Summary
Accurate chromosome segregation requires assembly of the multi-protein kinetochore complex at centromeres. Although prior work identified the centromeric histone H3-variant CENP-A as the important upstream factor necessary for centromere specification, in human cells CENP-A is not sufficient for kinetochore assembly. Here, we demonstrate that two constitutive DNA-binding kinetochore components, CENP-C and CENP-T, function to direct kinetochore formation. Replacing the DNA-binding regions of CENP-C and CENP-T with alternate chromosome-targeting domains recruits these proteins to ectopic loci, resulting in CENP-A-independent kinetochore assembly. These ectopic kinetochore-like foci are functional based on the stoichiometric assembly of multiple kinetochore components including the microtubule-binding KMN network, the presence of microtubule attachments, the microtubule-sensitive recruitment of the spindle checkpoint protein Mad2, and the segregation behavior of foci-containing chromosomes. We additionally find that CENP-T phosphorylation regulates the mitotic assembly of both endogenous and ectopic kinetochores. Thus, CENP-C and CENP-T form a critical regulated platform for vertebrate kinetochore assembly.
Keywords: Mitosis, Centromere, Kinetochore, Chromosome Segregation
Introduction
During mitosis, cells must accurately partition their genome such that genetic information is transferred unperturbed to the progeny. In eukaryotes, accurate chromosome segregation requires each chromosome to interact appropriately with microtubules from the mitotic spindle. This interaction is mediated by the macromolecular kinetochore complex that forms upon centromere DNA (Cheeseman and Desai, 2008). Ensuring proper chromosome segregation and kinetochore function requires three key activities. First, the centromere must be specified at a single site on each sister chromatid. Failure to specify a centromere prevents chromosome segregation, while multiple functional centromeric sites could result in chromosome fragmentation due to inappropriate attachments to the spindle. Second, a specific chromosome structure surrounding the centromere must be generated to ensure that this region can resist spindle forces and prevent chromosomal twisting that would result in merotelically attached kinetochores. Third, the macromolecular kinetochore structure, composed of almost 100 different proteins (Cheeseman and Desai, 2008), must assemble upon the centromere in a cell cycle-regulated manner to generate a structure capable of binding to microtubules. These proteins include the KNL1/Mis12 complex/Ndc80 complex (KMN) network, which is a core component of the outer kinetochore-microtubule interface (Cheeseman and Desai, 2008).
Previous work defined the histone H3 variant CENP-A (also called CenH3), which forms specialized nucleosomes found predominantly at centromeres, as the key upstream factor required for kinetochore specification (Gascoigne and Cheeseman, 2010). However, the mechanisms that act to direct the assembly of the remaining kinetochore proteins remain largely unknown (Gascoigne and Cheeseman, 2010). Despite the requirement for CENP-A in specifying the site of kinetochore assembly (Howman et al., 2000), it is unclear whether CENP-A acts directly in kinetochore assembly as a platform to associate with other kinetochore proteins (Carroll et al., 2010; Carroll et al., 2009), or if it generates a specialized chromatin environment that is permissive for kinetochore formation. In Drosophila, CENP-A is sufficient to drive kinetochore assembly, as its ectopic localization causes the formation of additional kinetochore structures (Heun et al., 2006). In contrast, in human cells, the presence of CENP-A does not appear to be sufficient for kinetochore assembly (Van Hooser et al., 2001).
In addition to CENP-A, a group of 16 chromatin proximal proteins termed the Constitutive Centromere Associated Network (CCAN) is present at centromeres in vertebrate cells throughout the cell cycle (Amano et al., 2009; Foltz et al., 2006; Hori et al., 2008; Okada et al., 2006; reviewed in Cheeseman and Desai, 2008), and is required for correct formation of the mitotic kinetochore. However, the precise role of the CCAN in kinetochore assembly remains unclear. Several CCAN proteins, including the CENPT/W complex and CENP-C, have been shown to possess DNA binding activity (Hori et al., 2008; Sugimoto et al., 1994), while others such as CENP-N interact directly with CENP-A containing nucleosomes (Carroll et al., 2010; Carroll et al., 2009). Defining how these proteins form the interface between centromeric chromatin and the outer kinetochore microtubule binding proteins and how kinetochore assembly is regulated to recruit outer kinetochore components during mitosis are key goals.
To define the mechanisms that direct kinetochore assembly downstream of CENP-A, we analyzed the constitutive kinetochore components, focusing on the DNA binding proteins CENP-C and the CENP-T/W complex. We find that ectopic targeting of CENP-C and CENP-T can generate foci that function as kinetochore-like structures in the absence of CENP-A, demonstrating that CENP-C and the CENP-T/W complex function as core kinetochore assembly factors in vertebrate cells.
