Abstract
Despite pharmacologic advances, the treatment of schizophrenia remains a challenge, and suboptimal outcomes are still all too frequent. Although treatment goals of response, remission, and recovery have been defined more uniformly, a good “effectiveness” measure mapping onto functional outcomes is still lacking. Moreover, the field has to advance in transferring measurement-based approaches from research to clinical practice. There is an ongoing debate whether, and which, first- or second-generation antipsychotics should be used. However, an individualized treatment approach needs to consider current symptoms, comorbid conditions, past therapeutic response, and adverse effects, as well as patient choice and expectations. Moreover, acute and long-term goals and effects of medication treatment need to be balanced. While the acute response to appropriately dosed first-generation antipsychotics may not differ much from second-generation antipsychotics, advantages of lower rates of extrapyramidal side effects, tardive dyskinesia, and, possibly, relapse may favor second-generation antipsychotics. However, when considering individual adverse effect prof iles, the differentiation into first- and second-generation antipsychotics as unified classes can not be upheld, and a more differentiated view and treatment selection is required. To date, clozapine is the only evidence-based treatment for refractory patients, and the role of antipsychotic polypharmacy and other augmentation strategies remains unclear, at best. To improve the treatment outcomes in schizophrenia, research efforts are needed that elucidate biomarkers of the illness and of treatment response (both therapeutic and adverse effects). Moreover, new treatment options are needed that affect nondopaminergic targets with relevance for symptom reduction, relapse prevention, enhanced efficacy for nonresponders, and reduced key adverse effects.
Keywords: schizophrenia, pharmacology, first-generation antipsychotic, second-generation antipsychotic, outcome
Abstract
A pesar de los progresos farmacológicos, el tratamiento de la esquizofrenia todavía constituye un desafío y los resultados insatisfactorios aun son bastante frecuentes. Aunque se han definido más uniformemente los objetivos terapéuticos de respuesta, remisión y récupération, aun falta una buena medición de “efectividad” para delimitar los resultados funcionales. Además, en esta área se tiene que avanzar en la transferencia de enfoques basados en mediciones que vayan desde la investigatión hasta la práctica clínica. Actualmente existe un debate orientado a si deben o no utilizarse antipsicóticos y cuáles de ellos, sean de primera o segunda generación. Sin embargo, un enfoque terapéutico individualizado requière tener en cuenta los síntomas actuales, las condiciones comórbidas, la respuesta terapéutica previa y los efectos adversos, como también la election del patente y sus expectativas. Por otra parte, se deben balancear los objetivos a corto y largo plazo, y los efectos del tratamiento medicamentoso. Aunque la respuesta aguda a dosis apropiadas de antipsicóticos de primera generacíon puede que no difiera mucho de los antipsicóticos de segunda generacíon, las ventajas de menores frecuencias de efectos secundarios extrapiramidales, disquinesia tardfa e incluso recaídas pueden darle ventaja a los antipsicóticos de segunda generation. Sin embargo, cuando se consideran los perfiles de efectos adversos individuales, puede que no sea suficiente la diferenciación entre antipsicóticos de primera y segunda generation como grupos de fármacos, y se requiera de un criterio más diferenciado y de una selection del tratamiento. A la fecha, la clozapina es el único tratamiento basado en la evidencia para los patentes refractarios, y aun no está del todo claro el papel de la polifarmacia antipsicótica y de otras estrategias potenciadoras. Para mejorar los resultados de los tratamientos en la esquizofrenia, se requière de esfuerzos que procedan de la investigation y que permitan identificar biomarcadores de la enfermedad y de la respuesta terapéutica (tanto los efectos deseables como los adversos). Además, se necesitan nuevas opciones terapéuticas que no estén dirigidas hacia los sistemas dopaminérgicos y que influyan en la reduction de los síntomas, la prevention de las recaídas, el aumento de la eficacia en los no respondedores y la reduction de los principales efectos adversos.
Abstract
Malgré des avancées pharmacologiques, la schizophrénie reste difficile à traiter et des résultats insatisfaisants sont encore trop fréquents. Les cibles thérapeutiques de réponse, rémission et rétablissement ont été définies de façon plus uniforme mais il manque encore une mesure convenable « d'efficacité » basée sur les capacités fonctionnelles des patients. De plus, dans ce domaine, il faut avancer en transférant les approches basées sur les mesures de la recherche à la pratique clinique. Savoir si il faut utiliser des antipsychotiques de 1re ou de 2e génération et lesquels, fait toujours l'objet de discussions. Un traitement personnalisé nécessite néanmoins d'envisager les symptômes actuels, la comorbidité, les réponses thérapeutiques antérieures, les effets indésirables ainsi que les choix et les attentes du patient. Il faut en outre mettre en balance les objectifs et les effets des traitements à court et à long termes. Alors qu'une réponse aiguë à des antipsychotiques de 1re génération correctement dosés peut ne pas différer beaucoup d'une réponse à des antipsychotiques de 2e génération, les bénéfices d'une fréquence moindre d'effets extrapyramidaux, de dyskinésie tardive et, peut-être, de rechute, font pencher la balance du côté des antipsychotiques de 2e génération. Cependant, si l'on considère les profils individuels des effets secondaires, la distinction dans les antipsychotiques de 1re et 2e générations en tant que classes unifiées, ne tient pas et il est nécessaire d'avoir une vision plus différenciée et une sélection thérapeutique plus fine. À ce jour, la clozapine est le seul traitement ayant apporté ses preuves pour les patients résistants, le rôle d'une polythérapeutique antipsychotique et des autres stratégies d'augmentation restant au mieux obscur. L'amélioration des résultats du traitement dans la schizophrénie passe par des efforts de la recherche pour trouver des biomarqueurs de la maladie et de la réponse au traitement (effets thérapeutiques et effets indésirables). Ajoutons que de nouveaux choix de traitement sont nécessaires en ce qui concerne des cibles non dopaminergiques en tenant compte de la diminution des symptômes, de la prévention des rechutes, de l'augmentation de l'efficacité chez les non-répondeurs et de la diminution des effets indésirables majeurs.
