000;214:579–584. doi: 10.1148/radiology.214.2.r00fe20579. [DOI] [PubMed] [Google Scholar]
- Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in the Passive Mechanical Properties of the Gastrocnemius Muscle at the Muscle Fiber and Joint Levels in Stroke Survivors. Arch Phys Med Rehabil. 2009;90:819–826. doi: 10.1016/j.apmr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhang L-Q. Altered contractile properties of the gastrocnemius muscle poststroke. J Appl Physiol. 2008;105:1802–1808. doi: 10.1152/japplphysiol.90930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon) 2006;21:54–58. doi: 10.1016/j.clinbiomech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Herbert RD. Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord. 2002;40:1–9. doi: 10.1038/sj.sc.3101241. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influence of static stretching on viscoelastic properties of human tendon structures in vivo. J Appl Physiol. 2001;90:520–527. doi: 10.1152/jappl.2001.90.2.520. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol. 2001;531:277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahieu NN, McNair P, De Muynck M, Stevens V, Blanckaert I, Smits N, Witvrouw E. Effect of static and ballistic stretching on the muscle-tendon tissue properties. Med Sci Sports Exerc. 2007;39:494–501. doi: 10.1249/01.mss.0000247004.40212.f7. [DOI] [PubMed] [Google Scholar]
- Maynard V, Bakheit AM, Shaw S. Comparison of the impact of a single session of isokinetic or isotonic muscle stretch on gait in patients with spastic hemiparesis. Clin Rehabil. 2005;19:146–154. doi: 10.1191/0269215505cr853oa. [DOI] [PubMed] [Google Scholar]
- McNair PJ, Dombroski EW, Hewson DJ, Stanley SN. Stretching at the ankle joint: viscoelastic responses to holds and continuous passive motion. Med Sci Sports Exerc. 2001;33:354–358. doi: 10.1097/00005768-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Moseley AM. The effect of casting combined with stretching on passive ankle dorsiflexion in adults with traumatic head injuries. Phys Ther. 1997;77:240–247. doi: 10.1093/ptj/77.3.240. discussion 248–259. [DOI] [PubMed] [Google Scholar]
- Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H. Elastic properties of human Achilles tendon are correlated to muscle strength. J Appl Physiol. 2005;99:665–669. doi: 10.1152/japplphysiol.00624.2004. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait & Posture. 1996;4:136–148. [Google Scholar]
- Otten E. A myocybernetic model of the jaw system of the rat. J Neurosci Methods. 1987;21:287–302. doi: 10.1016/0165-0270(87)90123-3. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait analysis: normal and pathological function. SLACK incorporated. 1992;vol. [Google Scholar]
- Roy RR, Pierotti DJ, Baldwin KM, Zhong H, Hodgson JA, Edgerton VR. Cyclical passive stretch influences the mechanical properties of the inactive cat soleus. Exp Physiol. 1998;83:377–385. doi: 10.1113/expphysiol.1998.sp004121. [DOI] [PubMed] [Google Scholar]
- Ryan ED, Herda TJ, Costa PB, Walter AA, Hoge KM, Stout JR, Cramer JT. Viscoelastic creep in the human skeletal muscle-tendon unit. Eur J Appl Physiol. 2010;108:207–211. doi: 10.1007/s00421-009-1284-2. [DOI] [PubMed] [Google Scholar]
- Sale D, Quinlan J, Marsh E, McComas AJ, Belanger AY. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982;52:1636–1642. doi: 10.1152/jappl.1982.52.6.1636. [DOI] [PubMed] [Google Scholar]
- Selles RW, Li X, Lin F, Chung SG, Roth EJ, Zhang LQ. Feedback-controlled and programmed stretching of the ankle plantarflexors and dorsiflexors in stroke: effects of a 4-week intervention program. Arch Phys Med Rehabil. 2005;86:2330–2336. doi: 10.1016/j.apmr.2005.07.305. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Svantesson U, Sunnerhagen KS. Stretch-shortening cycle in patients with upper motor neuron lesions due to stroke. Eur J Appl Physiol Occup Physiol. 1997;75:312–318. doi: 10.1007/s004210050166. [DOI] [PubMed] [Google Scholar]