Results
CENP-A mis-targeting results in the ectopic recruitment of a small subset of kinetochore proteins
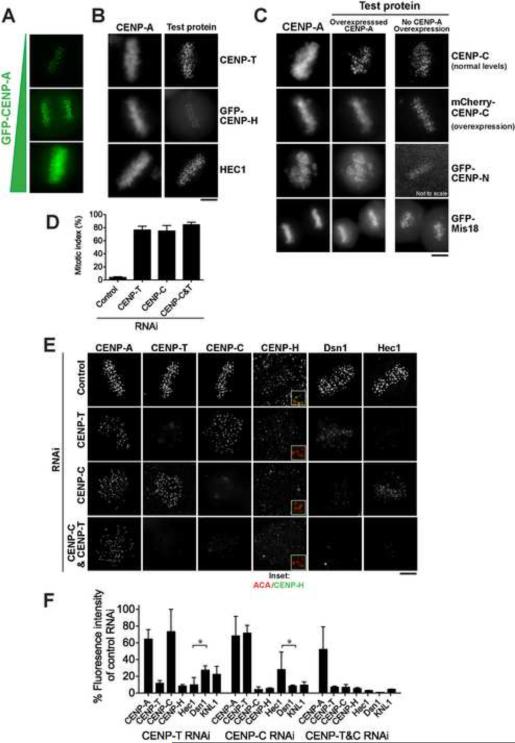
Specialized centromeric CENP-A nucleosomes are thought to provide the foundation for kinetochore assembly. To test the sufficiency of CENP-A for directing kinetochore assembly, we induced the mis-incorporation GFP-CENP-A into non-centromeric chromatin by overexpression in HeLa cells (Fig. 1A). Ectopically incorporated CENP-A resulted in the co-recruitment of only three proteins (CENP-C, CENP-N, and Mis18; Fig. 1C) out of 16 kinetochore proteins tested (Fig. 1B and S1). Thus, in human cells, mis-targeting of CENP-A does not result in ectopic kinetochore formation. CENP-C and CENP-N bind to CENP-A in vitro (Carroll et al., 2010; Carroll et al., 2009), and Mis18 functions as a CENP-A licensing factor (Fujita et al., 2007; Hayashi et al., 2004). In contrast to previous observations (Van Hooser et al., 2001), mis-targeting of CENP-C required co-overexpression of mCherry-CENP-C and GFP-CENP-A. CENP-A/CENP-C co-overexpression resulted in the mis-targeting of some additional kinetochore proteins, such as the Mis12 complex subunit Dsn1, to chromosome arms (Fig. S1B), but this occurred with lower frequency and at low levels. Thus, although CENP-A is essential for centromere specification, these data indicate that it is not sufficient for kinetochore assembly in humans.
Figure 1. Differential roles of CENP-A, the CENP-T/W complex, and CENP-C in kinetochore assembly.
(A) Images of HeLa cells with increasing levels of GFP-CENP-A expression. CENP-A localizes to chromosome arms at high expression levels. (B) Representative images of kinetochore components not mis-localized by CENP-A overexpression, visualized using anti-CENP-T and anti-Hec1 antibodies, or GFPLAP-CENP-H. (C) Images showing mis-localization of kinetochore components to chromosome arms in the presence of ectopic CENP-A relative to a GFP-H2B control. CENP-C was visualized by immunofluorescence, or by co-overexpression of mCherry-CENP-C. CENP-N or Mis18 were visualized using HeLa cell lines stably expressing GFPLAP fusions. (D) Graph showing quantification of mitotic index 48 h after the indicated depletions by RNAi. N=100 cells/condition, +/− SEM. (E) Representative immunofluorescence images of HeLa cells 48 h after RNAi depletion of the indicated proteins. Merge insets show ACA (red), CENP-H (green). (F) Quantification of kinetochore intensity 48 h following RNAi depletion of CENP-C, CENP-T, or CENP-C & CENP-T. N > 50 kinetochores, ≥ 5 cells, +/− SD. Asterisk indicates significant difference as determined by Mann Whitney U test P<0.005. Scale bars, 5 μm. Also see Fig. S1.
CENP-T/W and CENP-C are essential for kinetochore assembly
We next sought to define the contributions of other chromatin proximal proteins to kinetochore assembly. Depletion of CENP-C or CENP-T individually or in combination led to a mitotic arrest, but caused only a mild reduction in CENP-A and the reciprocal protein at centromeres (Fig. 1D–F), suggesting that these essential DNA-binding proteins are recruited independently. Importantly, co-depletion of CENP-C and CENP-T compromised the localization of all other kinetochore proteins tested, including representative components of the microtubule binding KMN network – KNL1, Dsn1 (Mis12 complex), and Ndc80/Hec1 (Fig. 1E), as well as the inner centromere protein Aurora B kinase (data not shown). However, CENP-C and CENP-T display potential differences in their relative requirement for the recruitment of different KMN network components (Fig. 1F). Thus, CENP-C and CENP-T are both required for kinetochore assembly.
The CENP-T N-terminus is required for kinetochore assembly
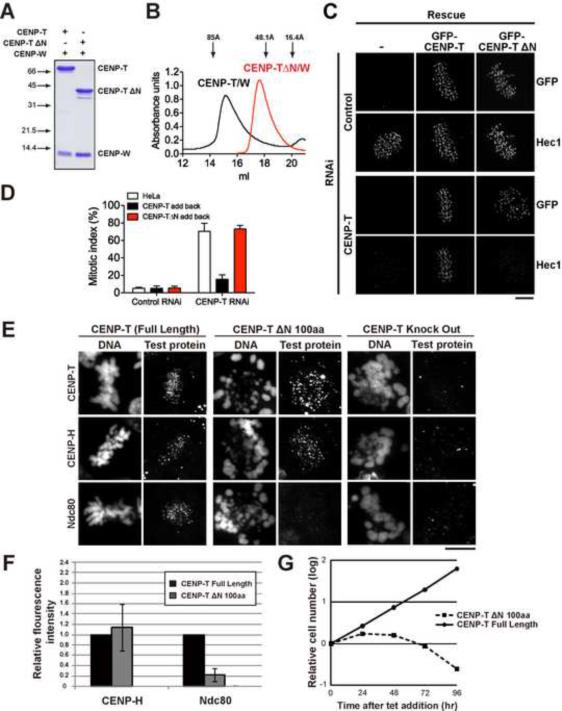
The DNA binding activity of CENP-T resides in a histone-like fold at its C-terminus (Hori et al., 2008; our unpublished results). Size exclusion chromatography of recombinant CENP-T/W complex indicated that it has an elongated structure, with a stokes radius of ~5.5 nm (Fig. 2B). Sucrose gradients indicated the complex does not exist as an oligomer (Fig. S2A), and removal of the N-terminal 288 amino acids dramatically shifted the elution profile of the complex (Fig. 2A and B), suggesting that the N-terminus of CENP-T has an extended structure. Analysis of the N-terminus of CENP-T in vivo indicated that is essential for kinetochore assembly and mitotic progression in both human cells and chicken DT40 cells. GFP-CENP-T ΔN-terminus (lacking amino acids 1–287 in human cells, or 1–100 in chicken DT40 cells), failed to rescue the mitotic arrest and delocalization of Hec1/Ndc80 from kinetochores following CENP-T depletion (Fig. 2C–G), suggesting that the N-terminus of CENP-T functions in recruiting downstream kinetochore components.
