Introduction
Babesiosis in humans is a malaria-like infection caused primarily by the protozoa Babesia microti. Infection typically occurs during the summer and fall months, when the tick vector is prevalent, and is characterized by erythrocyte invasion and destruction.1 Many patients have asymptomatic disease and recover spontaneously after babesia infection and those that do become symptomatic often improve following a short (7–10 day) course of therapy with either azithromycin and atovaquone or clindamycin and quinine.2, 3 However, immunosuppressed and splenectomized patients have both been shown to be at risk for more severe disease and in some instances these patients have had prolonged and apparently refractory disease despite appropriate antiparasitic coverage.4, 5
Recently, data have emerged suggesting that immunocompromised patients with babesia infection may develop resistance to the usual combination of azithromycin and atovaquone.6 These refractory cases have been managed with various combinations of antimicrobial agents and often have required red cell exchange transfusion to control their parasitemia. We describe the case of an immunocompromised man who underwent prior stem cell transplantation for chronic myelogenous leukemia (CML) and who later developed persistent babesiosis despite multiple weeks of appropriate therapy for his infection.
Case Report
A 48-year-old man from Rhode Island presented to hematology clinic in early October with new symptoms of fatigue following one episode of fever to 101 degrees Fahrenheit. He had received an allogeneic stem cell transplant 3 years prior for CML after failing therapy with imatinib and splenic irradiation. His post-transplant course was complicated by chronic graft versus host disease (GvHD) involving his skin and oral mucosa, for which he was treated with rituximab monthly until 7 months prior to his presentation and briefly with mycophenolate mofetil, which was discontinued 5 months prior. He remained on oral steroids with a baseline dose of 5–10 mg of prednisone daily.
On presentation, he was noted to have a mild hemolytic anemia in addition to the symptoms noted above. It was felt that these new findings represented worsening of his GvHD, so his steroid dose was increased to 40mg daily of prednisone. There were no parasites or schistocytes noted on blood smears performed at that time. Over the next 2 months, the patient remained on the increased dose of steroids, but had worsening of his anemia and rising values of serum lactate dehydrogenase, suggesting a hemolytic process. He had a brief hospitalization in mid November, where a bone marrow biopsy was performed. This showed a normocellular marrow with slight erythroid predominance. He received a blood transfusion and IVIG at that time.
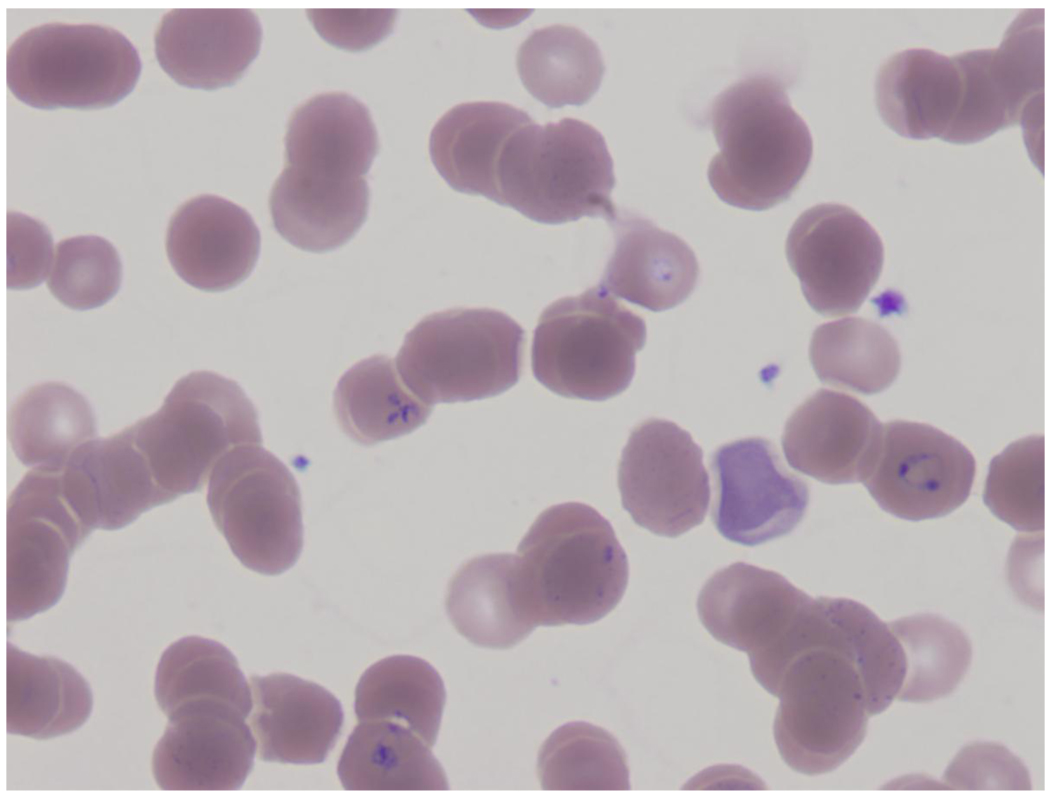
He returned in early December with worsening anemia and fatigue. His blood smear now revealed parasites consistent with Babesia sp. infection (figure 1) and he was initiated on therapy with azithromycin 500mg daily and atovaquone 750mg twice daily. His symptoms quickly improved with treatment and his hemolysis decreased significantly, though parasites remained present but decreased on smear. (figure 2)
Figure 1.