- Svantesson U, Takahashi H, Carlsson U, Danielsson A, Sunnerhagen KS. Muscle and tendon stiffness in patients with upper motor neuron lesion following a stroke. Eur J Appl Physiol. 2000;82:275–279. doi: 10.1007/s004210000216. [DOI] [PubMed] [Google Scholar]
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972;224:231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu C, Lespargot A, Tabary C, Bret MD. For how long must the Soleus muscle be stretched each day to prevent contraccture? Developmental Medicine & Child Neurology. 1988;30:3–10. doi: 10.1111/j.1469-8749.1988.tb04720.x. [DOI] [PubMed] [Google Scholar]
- Tsai KH, Yeh CY, Chang HY, Chen JJ. Effects of a single session of prolonged muscle stretch on spastic muscle of stroke patients. Proc Natl Sci Counc Repub China B. 2001;25:76–81. [PubMed] [Google Scholar]
- Vattanasilp W, Ada L, Crosbie J. Contribution of thixotropy, spasticity, and contracture to ankle stiffness after stroke. J Neurol Neurosurg Psychiatry. 2000;69:34–39. doi: 10.1136/jnnp.69.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L, Tirumalai AP, Lowery CM, Nock LF, Gustafson DE, Von Behren PL, Kim JH. US extended-field-of-view imaging technology. Radiology. 1997;203:877–880. doi: 10.1148/radiology.203.3.9169720. [DOI] [PubMed] [Google Scholar]
- Wiegner AW, Watts RL. Elastic properties of muscles measured at the elbow in man: I. Normal controls. J Neurol Neurosurg Psychiatry. 1986;49:1171–1176. doi: 10.1136/jnnp.49.10.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE. Effect of intermittent stretch on immobilised muscle. Ann Rheum Dis. 1988;47:1014–1016. doi: 10.1136/ard.47.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE. Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Ann Rheum Dis. 1990;49:316–317. doi: 10.1136/ard.49.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat. 1978;127:459–468. [PMC free article] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. vol. New York: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- Winters TF, Jr, Gage JR, Hicks R. Gait patterns in spastic hemiplegia in children and young adults. J Bone Joint Surg Am. 1987;69:437–441. [PubMed] [Google Scholar]
- Yeh CY, Chen JJ, Tsai KH. Quantitative analysis of ankle hypertonia after prolonged stretch in subjects with stroke. J Neurosci Methods. 2004;137:305–314. doi: 10.1016/j.jneumeth.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Butler J, Nishida T, Nuber G, Huang H, Rymer W. In vivo determination of the direction of rotation and moment-angle relationship of individual elbow muscles. Journal of biomechanical engineering. 1998;120:625. doi: 10.1115/1.2834754. [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Chung SG, Bai Z, Xu D, van Rey EM, Rogers MW, Johnson ME, et al. Intelligent stretching of ankle joints with contracture/spasticity. IEEE Trans Neural Syst Rehabil Eng. 2002;10:149–157. doi: 10.1109/TNSRE.2002.802857. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ren Y, Wu Y-N, Liu SQ, Zhang L-Q. Ultrasonic Evaluations of Achilles Tendon Mechanical Properties Post Stroke. Journal of Applied Physiology. 2009;106:843–849. doi: 10.1152/japplphysiol.91212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wu Y, Hwang M, Ren Y, Gaebler-Spira DJ, Zhang LQ. Changes of calf muscle-tendon biomechanical properties induced by passive stretching and active movement training in children with cerebral palsy. Developmental Medicine & Child Neurology. 2010;52:S41–S42. doi: 10.1152/japplphysiol.01361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]