Figure 2. The CENP-T N-terminus is required kinetochore assembly.
(A) Coomassie-stained gel showing bacterially purified recombinant full length 6xHis-CENP-T/CENP-W or CENP-T-ΔN/CENP-W, lacking aa 1–288. (B) Graph showing the elution profile (OD280) of recombinant CENP-T/W complex and CENP-T-ΔN/W on a Superose 6 size exclusion column. Arrows indicate the migration of standards with known Stokes radii: Thyroglobulin (85 Å), Aldolase (48.1 Å) and RNase A (16.4 Å). (C) Immunofluorescence images showing levels of Hec1/Ndc80 48 h after RNAi depletion of CENP-T in HeLa cells or HeLa cell lines expressing RNAi resistant GFP-CENP-T or GFP-CENP-T-ΔN. Scale bar, 5 μm. (D) Graph showing quantification of the mitotic index 48 h after RNAi depletion of CENP-T in HeLa cells, or HeLa cell lines expressing RNAi resistant CENP-T or CENP-T ΔN. N=100 cells, +/− SEM. (E) Representative immunofluorescence images of chicken DT40 cells after depletion of endogenous CENP-T and expression of the indicated protein fragments. Scale bar, 10 μm. (F) Quantification of kinetochore intensity after depletion of CENP-T and expression of the indicated proteins. N = 200, +/− SD. (G) Graph showing viability of CENP-T conditional-depletion in DT40 cells expressing the indicated proteins after addition of tetracycline. Also see Fig. S2.
Targeting of CENP-C and CENP-T to ectopic foci is sufficient for core kinetochore assembly
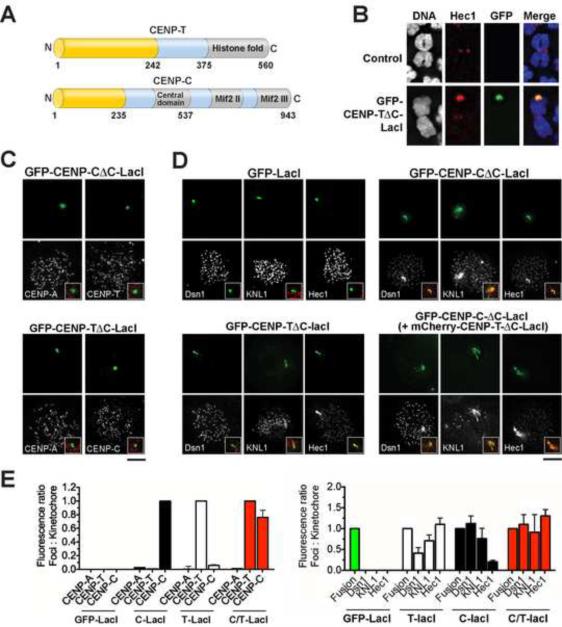
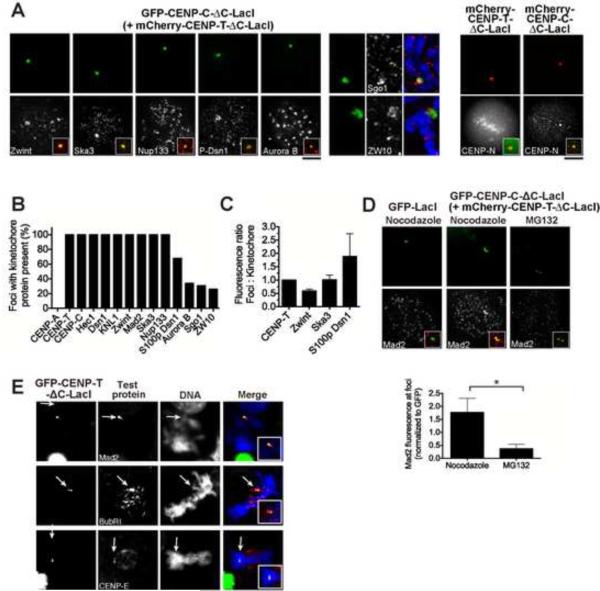
To determine whether CENP-C or CENP-T/W activity is sufficient for aspects of kinetochore assembly, we developed a strategy to target these proteins to ectopic loci. Overexpression of CENP-T was not sufficient to cause ectopic localization (Fig. S2B). We therefore replaced the C-terminal DNA-binding regions of CENP-T and CENP-C with alternate DNA binding domains, allowing us to target these proteins either throughout the chromosome using fusions to histone H2B (Fig. S3) or to a specific locus using a LacI fusion in a U2OS cell line with an array of Lac operator (LacO) repeats integrated into chromosome 1 (Janicki et al., 2004; Fig. 3A and 3B). In the experiments described below, the large number of LacO repeats present in this cell line resulted in foci that were 5–50 fold larger than endogenous kinetochores based on fluorescence intensity. Thus, while these ectopic LacI-directed foci allowed us to analyze the activity of CENP-T and CENP-C, these large foci are likely to differ from endogenous kinetochores.
Figure 3. Targeting of CENP-C-LacI and CENP-T-LacI to an ectopic focus causes mis-localization of the KMN network.