Blood smear
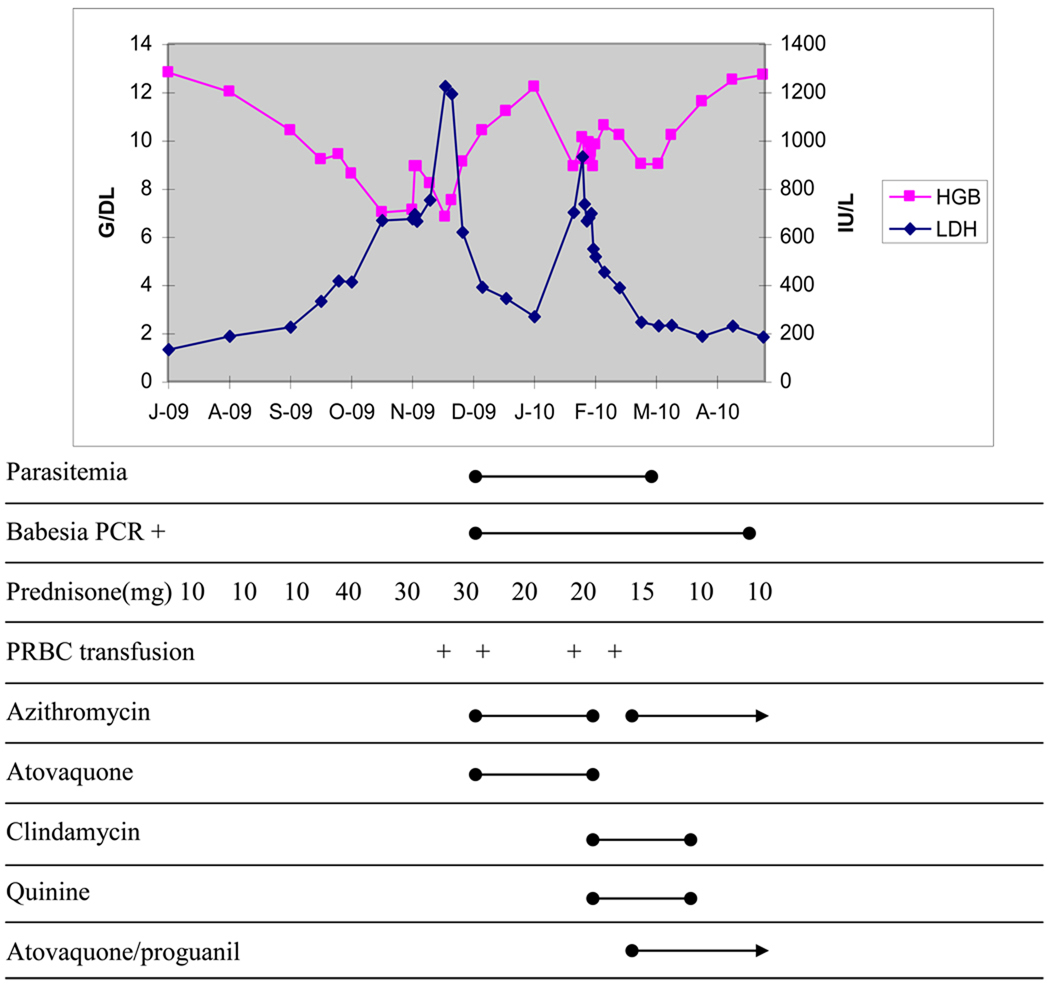
Figure 2.