The use of medication in the acute and long-term treatment of schizophrenia remains the cornerstone of disease management. This paper will attempt to review and highlight recent developments and current controversies in the pharmacologic treatment of schizophrenia. In that context, we will highlight areas where gaps in our knowledge continue to exist, and discuss the types of research ideally suited to fill these gaps. The treatment of the psychosis risk syndrome, also known as the “prodrome,” and cognitive dysfunction, an increasingly recognized core feature of schizophrenia, are dealt with elsewhere in this issue.
First- versus second-generation medications
A major focus of research over the past decade has been to establish the relative merits of first- and second-generation antipsychotics. With that, psychiatry has seen the completion of several large, government-funded trials, which attempted to apply some of the principles of effectiveness studies to complement data derived from more traditional efficacy research.
Before providing an overview of these results and discussing their implications, it will be useful to set the stage for the shift in prescribing practices and the ensuing costeffectiveness questions that emerged in the 1990s.
In the 1970s and 1980s, considerable effort went into solidifying our knowledge of the indications for and benefits and risks of long-term pharmacotherapy in schizophrenia. A large series of relapse-prevention studies were conducted demonstrating the ability of continuous antipsychotic drug administration to significantly reduce the risks of psychotic relapse and rehospitalization in comparison with placebo.1
Neurologic adverse effects
At the same time, increasing concern had developed regarding the potential of antipsychotic drugs to produce a variety of neurologic adverse effects, ranging from acute dystonic reactions to akathisia, parkinsonism, and tardive dyskinesia. Each one of these conditions were the subject of epidemiologic, treatment, and outcome studies which are beyond the scope of this review.
Tardive dyskinesia became a particular concern because of its potential severity, persistence, and psychosocial as well as medicolegal consequences. A number of prospective studies were conducted to determine the incidence and risk factors for tardive dyskinesia.2-4 In general, the risk of new cases of tardive dyskinesia was found to be 5% per year of cumulative drug exposure, with age and early occurring extrapyramidal side effects being two important risk factors. In elderly individuals (though with a different spectrum of diagnoses) receiving antipsychotic medications for the first time the incidence was generally fivefold higher.5
With the development of clozapine, the scientific and clinical community became convinced that it was possible to separate the therapeutic effects of antipsychotic medications from their neurologic extrapyramidal effects. Although it took many years after these early observations before clozapine was used on a wide scale for treatment-refractory patients,6 the awareness of its lack of propensity to cause extrapyramidal effects provided considerable impetus in drug development and served as a model for “atypicality” (a concept which has outlived its usefulness, but did serve a useful heuristic function).
With the introduction of other “second-generation” antipsychotics (risperidone, olanzapine, quetiapine, etc), there was considerable focus on differences in the propensity to cause extrapyramidal syndrome (EPS) between these agents and first-generation drugs.7 Overall, the differences were generally significant, but most often based on comparisons with haloperidol. Although haloperidol was the market leader at that point, making such comparisons somewhat logical, concerns have been raised that the choice of that medication, and its utilization in potentially higher than necessary doses, might have served to accentuate differences in the risk for neurologic adverse effects. The challenge of conducting studies, which take into account and adequately control for the relative dose equivalences of specific medications across a range of illness phases, patient ages, and outcome domains (ie, therapeutic and adverse effects), should not be minimized. In fact, one can easily argue that appropriately validated dose equivalences are generally lacking, and are usually derived from the analysis of large data sets from studies which were not necessarily designed to address these issues.
The largest study conducted comparing three different doses of haloperidol and three different doses of a second-generation medication with placebo8 provided an interesting perspective. Even doses of haloperidol as low as 4 mg were associated with significantly greater EPS than placebo or the “atypical” medication sertindole. In addition, a recent meta-analysis9 examined the effect of haloperidol dosage on the relative need for antiparkinsonian medication in trials comparing second-generation medications with haloperidol. Overall, the authors found that the superiority remained whether the dosages of haloperidol employed were above or below 12 mg/day. Similarly with tardive dyskinesia, the meta-analyses which have been conducted5 support the significantly reduced risk of tardive dyskinesia with the second-generation antipsychotics. Overall, the risk appears to be one fifth of what it had been with conventional medications. (We will return to this issue in the discussion of results from the effectiveness studies).
Metabolic adverse effects
At the same time that clinicians and patients benefited from a reduction in the risk of neurologic adverse effects, it became apparent that some of the second-generation medications had a strong propensity to contribute to an increase in weight and metabolic adverse effects, such as insulin resistance and dyslipidemia.10 It has taken several years to clarify this risk, the extent to which medications contributed and the relative risk associated with specific medications. In addition, it has also become apparent that drug-naïve patients are likely to show more pronounced effects, even with those medications on the lower end of the risk spectrum, in comparison with patients who have already been chronically treated.11
With accumulating data emphasizing the substantially shortened life expectancy of patients with schizophrenia,12 the prevention and management of cardiometabolic effects has taken on increasing salience. Given the challenges confronting patients with chronic psychiatric conditions in terms of diet, exercise, healthy lifestyle behaviors, smoking, substance use and abuse, etc, the proactive medical management of such individuals remains an enormous unmet need.13
Relative efficacy
Several literature reviews and meta-analyses have been published in recent years, which have attempted to determine the extent to which various drugs and drug classes differ in their efficacy.9,14,15
It appears that in the treatment of acute psychosis among patients with first-episode or recent-onset illness that there are not significant differences in overall response rates of psychotic signs and symptoms with different antipsychotic drugs or drug classes.16-19 In general, response rates among such patients are quite high.
However, in the maintenance phase of treatment following acute response among first-episode patients, differences do begin to emerge favoring second-generation medications, incuding olanzapine and risperidone,19-22 as well as amisulpride, quetiapine, and ziprasidone.19 In the treatment of multiepisode patients the picture becomes more complicated. The enthusiasm with which the second-generation drugs were received was fueled by unmet need, a long period without any new antipsychotics, vigorous marketing, and to some extent “wishful thinking” as clinicians would also like to believe that they have new and better tools with which to help their patients.
Over time as the cost of medications escalated, intense debate ensued about the relative merits of the different drugs and drug classes. Large “effectiveness” studies such as CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness),17 CUtLASS (Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia),3,24 and EUFEST (European First Episode Schizophrenia Trial)19 were intended, to some extent, to clarify this issue.