(A) Schematic representation of CENP-T and CENP-C. The N-terminal regions used in fusion protein experiments are indicated in yellow (amino acids 1–242 for CENP-T and 1–235 for CENP-C). DNA binding regions are indicated in gray (Hori et al., 2008; Yang et al., 1996). (B) Immunofluorescence images showing representative metaphase chromosome spreads from U2OS-LacO cells. Bottom, chromosome 1 with GFP-CENP-T-ΔC-LacI present at the LacO array. (C) Immunofluorescence images showing only centromeric localization of DNA binding kinetochore proteins in cells transiently expressing GFP-CENP-T-ΔC-LacI or GFP-CENP-C-ΔC-LacI after 15 h Nocodazole treatment. Insets show a merge of kinetochore protein (red) and LacI foci (green). (D) Immunofluorescence images showing co-localization of KMN network proteins with LacI foci in cells transiently expressing GFP-CENP-T-ΔC-LacI or GFP-CENP-C-ΔC-LacI, or co-expressing mCherry-CENP-T-ΔC-LacI (not shown) and GFP-CENP-C-ΔC-LacI. Insets show a merge of kinetochore protein (red) and LacI foci (green). (E) Quantitation of the relative fluorescence of the indicated kinetochore proteins at endogenous kinetochores versus ectopic foci in cells expressing LacI fusion proteins. Quantification was conducted after 15 h nocodazole treatments, >10 cells/condition, 20 kinetochores/cell, +/− SEM. Data is shown normalized to the foci/kinetochore ratio of the fusion protein. Also see Fig. S3 and Movie S4.
In the presence of either GFP-CENP-C-ΔC or GFP-CENP-T-ΔC fusions, endogenous CENP-A, CENP-T, and CENP-C did not co-localize with the fusion proteins (Fig. 3C and S3A). In contrast, representative KMN network subunits Dsn1 (Mis12 complex), KNL1, and Ndc80/Hec1 co-localized with GFP-CENP-C-ΔC and GFP-CENPT-ΔC fusions, but not with GFP controls (Fig. 3D and S3B). We also observed co-localization of Ndc80 and Nuf2 with GFP-CENP-T-ΔC-LacI fusions in chicken DT40 cells (Fig. S3F). Importantly, simultaneous expression of mCherry-CENP-T-ΔC and GFP-CENP-C-ΔC fusions resulted in levels of KMN network components at ectopic foci that were stoichiometrically proportional to the amount of CENP-T and CENP-C present at the foci relative to endogenous kinetochores (Fig. 3D and 3E; Fig. S3B). For example, if 5× more CENP-T was present at an ectopic focus relative to an endogenous kinetochore, 5× Dsn1, KNL1, and Ndc80/Hec1 were also present. Thus, in the absence of CENP-A, CENP-T and CENP-C can properly assemble the microtubule binding KMN network at non-centromeric sites.
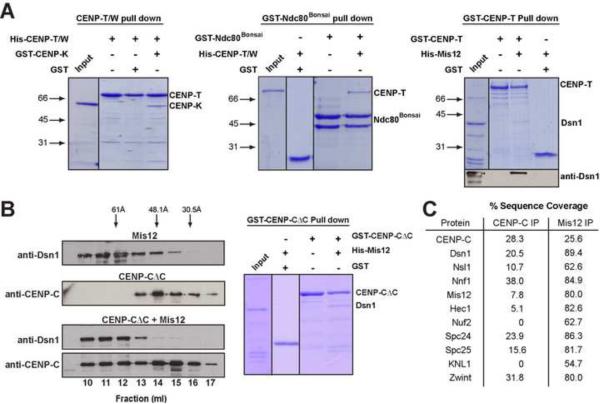
As for our depletion analysis (Fig. 1), we observed an asymmetric behavior in the mis-targeting of KMN network components in this ectopic assay; CENP-C-ΔC fusions preferentially recruited Dsn1 and KNL1, while CENP-T-ΔC-fusions preferentially recruited Ndc80/Hec1 (Fig. 3D, 3E and S3B). Consistent with this, our biochemical analysis indicated that CENP-T/W can bind directly to a shortened Ndc80 complex (Ndc80Bonsai; Ciferri et al., 2008), CENP-K, and weakly to the Mis12 complex (Fig. 4A). Similarly, we found that CENP-C can interact with the Mis12 complex in vivo and in vitro (Fig. 4B and C; also see Przewloka et al., 2011; Screpanti et al., 2011). These data demonstrate that the N-terminal regions of CENP-T and CENP-C can each act directly to recruit the KMN network to kinetochores during mitosis.
Figure 4. The CENP-T/W complex and CENP-C interact directly with KMN network components in vitro.
(A) The CENP-T/W complex binds directly to CENP-K, GST-Ndc80Bonsai, and the Mis12 complex in vitro. Left, Coomassie-stained gel showing 6xHis-CENP-T/W complex immobilized on Ni-NTA agarose resin, alone or in the presence of GST or GST-CENP-K. Middle, Coomassie-stained gel showing 6xHis-CENP-T/W complex binding to either GST-Ndc80Bonsai or GST immobilized on glutathione agarose. Right, Coomassie-stained gel (top) and Western blot probed with anti-Dsn1 antibodies (bottom) showing GST-CENP-T or GST immobilized on glutathione agarose in the presence of 6xHis tagged Mis12 complex. (B) CENP-C binds directly to the Mis12 complex in vitro. Western blot probed with the indicated antibodies showing the elution profile by size exclusion chromatography for GST-CENP-C ΔC (amino acids 1–235) alone (top panel) or shifted in the presence of pre-assembled Mis12 complex (bottom panels). Arrows indicate the migration of standards with known Stokes radii: Ferritin (61 Å), Aldolase (48.1 Å) and Ovalbumin (30.5 Å). Bottom figure, Coomassie-stained gel showing GST-CENP-C ΔC or GST immobilized on glutathione agarose, alone or in the presence of the Mis12 complex. (C) Percent sequence from the mass spectrometric analysis of one step immunoprecipitations of either CENP-C, or LAPGFP-Mis12. Diverse samples prepared using identical conditions serve as negative controls for these purifications.