Time course of babesiosis and interventions
After roughly 8 weeks of therapy, he represented with recurrent fatigue and low-grade fever. He was found to have worsening hemolysis with persistent parasitemia, so was switched to clindamycin 600mg every 8 hours orally and quinine 628mg every 8 hours orally. Despite this switch in medication, his symptoms worsened and he was readmitted to the hospital in early February. He was restarted on azithromycin at 1000mg orally daily, had atovaquone/proguanil 500/100mg twice daily initiated and was continued on clindamycin and quinine, with the clindamycin being switched temporarily to intravenous route. He was discharged on this regimen roughly 7 days later, but with the atovaquone/proguanil dose being reduced to 250/100mg twice daily. On this new regimen, his parasitemia decreased over time and became undetectable on smear in mid March, which was his first undetectable smear since early December, when he first initiated treatment for babesiosis. He subsequently developed mild auditory disturbances on quinine and after roughly 6 weeks of this 4-drug therapy, he was taken off clindamycin and quinine and continued on azithromycin and atovaquone/proguanil. His parasitemia has remained undetectable and his anemia and hemolysis have resolved. His serum babesia PCR remained positive until the end of April, when it too became undetectable.
Discussion
Immune dysfunction is a known risk factor for persistent, refractory, and relapsing babesia infection. A recent case control study comparing patients with persistent symptoms, anemia, and parasitemia to controls who cleared the infection quickly found that a high proportion of the cases had B cell lymphoid malignancies, were asplenic, and had received rituximab as part of their therapy.4 The authors suggested that these disorders, and the treatment with rituximab, likely limited the development of anti-babesial antibody to help clear the parasites in these patients. However, the role of humoral immunity in the control of babesia infection is not completely understood. Although refractory cases of babesia infection appear to occur in human hosts who have humoral deficiencies, other animal models suggest that cell-mediated immunity may play a more important role. For example, an experiment in B-cell deficient mice showed similar control of the parasite compared to wild-type mice, whereas SCID mice and mice with T-cell deficiencies had significantly higher parasite burdens.7 Our patient had deficiencies in both humoral and cell-mediated immunity as a result of his underlying disease and the medications he had received. Interestingly, his parasitemia only became apparent after his steroid dose was increased and resolution of his infection seemed to correlate with tapering of his steroids, suggesting the importance of the cell-mediated immune response in the control of his infection.
To the best of our knowledge this is only the second case of babesiosis reported in a stem cell transplant patient. The previous case report was in a patient who received a stem cell transplant for sickle cell disease and was presumed to have acquired babesia infection through blood transfusion.8 Cases of transfusion-related babesiosis have been well described. A recent series conducted through the American Red Cross Hemovigilance Program found 7 definite and 11 probable cases of babesia transmission through blood transfusion during the 3-year period from 2005–2007.9 Further, this number was felt to be an underestimate of the true rate given that only symptomatic cases were investigated. Given the higher than average rate of transfusion in the stem cell transplant population, the risk for babesia infection in this vulnerable group of patients is likely greater than would be expected from exposures through their environment alone. Our patient had not received any blood products in the months preceding his infection, so likely had an environmental source rather than transfusion related babesiosis.
The incidence of babesia infection has dramatically increased over the past decade in the United States and a wider geographic range of infection has been seen in recent years.10 Therefore, it is reasonable to expect that a greater number of babesia cases will be seen among high risk populations and as a result, the prevalence of refractory or persistent disease will increase over time as well. In these cases, longer treatment courses with multiple agents, red cell exchange transfusion, and decreases in immunosuppressive therapy may be necessary to ultimately clear the parasite.
Acknowledgements
The authors would like to thank Peter J Krause for his advice and expert guidance regarding the treatment of this complicated infection.
Funding Support
NIH training grant 5 T32 AI055412 to ASL and Tufts Medical Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors have no conflict of interest to declare.
References
- 1.Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2008;22:469–488. doi: 10.1016/j.idc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The Clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 3.Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343:1454–1458. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 4.Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 5.Clark IA, Budd AC, Hsue G, et al. Absence of erythrocyte sequestration in a case of babesiosis in a splenectomized human patient. Malar J. 2006;5:69. doi: 10.1186/1475-2875-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to Azithromycin-Atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis. 2010;50:381–386. doi: 10.1086/649859. [DOI] [PubMed] [Google Scholar]
- 7.Clawson ML, Paciorkowski N, Rajan TV, et al. Cellular immunity, but not gamma interferon, is essential for resolution of Babesia microti infection in BALB/c mice. Infect Immun. 2002;70:5304–5306. doi: 10.1128/IAI.70.9.5304-5306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirino CM, Leitman SF, Palmore TN, et al. Transfusion-associated babesiosis with an atypical time course after nonmyeloablative transplantation for sickle cell disease. Ann Intern Med. 2008;148:794–795. doi: 10.7326/0003-4819-148-10-200805200-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonnetti L, Eder AF, Kennedy J, et al. Transfusion-transmitted Babesia microti identified through hemovigilance. Transfusion. 2009;49:2557–2563. doi: 10.1111/j.1537-2995.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 10.Vannier E, Krause PJ. Update on babesiosis. Interdiscip Perspect Infect Dis. 2009;2009:984568. doi: 10.1155/2009/984568. Epub 2009 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]