The data from these trials must be considered along with the data from all other trials which have been the subject of a series of meta-analyses. Single studies, no matter how large, and meta-analyses, no matter how comprehensive, all have their limitations, but it is incumbent upon us to assimilate, objectively integrate and draw relevant conclusions from the evidence, such as it is.
In comparing first and second-generation antipsychotics (FGAs and SGAs),9 Leucht et al found four SGAs (amisulpride, clozapine, olanzapine, and risperidone) to be more efficacious than FGAs with effect sizes ranging from small to medium (0.13 for risperidone and 0.52 for clozapine). Leucht et al emphasized that the SGAs which were more efficacious showed these advantages for both of the specific domains of positive and negative symptoms, suggesting that their superiority for negative symptoms does not represent a “core component of atypicality.” As noted previously, all SGAs had fewer EPS than haloperidol, even when the latter was used in doses below 7.5 mg/day.9
When Leucht et al9 compared their results to those of other meta-analyses by Geddes et al, Davis et al, and the Cochrane Schizophrenia Group, 25-27 the effect sizes were generally comparable. All meta-analyses - analyzing similar data sets - found that amisulpride, clozapine, olanzapine, and risperidone were significantly more efficacious than FGAs.
When Leucht and colleagues14 reviewed the head-tohead comparisons of SGAs they reported a similar pattern. Regarding total PANSS score change, olanzapine was more efficacious than aripiprazole, quetiapine, risperidone, and ziprasidone; risperidone was more efficacious than quetiapine and ziprasidone, and amisulpride was not statistically different from olanzapine or risperidone. However, regarding dropouts due to inefficacy, olanzapine was only superior to quetiapine and ziprasidone, two SGAs often dosed inappropriately low in schizophrenia, and amisulpride was more efficacious than ziprasidone. Surprisingly, clozapine only proved to be superior to zotepine and to risperidone in dropouts due to inefficacy. As the authors suggest, possibly inadequate doses of clozapine used in some studies might have contributed to these findings, which are inconsistent with most of the large individual studies.
In comparing these analyses to the results of CATIE and CUtLASS, there are some consistencies. In CATIE,17 olanzapine was superior in dropout rates due to inefficacy and time on effective treatment (at least when including the 23% of patients who were rerandomized to olanzapine), and clozapine (though given openly) was superior to all other studied SGAs in phase 2.28 In CUtLASS,24 clozapine was superior to other SGAs as well, but in this study, quality of life, and not all-cause discontinuation, was the primary outcome measure. This difference highlights the importance of the choice of the primary outcome measure, particularly in effectiveness studies, where the outcome is supposed to address elements of efficacy, tolerability, patient acceptability, and functioning.
Although the meta-analyses tended to find more support for risperidone's superiority than CATIE did, the dose equivalences used in CATIE have been challenged where the modal dose of risperidone was only 3.9 mg/day.
However, there were no differences between the individually studied SGAs and the FGA comparator perphenazine in CATIE,17 as well as between the class of clinician's choice SGAs and FGAs (mainly consisting of sulpiride, which some consider to be an SGA) in CUtLASS.23
The large effectiveness trials have been discussed in great detail elsewhere. Despite the rigorousness with which we try to design trials, there are always compromises in both design and execution that are unavoidable, particularly when multiple outcome measures are included and multiple questions are addressed simultaneously.
A particularly important issue which impacts all of the effectiveness trials was stressed by Kraemer et al.29 Whether a study is designed to establish superiority of one treatment or groups of treatments over others or to establish equivalence is critical. As Kraemer et al suggest, if a study is designed to demonstrate one treatment's superiority, then statistically nonsignificant results should not be assumed to be evidence of “equivalence.” To test this, a true noninferiority design is needed that generally requires larger samples.
The inevitable conclusions from these data are that drugs and drug classes are heterogeneous, and that we should not assume commonalities based on anything except appropriate comparisons. It is also obvious that every drug involves its own risks and benefits, and that clinicians have to evaluate data and make decisions based on the individual patients' presentation, history, sensitivities, preferences, responses, adverse effects, etc (Table I).
Table I. Considerations in choosing antipsychotic medications.
| Illness profile | Patient profile | Medication profile |
| History of illness onset and course | Vulnerability to adverse effects | Efficacy |
| Tolerance of adverse effects | Tolerability (short and long-term) | |
| Insight and attitude toward illness | Delivery methods/formulations available | |
| Presenting signs and symptoms | Need for monitoring | |
| Preference for treatment approaches | Availability/cost | |
| Pharmacokinetics | ||
| Past treatment response | Comorbid medical conditions | |
| Comorbid psychiatric conditions | ||
| Comorbid substance abuse | ||
| Social support network |
This serves as a segue into the next section of this discussion, which focuses not so much on which drug to choose, but how to conceptualize and evaluate response (both therapeutic and adverse) in order to inform treatment decisions (which may or may not involve changing medication). It is our firm belief that the real challenges and opportunities in treating patients with schizophrenia lie in how treatments are managed, evaluated, and potentially altered, rather than which drug one chooses for an initial trial.
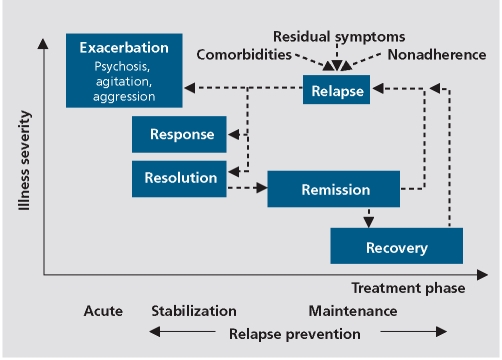
As with all treatment planning, formulating and tracking treatment goals and outcomes is important (Figure 1).
Figure 1. Treatment stages.
Treatment outcome
Response
An important issue for clinicians is how to decide when and if a particular treatment is having the desired effect or is producing adverse effects that are not acceptable. In psychiatry there are few objective measures comparable to the laboratory tests, physical signs, or imaging results that can inform treatment decisions in other areas of medicine. We tend to rely on our subjective impressions of a patient's (subjective) report and our observations of changes in their affect, thoughts/speech, and behavior. We would be better served by using (even brief) quantitative assessment instruments, but this has yet to be accepted on a wide scale.