Induced kinetochore-like foci recruit multiple kinetochore components
Induced targeting of CENP-C and CENP-T to ectopic foci results in formation of kinetochore-like structures based on the recruitment of KMN network components (Fig. 3). To evaluate the functionality of these ectopic kinetochore protein foci, we next tested for the presence of outer kinetochore and regulatory proteins (see Cheeseman and Desai, 2008). Multiple kinetochore proteins co-localized with GFP-CENP-C-ΔC-LacI and GFP-CENP-T-ΔC-LacI foci, but not GFP-LacI foci, including the KMN network binding protein Zwint, the microtubule binding Ska1 complex subunit Ska3, the kinetochore nucleoporin Nup107–160 complex subunit Nup133, and CENP-N (Fig. 5A and Fig. S4A). The majority of these proteins were observed at 100% of foci (Fig. 5B), and Ska3 targeted to ectopic foci with a stoichiometry similar to that at endogenous kinetochores. We also obtained similar results using H2B fusions (Fig. S3C).
Figure 5. Induced CENP-T and CENP-C foci recruit regulatory and outer kinetochore proteins.
(A) Left, immunofluorescence images showing co-localization of kinetochore proteins with LacI foci in cells transiently co-expressing mCherry-CENP-T-ΔC-LacI (not shown) and GFP-CENP-C-ΔC-LacI after 15 h Nocodazole treatment. Inserts show a merge of kinetochore protein (red) and LacI foci (green). Right, co-localization of GFP-CENP-N in cells expressing mCherry-CENP-T-ΔC-LacI or mCherry-CENP-C-ΔC-LacI. (B) Quantification of the percentage of focus-containing cells with the indicated kinetochore protein at the ectopic site. N ≥ 20. (C) Quantification of the relative fluorescence of the indicated kinetochore proteins at kinetochores versus ectopic foci, in cells expressing LacI fusion proteins. Quantification was done after 15 h nocodazole treatment, N ≥ 10 cells/condition, 20 kinetochores/cell, +/− SEM. Data are shown normalized to the foci/kinetochore ratio of the fusion protein. (D) Top, Immunofluorescence images showing co-localization of Mad2 with LacI foci in cells transiently co-expressing mCherry-CENP-T-ΔC-LacI (not shown) and GFP-CENP-C-ΔC-LacI after 15 h Nocodazole treatment, or 1 h treatment with MG132. Bottom, Quantification of Mad2 fluorescence at ectopic foci after 15 h nocodazole treatment or 1 h treatment with MG132, N ≥ 10 cells/condition, 20 kinetochores/cell, +/− SEM. Asterisk indicates significant difference as determined by Mann Whitney U test P<0.005. Data are shown normalized to GFP fluorescence at ectopic foci. (E) Immunofluorescence images showing co-localization of SAC proteins with LacI foci in chicken DT40 cells containing a LacO array, and expressing GFP-CENP-T-ΔC-LacI. Scale bar, 10 μm. Also see Fig. S4
We next examined CENP-T/C foci for the presence of kinetochore regulatory proteins. We found that Aurora B kinase and INCENP, components of the Chromosomal Passenger Complex (CPC), localized to both ectopic foci (Fig. 5A) and chromosome arms in the H2B fusions (Fig. S3C). However, we note that Aurora B signals were only observed at 34% of mitotic LacO foci (Fig. 5B). Consistent with the recruitment of Aurora B, Dsn1 present at ectopic LacO foci was phosphorylated at an established Aurora B phosphorylation site based on phospho-antibody localization (Fig. 5C; Welburn et al., 2010). Finally, we observed the MEI-S332/Shugoshin-family protein Sgo1 at ectopic CENP-T/C foci in 31% of cells. In contrast, several kinetochore proteins were not observed at ectopic CENP-T/C foci in U2OS LacO-containing cells, or H2B fusion protein expressing cells, including CENP-O, MCAK, Bub1 and Mis18 (Fig. S4 BF).
We also analyzed the ectopic kinetochore-like foci for components of the spindle assembly checkpoint (SAC), and observed the recruitment of Mad2 and ZW10 to ectopic CENP-T/C foci in human cells (Fig. 5A and D), and Mad2, BubR1, CENP-E, and ZW10 in chicken DT40 cells (Fig. 5E data not shown). Importantly, we observed a dramatic increase in the recruitment of Mad2 to the ectopic CENP-T/C foci in absence of microtubules (Fig. 5D), similar to what is observed at endogenous kinetochores. The targeting of these diverse regulatory kinetochore proteins, and the microtubule-sensitive recruitment of Mad2, is consistent with functional kinetochore-like structures at these sites.
Induced kinetochore-like foci associate with microtubules
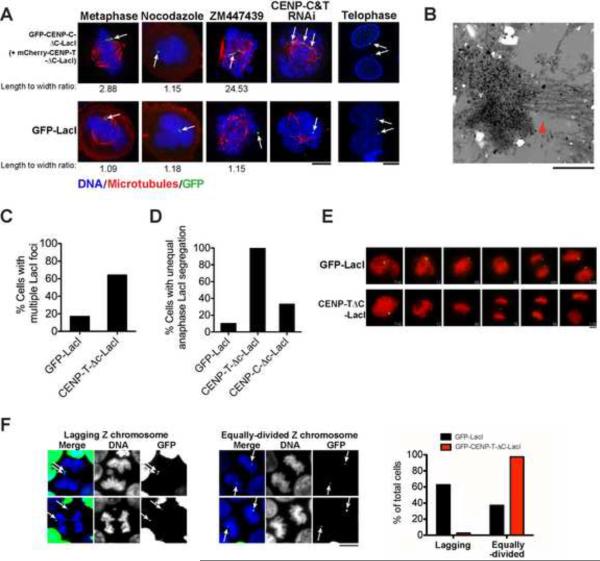
We next tested the interaction of ectopic kinetochore-like foci with microtubules. Although GFP-LacI foci remain circular during mitosis, GFP-CENP-T-ΔC-LacI foci formed a bar-like shape, suggestive of a force being applied across the region (Fig. 6A, arrow). Deformation of the GFP-CENP-T-ΔC-LacI focus was exacerbated by treatment with the Aurora B inhibitor ZM447439, which has been proposed to regulate both kinetochore-microtubule attachments (Cheeseman and Desai, 2008) and chromatin structure (Lipp et al., 2007). The deformed shape of the GFP-CENP-T-ΔC-LacI focus was dependent on microtubules, as nocodazole treatment relaxed this to a circular morphology (Fig. 6A). In the absence of endogenous kinetochores (using RNAi-based depletion of endogenous CENP-T and CENP-C), robust interactions with microtubules were observed for ectopic CENP-T/C-LacI foci, but not GFP-LacI controls (Fig. 6A). These foci often appeared broken, indicating that interactions with microtubules are maintained and may cause damage due to merotelic attachments. Electron microscopy analysis of CENP-T-LacI foci also indicated the presence of microtubule attachments at these sites (Fig. 6B) and the formation of constriction similar to that found at endogenous kinetochores (Fig. S5A). The interaction of CENP-T-based structures with microtubules does not appear to require the presence of chromosomal DNA, as when CENP-T was targeted to the mitochondrial outer membrane, mitochondria redistributed to the mitotic spindle suggesting the presence of microtubule interactions (Fig. S5B). Taken together, these data suggest that induced ectopic CENP-T foci can form interactions with microtubules.
Figure 6. Induced CENP-T and CENP-C foci interact with microtubules and function in chromosome segregation.
(A) Representative immunofluorescence images of cells expressing GFP-LacI, or co-expressing RNAi resistant mCherry-CENPT-ΔC-LacI (not shown) and GFP-CENP-C-ΔC-LacI. Microtubules are shown in red. Cells were treated with 2 μM ZM447439 or 3.3 μM Nocodazole, or 48 h CENP-C and CENP-T RNAi as indicated. In all cases, cells were cold treated for 20 min prior to fixation to visualize stable kinetochore microtubule fibers. Arrows indicate the foci. The mean length/width ratio for the foci is shown below the indicated images. N >5 cells. (B) Electron micrographs of an ectopic CENP-T-LacI foci showing the presence of microtubule attachments (red arrow). Dark spots indicate immuno-labeling with anti-Hec1 antibody. This ectopic CENP-T-LacI foci was defined by the size and extensive anti-Hec1 labeling of this structure relative to an endogenous kinetochore. Direct correlative light-EM is shown to analyze ectopic kinetochore structure in Fig. S5. (C) Graph showing the percentage of cells with more than one LacI foci 72 h after expression of GFP-LacI or GFP-CENP-T-ΔC-LacI. N=200 cells (D) Graph showing quantification of LacI foci segregation in live cells expressing GFP-LacI, GFP-CENP-T-ΔC-LacI, or GFP-CENP-C-ΔC-LacI. (E) Selected images from time-lapse movies of U2OS-LacO cells expressing mCherry-histone-H2B to visualize chromatin, and GFPCENP-T-ΔC-lacI or GFP-LacI showing segregation of LacI foci at anaphase. Time in shown in minutes after NEBD. Scale bars, 5 μm. (F). Centromere replacement assay in chicken DT40 cells (see Fig. S6 for a schematic of this strategy). Left and middle panels; representative images of DT40 cells with GFP-LacI fusion protein localized to a LacO array on the Z chromosome (arrows), after Cre recombinase mediated excision of the centromeric region of the same chromosome. Left, representative images of the effected chromosome lagging at anaphase. Middle, representative images of correct segregation of the Z chromosome. Right panel; graph showing the percentage of GFP foci containing cells with lagging or equally dividing Z chromosomes 18 h after addition of tamoxifen to induce excision of the endogenous centromere cells. N=78 cells per condition. Also see Fig. S5 and movies S1–S4.
To evaluate the interaction of CENP-T/CENP-C foci with microtubules, we assessed the segregation behavior of foci-containing chromosomes. Cell lines stably expressing the GFP-LacI control protein could be easily generated in U2OS-LacO cells. Control GFP-LacI expressing cells segregated the LacO-containing chromosome correctly in 90% of cases based on the number of foci in daughter cells (Fig. 6C) and live cell imaging (Fig. 6D and E; Movie S1). In contrast, although we were able to generate stable GFP-CENP-T-ΔC-LacI cell lines in U2OS cells lacking the LacO array, cell lines could only be generated in the presence of a LacO array if cells were grown in 1 mM IPTG to prevent binding of the LacI fusion protein to the LacO array. In cells transiently expressing GFP-CENP-T-ΔC-LacI, segregation of the LacO-containing chromosome was impeded, with the GFP-CENP-T-ΔC-LacI foci lagging behind the separating chromatin masses at anaphase (Fig. 6D and E; Supplementary movies S2 and S3) resulting in multiple foci in the daughter cells (Fig. 6C). Similarly, CENP-C-ΔCH2B or CENP-T-ΔC-H2B expressing cells showed rapid chromosome movement, but failed to align chromosomes at a metaphase plate or complete a normal anaphase (Fig. S3D and S3E; Movie S4). Thus, the presence of ectopic CENP-T foci perturbs the segregation of chromosomes that contain an endogenous kinetochore.
Induced CENP-T-ΔC-LacI foci can direct chromosome segregation in the absence of an endogenous kinetochore
To assess the function of an ectopic kinetochore focus in the absence of an endogenous kinetochore, we developed a centromere replacement system in chicken DT40 cells. We modified the chicken Z chromosome to insert LacO repeats ~50 kb from the endogenous Z centromere (Fig S5C and D), and placed loxP sites flanking the centromere region. Activation of Cre recombinase resulted in excision of the endogenous centromere (Shang et al., 2010) leaving only the LacO repeats (Fig S5E). We assessed the segregation behavior of the Z chromosome in the first division after centromere removal. Over 60% of cells expressing GFP-LacI displayed lagging chromosomes following centromere excision (Fig. 6F). In contrast, in cells with GFPCENP-T-ΔC-LacI foci, lagging centromeres were observed in less than 3% of cells. Although expression of GFP-CENP-T-ΔC-LacI does not rescue the long-term viability of these cells (most likely for technical reasons due to a lower frequency of foci formation), these data suggest that CENP-T-induced ectopic foci are able to partially replace endogenous kinetochore function.