Response to treatment is generally assumed to mean a clinically significant improvement in the “chief complaint” or the psychopathology associated with the condition. How do clinicians (and patients) decide when improvement is “enough,” or whether the treatment should be altered in some fashion? This requires attention to issues related to dosage and duration of treatment as well as adherence in medication-taking, bioavailability, and metabolism.
Although clinical trials often use percentage improvement over baseline to measure treatment “response,” we are ultimately most interested in where patients end up in terms of the degree of residual psychopathology. This in no way diminishes the importance of a major improvement, but it is likely that the degree of residual psychopathology will be the critical factor in determining subsequent treatment decisions.
Remission
From this perspective, the remission criteria30 are a valuable tool. Although some clinicians might assume it unrealistic to expect remission to occur during a relatively short-term (4- to 6-week) treatment trial, data from large meta-analyses31 suggest that a substantial proportion of patients can achieve remission within 4 to 6 weeks.
The proposed remission criteria30 focused on seven characteristic signs and symptoms associated with the diagnosis of schizophrenia and selected the corresponding items on validated rating scales, such as the Positive and Negative Syndrome Scale (PANSS),32 or the Scale for the Assessment of Positive Symptoms (SAPS)33 and the Scale for the Assessment of Negative Symptoms (SANS),34 which assessed all of these positive and negative symptoms. According to the criteria, a patient is in remission if for example, the eight corresponding PANSS items are rated as no greater than mild, concurrently for at least 6 months. (The criteria have also been used on a cross-sectional basis as a measure of absolute treatment response as referenced to previously).
If a patient does not achieve remission, the clinician has to conduct a thorough evaluation of potential reasons, eg, diagnostic error, nonadherence, inadequate dosage, inadequate blood level, comorbid condition(s), substance abuse, drug-drug interaction, adverse effects interfering with clinical response, ineffective drug, etc. After conducting such an evaluation, a decision must be made as to what action to take based on the results. Assuming that the only reasonable explanation remains the drug's lack of efficacy for that particular patient, then whether to wait for additional response, add a second drug (of the same or different class) or carry out a complete switch to an alternative agent, is the decision that must be made.
Recovery
To capture more than just symptom reduction (response) or an absolute level of psychopathology (remission), the concept of recovery has gained more acceptance. This is due to the fact that functional outcomes are the ultimate goal of interventions in schizophrenia. In this context, Liberman and Kopelewicz35 proposed what has come to be a widely accepted definition of recovery, including 4 domains with criteria that must all be met concurrently for at least 2 years. In addition to symptomatic remission as defined above, it also includes a minimum level of educational/vocational functioning, the ability to perform day-to-day living tasks without supervision, and a minimum level of social interactions of at least one social contact per week outside of the family. Unfortunately, even in a well-staffed first episode program, as few as 13.7% of patients were able to meet these criteria at least once during a 5-year follow-up period.36 This indicates that in addition to pharmacologic treatments and broad psychosocial interventions, targeted psychosocial treatments will need to be combined in an individualized way to enhance psychosocial and educational/vocational functioning. Since data from the same group indicated that stopping medications was the primary reason for symptomatic relapse,37 strategies to enhance the level of medication adherence are also a critical component for achieving remission and recovery.
Time course of antipsychotic effect
If all of the above considerations are addressed, one of the most challenging issues remains whether or not the patient has had an “adequate” trial. The response to medications varies considerably between patients. When we asked experts38 how long an adequate initial trial should last, the responses ranged from 2.6 to 5.5 weeks. Textbooks of psychiatry had generally stated that response might be delayed for weeks rather than days.39 Recent meta-analyses have challenged that assumption. Agid et al40 evaluated 42 studies including 7450 patients and found that the greatest proportion of improvement in psychotic signs and symptoms (even controlling for placebo response) in short-term trials occurred in the first week. Leucht et al41 replicated these results utilizing individual patient data. In addition, when examining data available in a subset of patients at 1 year, they found that most of the drug effect observed at 1 year had already occurred by week 4. Subsequent post-hoc analyses42 found significant separation between drug and placebo effects on positive psychotic symptoms even after only 24 hours.
These data have reinvigorated the effort to use early response/nonresponse as a predictor of subsequent response.43,44 Correll et al45 were the first to attempt to predict nonresponse at 4 weeks using the change of symptoms at 1 week in a sensitivity-specificity analysis in 131 patients receiving uniform treatment with fluphenazine. When Leucht et al46 conducted a receiver-operator analysis to answer this question, a response of less than 20% improvement on the total Brief Psychiatric Rating Scale47 (BPRS) best predicted nonresponse at 4 weeks. Chang et al48 reported similar results in 123 patients treated with risperidone, and Leucht et al49 replicated their earlier findings in 1996 patients from pooled olanzapine clinical trials.
Kinon et al50 and Ascher-Svanum et al51 reported on post-hoc analyses of 1077 patients who had participated in a series of double-blind trials involving olanzapine and found that a less than 20% reduction in PANSS scores at 2 weeks was associated with good predictive power to identify patients unlikely to respond by 12 weeks. Patients with poor early response were also found to be more likely to discontinue from the trial and their cost of care was significantly higher than those with more robust early response.51
Kinon et al52 conducted a prospective study of 630 patients treated with risperidone (2 to 6 mg/day). The 192 patients who completed 2 weeks and experienced a less than 20% improvement on the PANSS at 2 weeks were then randomized to either continue on risperidone or be switched to olanzapine 10 to 20 mg/day for an additional 10 weeks. This first prospective study of its kind replicated the results of the retrospective, post hoc analysis described previously. The initial nonresponders were unlikely to respond when given up to 10 weeks additional time on the same medication. The authors found a very modest gain in switching to olanzapine; however, specific subgroups of patients might be identified who benefit more dramatically from a switch, ie, those who were more severely ill.
Although the thresholds of minimal response and ultimate response are debatable, these results are generally consistent in suggesting that lack of at least minimal response after 2 weeks of treatment is a strong indicator that the current treatment is not likely to bring about substantial response. (The decision as to what change measure to use will depend on the goals of the analysis and such choices should be guided by empirical data whenever possible). The challenge at this point is to determine what alternative treatments are likely to have a greater likelihood of success.
Managing poor or partial responders
There are remarkably few studies which have identified poor or partial responders and randomly assigned consenting subjects to alternative strategies while also controlling for the passage of time by including a control group that stays on the original treatment. This type of design is needed to determine the efficacy of raising the dose, adding a second drug, or completely changing the medication.