Phospho-regulation of mitotic kinetochore assembly
Kinetochore assembly is a highly regulated process. Whereas CENP-C and CENP-T are present at the centromere throughout the cell cycle, the majority of outer kinetochore proteins are recruited only upon entry into mitosis. As CENP-T is constitutively localized, but preferentially recruits the KMN network components during mitosis, we reasoned that these interactions might be regulated. An ideal candidate for controlling such interactions to distinguish mitosis from interphase functions is phosphorylation by cyclin dependent kinase (CDK). In an ongoing proteomic analysis of kinetochore phosphorylation, and consistent with reports from proteome-wide phosphorylation studies (Dephoure et al., 2008; Nousiainen et al., 2006; Santamaria et al., 2010), we found that CENP-T is phosphorylated on multiple potential CDK consensus sites in HeLa cells (Fig. S6A). In addition, mass spectrometry analysis of recombinant CENP-T/W complex demonstrated that CDK directly phosphorylates these sites in vitro (Fig. S6A).
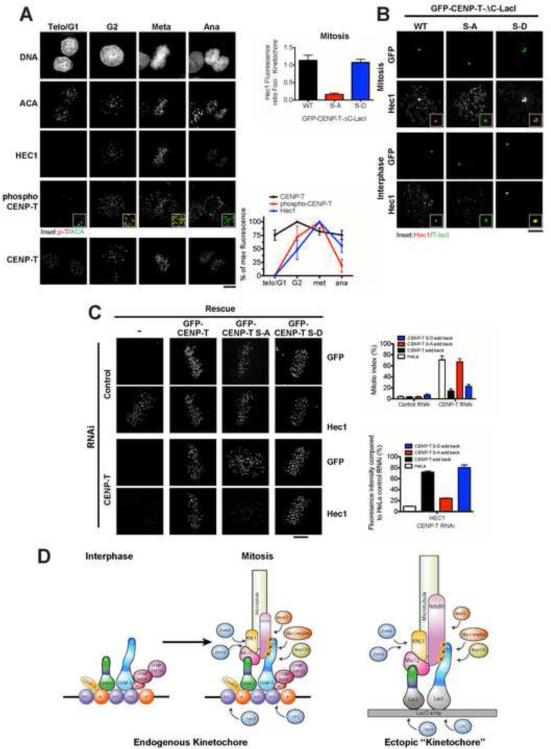
To analyze the dynamics of CENP-T phosphorylation, we generated an antibody specific to CENP-T phosphorylated at one CDK site (serine 47; Fig. S6B). Immuno-staining of HeLa cells indicated that CENP-T becomes phosphorylated in G2, concomitant with the localization of Hec1 to centromeres (Fig.7A). CENP-T remained phosphorylated until anaphase, when fluorescence levels dropped to ~20% of their metaphase maximum, consistent with a decrease in CDK activity at this time. In contrast, Hec1 levels remained relatively high at anaphase, and were not reduced until telophase, consistent with additional regulatory mechanisms for kinetochore disassembly.
Figure 7. Mitotic kinetochore assembly is regulated by phosphorylation of CENPT by CDK.
(A) Immunofluorescence images of Hela cells at the indicated cell cycle stages. Right panel shows quantification of CENP-T, phospho-CENP-T and Hec1 levels at centromeres, N ≥ 10 cells/condition, 20 kinetochores/cell, +/− SEM. Scale bars, 5 μm. (B) Immunofluorescence images showing levels of Hec1/Ndc80 at ectopic foci in mitotic and interphase U20S LacO cells expressing LacI fusions of: GFP-CENP-T, GFP-CENPT S-A (S or T to A mutations at amino acids 11, 27, 47, 85 and 195), or GFP-CENP-T S-D (S or T to D mutations at amino acids 11, 27, 85). Graph shows the relative fluorescence of Hec1/Ndc80 at kinetochores versus ectopic foci, in mitotic cells expressing the indicated CENP-T LacI fusion proteins. Quantification was done after 15 h nocodazole treatment, N ≥ 10 cells/condition, 20 kinetochores/cell, +/− SEM. Data is shown normalized to the foci/kinetochore ratio of CENP-T. (C) Left panels: Immunofluorescence images showing levels of Hec1/Ndc80 48 h after RNAi depletion of CENP-T in HeLa cells or HeLa cell lines expressing RNAi resistant GFP-CENP-T, GFP-CENP-T S-A or GFP-CENP-T S-D. Right panels: Quantification of mitotic index and Hec1/Ndc80 levels, after RNAi depletion of CENP-T and add back of the indicated GFP-CENP-T proteins, +/− SEM. (D) Model depicting interactions at endogenous and ectopic kinetochores. Also see Fig. S6.
To test the role of CENP-T phosphorylation in controlling mitotic kinetochore assembly, we mutated the CDK phosphorylation sites at the N-terminus of CENP-T to alanine to prevent phosphorylation, and to aspartate to mimic constitutive phosphorylation. Preventing CENP-T phosphorylation abolished the targeting of Ndc80/Hec1 to ectopic CENP-T-LacI foci during both interphase and mitosis (Fig. 7B). Preventing phosphorylation of CENP-T also disrupted the targeting of Dsn1 to ectopic foci during mitosis, although Dsn1 continued to localize to foci in a subset of interphase cells (data not shown). In contrast, constitutively mimicking CENP-T phosphorylation results in the targeting of Ndc80/Hec1 to the majority of both interphase and mitotic ectopic foci (Fig. 7B; data not shown).
In addition to regulating assembly of ectopic CENP-T foci, these phosphorylation sites are critical for controlling the function of endogenous kinetochores. Eliminating CENP-T phosphorylation prevented Ndc80/Hec1 localization and caused defects in chromosome segregation that result in a mitotic arrest (Fig. 7C). Similarly, expression of phospho-inhibitory CENP-T in chicken DT40 cells lacking endogenous CENP-T greatly reduced Ndc80/HEC1 levels at kinetochores as well as cell viability (Fig. S6C and D). However, in the context of the endogenous kinetochore, phospho-mimetic CENP-T did not cause constitutive kinetochore assembly (Fig. 7C). Similarly, treatment of mitotic cells with a CDK inhibitor caused a rapid loss of CENP-T phosphorylation at kinetochores, but did not cause premature disassembly of Hec1 (data not shown), suggesting that additional factors likely contribute to kinetochore disassembly.