Kinon et al53 reported on 115 newly admitted schizophrenia patients treated for 4 weeks with fluphenazine 20 mg/day. Those who failed to achieve the a priori defined remission level of positive symptoms (ie, no more than mild positive symptoms) were randomly assigned, double-blind, to continue on the same treatment, have the dose quadrupled, or be switched to haloperidol at an equivalent (20 mg/day) dose and followed for an additional 4 weeks. There were no significant differences in the outcomes between the three groups and, overall, only an additional 9% of patients met remission criteria at 8 weeks. This design needs to be applied with second-generation medications and ideally should also include polypharmacy and clozapine as comparators.
There is some suggestion that if a switch from one antipsychotic to another is implemented that choosing an agent with a different receptor binding profile would make the most sense. In a post-hoc analysis of CATIE, Stroup et al54 reported that among the 114 subjects who had been considered unsuccessfully treated with perphenazine in Phase I, those who were randomized to olanzapine or quetiapine did better than those randomized to risperidone, which has a more similar pattern of tight D2 blockade.
Antipsychotic polypharmacy
The notion that switching to a medication with a different receptor binding profile might be helpful in poor or partial responders is also the rationale for antipsychotic polypharmacy. In addition, the complex receptor binding profile of clozapine has suggested to some that attempts to simulate this effect should involve combining multiple drugs.
There are several reports indicating a high prevalence of antipsychotic polypharmacy in recent clinical practice.55 Despite widespread use, there is relatively little evidence that this strategy is helpful, particularly when clozapine is not involved.
Correll et al56 reviewed 19 randomized trials including 1216 subjects. Although the overall results could be interpreted as suggesting an advantage for combination therapy in comparison with monotherapy, the factors which appeared to contribute to this effect limit the conclusions that can be drawn for the present context. Combination therapy was more likely to be efficacious when administered from the start of treatment rather than waiting to identify poor or partial responders and when clozapine was involved. Studies which took place in China and studies which lasted longer than 10 weeks were also more likely to be positive. This leaves the question unresolved as to whether or not adding a second antipsychotic is likely to be helpful when a patient fails to derive an adequate response from an initial trial of monotherapy with drugs other than clozapine.
Adding a second antipsychotic to mitigate adverse effects from another medication is a different situation, and there is some suggestion that, for example, adding aripiprazole to clozapine can lead to a reduction in weight and/or lipid abnormalities.57,58
Clozapine
Since the Kane et al6 study, clozapine has been considered the best established treatment for refractory patients. In addition, clozapine has been shown to be superior to second-generation antipsychotics even among patients who were only moderately ill7 and would not necessarily have met criteria for true treatment resistance. The superiority of clozapine has been demonstrated in subsequent individual studies59,28,24 and metaanalysis.60,61,62
However, as mentioned previously, in a recent metaanalysis by Leucht et al14 of 28 randomized, head-to-head comparisons of clozapine with other second-generation antipsychotic drugs, clozapine did not show consistent superiority. Nevertheless, many if not most of these studies used low or very low doses of clozapine or patients were not truly poor responders.
Other pharmacologic classes in augmentation strategies
Numerous other classes of agents have been studied to determine their ability to augment the effects of antipsychotics in the treatment of patients with schizophrenia, either in general or in poor or partial responders. Cochrane reviews of benzodiazipines,63 lithium,64 and valproate65 could find no clear evidence of efficacy. A systematic review of carbamazepine66 was also negative.
Lamotrigine has been examined in a Cochrane review including five studies involving 537 participants.67 Some evidence of efficacy was found, but the results were not considered robust. Tiihonen et al68 have reported evidence from a post-hoc analysis of patients from five trials involving lamotrigine augmentation of clozapine which suggested some significant effects, but interpretation remains difficult because of different designs and inclusion criteria. Further well-designed studies involving lamotrigine would be very valuable.
Since available antipsychotics generally have only limited efficacy for negative symptoms and since negative symptoms are closely related to functional outcomes, various augmentation strategies of antipsychotics have been tested in this domain. Despite positive results in initial, small scale trials with N-methyl-D-aspartate (NMDA) receptor agonistic treatments, such as glycine, d-alanine, d-serine, dcycloserine, the largest placebo-controlled study of glycine and d-cycloserine was negative.69 However, recent trials of augmentation treatment with glycine transporter inhibitors have been positive,70 suggesting that this mechanism may be more promising. In addition a meta-analysis of 5 smallscale trials of adjunctive treatment with antidepressants concluded that these agents may reduce negative symptoms in patients with a predominantly negative symptom profile.71 However, since depression can mimic negative symptoms and since these trials had only 16 or less patients in each treatment arm, more data are needed.
Maintenance treatment
Once the maximum degree of therapeutic response is achieved after an acute exacerbation, the challenge becomes maintaining those gains, preventing relapse and facilitating the ongoing application of appropriate psychosocial and vocational therapies.
There is little question about the indications for continuing antipsychotic medication on an indefinite basis, except perhaps in patients who have only experienced one episode. Even there, however, relapse rates are 82% after 5 years,37 and discontinuing medication is associated with a five times higher risk of relapse than staying on medication. This does suggest, however, that a small subgroup of patients might remain free of relapse, but at present we have no means to identify such individuals prior to making the decision to stop antipsychotic maintenance therapy.
The choice of medication takes on particular importance when long-term treatment is the focus, as the benefitto-risk ratio may change substantially. Some drugs are associated with greater or lesser degrees of specific longterm risks, eg, tardive dyskinesia, weight gain, type 2 diabetes, dyslipidemia, etc. Risk not only varies by drug, but of course also from individual to individual. At present, taking a good history and appropriate ongoing monitoring is the best strategy to identify particular risk profiles, but it is hoped that in the not-too-distant future pharmacogenetics might help in informing choice of optimum treatment(s).72
With regard to relapse prevention, there is some evidence that the second-generation antipsychotics are associated with lower relapse rates than first-generation antipsychotics. Leucht et al73 conducted a meta-analysis of doubleblind random assignment studies which lasted at least one year and compared relapse rates between the respective drugs. The average relapse rate among second-generation drugs after 1 year was 15% compared with 23% among first-generation medications, a statistically significant difference (P<.001) and a relative risk reduction of 35%. We do not have a definitive explanation for this difference, and although improved adherence might seem like the most parsimonious explanation, the data reported from the trials included in the meta-analysis do not support the assumption that improvements in adherence are a sufficient explanation. It is possible that the differences in receptor binding profile might explain this effect, but again clear evidence of any specific receptor effect is lacking.
Adverse effects
The appropriate recognition and treatment of adverse effects of antipsychotics is relevant in the overall management of schizophrenia. Adverse effects can interfere with treatment adherence, functional capacity, subjective well-being, quality of life, and life expectancy14 Like for efficacy, the measurement and monitoring of side effects should be part of routine treatment. With regard to antipsychotics, key adverse effects that should be assessed regularly include sedation, sleep difficulties, sexual and reproductive system problems, extrapyramidal side effects and involuntary movements, and weight change, as well as abnormalities in blood pressure and in blood lipid and glucose levels.10,13
Unfortunately, recent data have shown that particularly the monitoring of potentially problematic metabolic side effects, such as elevations in fasting blood glucose and lipids, is quite suboptimal. This is a particular concern, as people with schizophrenia have been found to have elevated risk factors for cardiovascular morbidity and mortality compared with the general population.13 It appears that despite clear warnings and treatment recommendations,10 clinician's monitoring behavior has not increased in a relevant way, and the monitoring frequency is as low as in a nonpsychiatric control population treated with albuterol.74 Clearly, the field needs to consider reasons for this and take steps toward comprehensive education and quality improvement programs.
Switching strategies
As stated above, with few exceptions (eg, in treatmentrefractory patients or to avoid cardiovascular risk factor accumulation), it may be more important how the currently available medications are used and sequenced, rather than which particular medication is used. Due to the fact that a substantial proportion of patients with schizophrenia remains symptomatic and functionally impaired, develop treatment intolerability, or are dissatisfied with their treatment, switching between medications is frequent.
Most clinicians know from experience that changing medications can be a destabilizing event. In fact, even in the CATIE phase 1 trial, patients rerandomized in a double-blind fashion to the same antipsychotic stayed in the study longer than those who were switched to a different antipsychotic.75 Unless patients require a switch for an acute destabilization or life-threatening adverse event, clinicians need to consider the current psychosocial situation, level of support and symptomatic status of the primary disorder and of comorbid conditions when planning for a change in the medication regimen. In addition, consideration of the pharmacologic profiles of the pre-switch and the post-switch antipsychotic can also be helpful to predict the potential emergence of rebound phenomena that can complicate the switch process. The potential for rebound and withdrawal phenomena is greatest when the pre- and post-switch antipsychotics differ considerably regarding binding affinity for specific receptors (ie, pharmacodynamic dopaminergic, histaminergic, or cholinergic rebound) and/or when they differ considerably regarding their respective half-life (ie, pharmacokinetic dopamine rebound when the new antipsychotic has a much longer half-life and the prior antipsychotic is discontinued too quickly.)76
Histaminergic rebound is characterized by a reversal of the antihistaminergic anxiolytic, calming, sleep-inducing, and EPS-reducing effects, potentially resulting in rebound insomnia, anxiety, agitation, EPS, and restlessness. Similarly, cholinergic rebound is characterized by agitation, confusion and EPS, and dopaminergic rebound can manifest as worsening or newly emerging agitation, aggression, psychosis, mania, akathisia, or withdrawal dyskinesia.
Pharmacodynamic and pharmacokinetic rebound phenomena can be avoided in many cases by implementation of an overlapping, or “plateau” crosstitration,77 or by treating withdrawal/rebound symptoms with timelimited, targeted use of benzodiazepines, antihistamines, mirtazapine, anticholinergics, gabapentin, or nonbenzodiazepine anxiolytics/sedatives.
Conclusions
Despite pharmacologic advances, the treatment of schizophrenia remains challenging, and suboptimal outcomes are still too frequent. Even though treatment goals of response, remission, and recovery have been defined more uniformly, the field is lacking a functionally relevant “effectiveness” measure as used in large, pragmatic trials in cardiology or oncology. Moreover, measurement-based approaches, which are standard in research, need to be applied more broadly to clinical practice.
There is an ongoing debate as to whether and which first- or second-generation antipsychotics should be used, and in which particular patients. However, an individualized treatment strategy needs to consider both current symptoms, comorbid conditions, past therapeutic and adverse effect response, and patient choice and expectations. Moreover, acute and long-term goals and medication effects need to be balanced. While the acute response to appropriately dosed first-generation antipsychotics may not differ much from second-generation antipsychotics, advantages of lower rates of extrapyramidal side effects, tardive dyskinesia, and, possibly, relapse may favor second-generation antipsychotics. However, when considering individual adverse effect profiles, the differentiation into first- and second-generation antipsychotics as unified classes cannot be supported, and a more differentiated view and treatment selection is required.
To date, clozapine is the only evidence-based treatment for refractory patients, and the role of antipsychotic polypharmacy and other augmentation strategies remains unclear, at best. To improve the treatment outcomes in schizophrenia, research efforts are needed that elucidate biomarkers of the illness and of treatment response (both therapeutic and adverse effects). Moreover, new treatment options are needed that affect nondopaminergic targets with relevance for symptom reduction in various domains, relapse prevention, enhanced efficacy for nonresponders, and reduced key adverse effects. Effective treatments for cognitive dysfunction and negative symptoms and those positively affecting functional outcomes are sorely needed. Furthermore, the combined use of pharmacologic and nonpharmacologic cognitive, behavioral, vocational, and psychosocial approaches needs to be studied more, and management strategies for lack of illness insight and adherence should be developed further.
Acknowledgments
Grant support: The Zucker Hillside Hospital Advanced Center for Intervention and Services Research for the Study of Schizophrenia (MH074543-01) from the National Institute of Mental Health, Bethesda, Maryland.
Disclosures: Dr Kane has been a consultant to Astra-Zeneca, Janssen, Pfizer, Eli Lilly, Bristol-Myers Squibb, Otsuka, Vanda, Proteus, Takeda, Targacept, Intracellular Therapeutics, Rules Based Medicine , and has received honoraria for lectures from Otsuka, Eli Lilly, Bristol-Myers Squibb, and Janssen. Dr Correll has been a consultant and/or advisor to Actelion, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Janssen/J&J, GSK, Hoffmann-La Roche, Medicure, Otsuka, Pfizer, Schering-Plough, Supernus, Takeda, and Vanda.
Contributor Information
John M. Kane, The Zucker Hillside Hospital, North Shore-Long Island Jewish Health System, Glen Oaks, NY and Albert Einstein College of Medicine, Bronx, NY, USA.
Christoph U. Correll, The Zucker Hillside Hospital, North Shore-Long Island Jewish Health System, Glen Oaks, NY and Albert Einstein College of Medicine, Bronx, NY, USA.
REFERENCES
- 1.Davis JM. Maintenance therapy and the natural course of schizophrenia. J Clin Psychiatry. 1985;46:18–21. [PubMed] [Google Scholar]
- 2.Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–526. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- 3.Saltz BL, Woerner MG, Kane JM, et al. Prospective study of tardive dyskinesia incidence in the elderly. JAMA. 1991;266:2402–2406. [PubMed] [Google Scholar]
- 4.Morgenstern H, Glazer WM. Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Results of the Yale Tardive Dyskinesia Study. Arch Gen Psychiatry. 1993;50:723–733. doi: 10.1001/archpsyc.1993.01820210057007. [DOI] [PubMed] [Google Scholar]
- 5.Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of oneyear studies. Am J Psychiatry. 2004;161:414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- 6.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatmentresistant schizophrenic. A double-blind comparison with chlorpromazine. . Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 7.Kane JM. Extrapyramidal side effects are unacceptable. Eur Neuropsychopharmacol. 2001;11(suppl 4):S397–S403. doi: 10.1016/s0924-977x(01)00109-2. [DOI] [PubMed] [Google Scholar]
- 8.Zimbroff DL, Kane JM, Tamminga CA, et al. and The Sertindole Study Group: A controlled, dose-response study of sertindole and haloperidol in schizophrenia. Am J Psychiatry. 1997;1154:782–791. doi: 10.1176/ajp.154.6.782. [DOI] [PubMed] [Google Scholar]
- 9.Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol Med. 2009;39:1591–1602. doi: 10.1017/S0033291709005455. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipyschotic Drugs and Obesity and Diabetes. J Clin Psychiatry. 2004;65;2:267–273. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 11.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 13.Correll CU. Balancing efficacy and safety in treatment with antipsychotics. CNS Spectr. 2007;12(10 suppl 17):12–20, 35. doi: 10.1017/s1092852900026298. [DOI] [PubMed] [Google Scholar]
- 14.Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of headto-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–163. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 15.Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- 16.Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163:2096–2102. doi: 10.1176/ajp.2006.163.12.2096. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 18.McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1050–1060. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- 19.Kahn RS, Fleischhacker WW, Boter H, et al. EUFEST Study Group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomized clinical trial. . Lancet. 2008; 371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 20.Schooler N, Rabinowitz J, Davidson M, et al. Risperidone and haloperidol in first episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162:947–953. doi: 10.1176/appi.ajp.162.5.947. [DOI] [PubMed] [Google Scholar]
- 21.Green AI, Lieberman JA, Hamer RM, et al. HGDH Study Group. Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophr Res. 2006;86:234–243. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1366–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 23.Jones PB, Barnes TRE, Davis L, et al. Randomized controlled trial of the effect on quality of life of second vs first generation antipsychotic drugs in schizophrenia. Cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SW, Barnes TR, Davies L, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32:715–723. doi: 10.1093/schbul/sbj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and metaregression analysis. BMJ. 2000;2;321:1371–1376. doi: 10.1136/bmj.321.7273.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of secondgeneration antipsychotics. Arch Gen Psychiatry. 2003;60:553–564. doi: 10.1001/archpsyc.60.6.553. [DOI] [PubMed] [Google Scholar]
- 27.Adams CE, Coutinho E, Davis JM, et al. Chichester, UK. John Wiley & Sons. Cochrane Schizophrenia Group in The Cochrane Library. 2006 [Google Scholar]
- 28.McEvoy JP, Lieberman JA, Stroup TS, et al. CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypicalanti psychotic treatment. Am J Psychiatry. 2006;163:600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer HC, Glick ID, Klein DF. Clinical trials design lessons from the CATIE study. Am J Psychiatry. 2009;166:1222–1228. doi: 10.1176/appi.ajp.2009.08121809. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 31.Beitinger R, Lin J, Kissling W, Leucht S. Comparative remission rates of schizophrenic patients using various remission criteria. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1643–1651. doi: 10.1016/j.pnpbp.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 33.Andreasen NC. Iowa City, IA: The University of Iowa. The Scale for the Assessment of Positive Symptoms (SAPS) 1984 [Google Scholar]
- 34.Andreasen NC. Iowa City, Iowa: The University of Iowa. The Scale for the Assessment of Negative Symptoms (SANS) 1983 [Google Scholar]
- 35.Liberman RP, Kopelowicz A. Teaching persons with severe mental disabilities to be their own case managers. Psychiatr Serv. 2002;53:1377–1379. doi: 10.1176/appi.ps.53.11.1377. [DOI] [PubMed] [Google Scholar]
- 36.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161:473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 37.Robinson D, Woerner M, Alvir J, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56: 241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- 38.Kane JM, Leucht S, Carpenter D, Docherty JP. Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5–19. [PubMed] [Google Scholar]
- 39.Gelder, M. G., Lopez-Ibor, J. J., Andreasen, N. New York, NY: Oxford University Press. . New Oxford Textbook of Psychiatry. 2000 [Google Scholar]
- 40.Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- 41.Leucht S, Busch R, Hamann J, Kissling W, Kane JM. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57:1543–1549. doi: 10.1016/j.biopsych.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 42.Agid O, Kapur S, Warrington L, Loebel A, Siu C. Early onset of antipsychotic response in the treatment of acutely agitated patients with psychotic disorders. Schizophr Res. 2008;102:241–248. doi: 10.1016/j.schres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Nedopil N, Pflieger R, Ruther E. The prediction of acute response, remission and general outcome of neuroleptic treatment in acute schizophrenic patients. Pharmacopsychiatria. 1983;16:201–205. doi: 10.1055/s-2007-1019499. [DOI] [PubMed] [Google Scholar]
- 44.Bartko G, Herczeg I, Bekesy M. Predicting outcome of neuroleptic treatment on the basis of subjective response and early clinical improvement. J Clin Psychiatry. 1987;48:363–365. [PubMed] [Google Scholar]
- 45.Correll CU, Malhotra AK, Kaushik S, McMeniman M, Kane JM. Early prediction of antipsychotic response in schizophrenia. Erratum in: Am J Psychiatry. 2005;162:1774. Am J Psychiatry. 2003;160:2063–2065. doi: 10.1176/appi.ajp.160.11.2063. [DOI] [PubMed] [Google Scholar]
- 46.Leucht S, Busch R, Kissling W, Kane JM. Early prediction of antipsychotic nonresponse among patients with schizophrenia. J Clin Psychiatry. 2007;68:352–360. doi: 10.4088/jcp.v68n0301. [DOI] [PubMed] [Google Scholar]
- 47.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 48.Chang YC, Lane HY, Yang KH, Huang CL. Optimizing early prediction for antipsychotic response in schizophrenia. J Clin Psychopharmacol. 2006;26:554–559. doi: 10.1097/01.jcp.0000246211.95905.8c. [DOI] [PubMed] [Google Scholar]
- 49.Leucht S, Shamsi SA, Busch R, Kissling W, Kane JM. Predicting antipsychotic drug response - replication and extension to six weeks in an international olanzapine study. Schizophr Res. 2008;101:312–319. doi: 10.1016/j.schres.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Kinon BJ, Chen L, Ascher-Svanum H, et al. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. . Schizophr Res. 2008;102:230–240. doi: 10.1016/j.schres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Ascher-Svanum H, Nyhuis AW, Faries DE, Kinon BJ, Baker RW, Shekhar A. Clinical, functional, and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophrenia. Schizophr Bull. 2008;34:1163–1171. doi: 10.1093/schbul/sbm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35:581–590. doi: 10.1038/npp.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinon BJ, Kane JM, Johns C, et al. Treatment of neuroleptic-resistant schizophrenic relapse. Psychopharmacol Bull. 1993;29:309–314. [PubMed] [Google Scholar]
- 54.Stroup TS, Lieberman JA, McEvoy JP, et al. CATIE Investigators. Effectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: a CATIE study. Am J Psychiatry. 2007;164:415–427. doi: 10.1176/ajp.2007.164.3.415. [DOI] [PubMed] [Google Scholar]
- 55.Correll CU, Kane JM. Is there a rationale for antipsychotic polypharmacy in schizophrenia? In: Fleischhacker WW, Hummer M, eds. Innsbruck, Austria: Verlag Integrative Psychiatrie. Schizophrene Stoerungen - State of the art III. 2004:95–112. [Google Scholar]
- 56.Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35:443–57. doi: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:720–731. doi: 10.4088/jcp.v69n0505. [DOI] [PubMed] [Google Scholar]
- 58.Fleischhacker WW, Heikkinen ME, Olié J-P, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Available on CJO. Int J Neuropsychopharmacol. 2010 May 12;doi:10.1017/S1461145710000490 doi: 10.1017/S1461145710000490. [DOI] [PubMed] [Google Scholar]
- 59.Rosenheck R, Cramer J, Xu W, et al. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. N Engl J Med. 1997;337:809–815. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- 60.Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990–999. doi: 10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- 61.Chakos MH, Alvir JM, Woerner MG, et al. Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Arch Gen Psychiatry. 1996;53:313–319. doi: 10.1001/archpsyc.1996.01830040049009. [DOI] [PubMed] [Google Scholar]
- 62.Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009;CD000059 doi: 10.1002/14651858.CD000059.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volz A, Khorsand V, Gillies D, Leucht S. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;CD006391 doi: 10.1002/14651858.CD006391. [DOI] [PubMed] [Google Scholar]
- 64.Leucht S, Kissling W, McGrath J. Lithium for schizophrenia. Review. PubMed PMID: 17636738. Cochrane Database Syst Rev. 2007;CD003834 doi: 10.1002/14651858.CD003834.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Schwarz C, Volz A, Li C, Leucht S. Valproate for schizophrenia. Cochrane Database Syst Rev. 2008;CD004028 doi: 10.1002/14651858.CD004028.pub3. [DOI] [PubMed] [Google Scholar]
- 66.Leucht S, Kissling W, McGrath J, White P. Carbamazepine for schizophrenia. Cochrane Database Syst Rev. 2007;CD001258 doi: 10.1002/14651858.CD001258.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Premkumar TS, Pick J. Lamotrigine for schizophrenia. Cochrane Database Syst Rev. 2006;CD005962 doi: 10.1002/14651858.CD005962.pub2. [DOI] [PubMed] [Google Scholar]
- 68.Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109:10–14. doi: 10.1016/j.schres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Buchanan RW, Javitt DC, Marder SR, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 70.Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and d-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2009:1–10. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- 71.Rummel C, Kissling W, Leucht S. Antidepressants for the negative symptoms of schizophrenia. Cochrane Database Syst Rev. 2006;3:CD005581. doi: 10.1002/14651858.CD005581.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malhotra AK, Lencz T, Correll CU, Kane JM. Genomics and the future of pharmacotherapy in psychiatry. Int Rev Psychiatry. 2007;19:523–530. doi: 10.1080/09540260701563460. [DOI] [PubMed] [Google Scholar]
- 73.Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry. 2003;160:1209–1222. doi: 10.1176/appi.ajp.160.7.1209. [DOI] [PubMed] [Google Scholar]
- 74.Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry. 2010;67:17–24. doi: 10.1001/archgenpsychiatry.2009.179. [DOI] [PubMed] [Google Scholar]
- 75.Essock SM, Covell NH, Davis SM, Stroup TS, Rosenheck RA, Lieberman JA. Effectiveness of switching antipsychotic medications. Am J Psychiatry. 2006;163:2090–2095. doi: 10.1176/ajp.2006.163.12.2090. [DOI] [PubMed] [Google Scholar]
- 76.Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47:9–20. doi: 10.1097/chi.0b013e31815b5cb1. [DOI] [PubMed] [Google Scholar]
- 77.Correll CU. Real-life switching strategies with second-generation antipsychotics. J Clin Psychiatry. 2006;67:160–161. doi: 10.4088/jcp.v67n0122. [DOI] [PubMed] [Google Scholar]