Taken together, these data demonstrate that in the absence of CENP-A, CENPC and CENP-T are sufficient to direct the assembly of mitotically regulated kinetochore-like structures (Fig. 7D). Thus, CENP-C and CENP-T can provide a regulated platform for mitotic kinetochore assembly in vertebrate cells.
Discussion
The observations described here reveal that two key DNA-binding kinetochore components, CENP-C and the CENP-T/W complex, can function to direct kinetochore formation in vertebrate cells in the absence of the specialized centromeric CENP-A nucleosomes. Rather than relying on protein depletion to assess the requirement of proteins in kinetochore assembly, we have developed an ectopic targeting assay to analyze the sufficiency of kinetochore components for assembly of this structure. Our results demonstrate that, although ectopic localization of CENP-A results in mis-localization of a small subset of kinetochore proteins, ectopic localization of CENP-T and CENP-C results in recruitment of multiple additional proteins, including the microtubule binding KMN network. These structures are at least partially functional based on the stoichiometric recruitment of diverse outer kinetochore proteins to ectopic CENP-T and CENP-C foci, evidence of interactions with microtubules, the ability of the foci to direct chromosome segregation, and the proper regulation of mitotic kinetochore assembly. Taken together, these data indicate that the presence of CENP-C and CENP-T/W can provide a platform for outer kinetochore assembly in vertebrate cells (Fig. 7D).
CENP-T/W and CENP-C form a platform for kinetochore assembly in vertebrate cells
Centromere proteins play two important, yet distinct functions in the recruitment of kinetochore proteins to chromatin during mitosis; specifying the site of kinetochore formation on the chromosome, and assembling the protein architecture of this structure. Previous work demonstrated that CENP-A is essential for specifying the site of kinetochore formation. However, whether CENP-A plays a direct molecular role in kinetochore assembly was unclear, as data on its sufficiency for kinetochore formation varied among eukaryotes. In Drosophila, the presence of ectopic CENP-A foci is sufficient for kinetochore formation (Heun et al., 2006). This contrasts with our work in human cells, in which ectopic localization of CENP-A is sufficient to mis-localize only a small subset of kinetochore proteins. Thus, in human cells, CENP-A is important for specifying the site of kinetochore formation, but is not sufficient for assembly of this structure. Instead of functioning itself to direct kinetochore assembly, the specialized CENP-A nucleosomal structure may be required to generate an environment that is permissive for kinetochore assembly, or that provides structural rigidity to this chromosomal region.
We have defined an essential role for the CCAN proteins CENP-C and CENP-T (the latter of which is absent in Drosophila; (Cheeseman and Desai, 2008) in directing kinetochore assembly in vertebrate cells (this work; Hori et al., 2008). Ectopic localization of CENP-A in human cells does not cause recruitment of CENP-T/W, explaining at least in part why CENP-A alone is not sufficient for kinetochore assembly. Consistent with this, we previously found that the CENP-T/W complex coimmunoprecipitates with H3, but not CENP-A containing nucleosomes (Hori et al., 2008). Thus, our results demonstrate that additional proteins, including CENP-T, are essential factors for kinetochore formation and can act independently of CENP-A to drive kinetochore assembly (Fig. 7C). Defining the mechanism by which the DNA binding activity of the CENP-T/W complex targets these proteins to centromeres represents a goal for future work.
Our finding that the KMN network proteins are recruited to kinetochores by CENP-C and CENP-T provides insight into the mechanism by which the microtubule binding activity of the kinetochore assembles onto this structure. Based on our analysis of CENP-C and CENP-T depletion, CENP-C and CENP-T activity in induced ectopic kinetochore assembly, and the protein interactions defined by our biochemical analysis, both CENP-C and CENP-T can act directly to recruit components of the KMN network. In addition, electron microscopy analysis of kinetochore proteins in chicken DT40 cells indicates that the N-terminus of CENP-T co-localizes with outer kinetochore components such as Ndc80 during mitosis, supporting an interaction between these proteins (Suzuki et al., 2011). CENP-C and CENP-T can directly or indirectly recruit multiple additional outer kinetochore proteins to ectopic foci based on our analysis of selected subunits. Thus, CENP-C and CENP-T can provide a platform for kinetochore assembly.
Phospho-regulation of mitotic kinetochore assembly
Of the almost 100 vertebrate kinetochore proteins, only 17 are constitutively present at centromeres. During a period of less than 2 hours during a 24 hour cell cycle in human cells, more than 60 mitotic-specific kinetochore proteins must be assembled onto this platform, and then subsequently be removed from these sites. However, very little is known about the mechanisms that control this highly ordered assembly process. Here, we demonstrate that phosphorylation of CENP-T by cyclin dependent kinase can control kinetochore assembly (Fig. 7). Although the regulation of mitotic kinetochore assembly is likely to be a multi-step process, CENP-T regulation in controlling the kinetochore assembly state represents an important step and provides additional support for the central role of CENP-T as a key kinetochore assembly factor.
Towards sequence-dependent vertebrate kinetochores
We have demonstrated that foci generated by the artificial tethering of CENP-C and CENP-T to ectopic loci can participate in chromosomes segregation, and can at least partially complement removal of the endogenous centromere. This suggests the possibility that such a system could be used to generate human artificial chromosomes (HACs). Previous attempts to generate artificial human chromosomes have been hampered by difficulties generating a functional centromeric region, which is thus far only possible in a small subset of cell lines (Harrington et al., 1997; Masumoto et al., 1998; Ohzeki, 2002). The system we describe here bypasses both the requirement for alpha satellite sequence and CENP-A, and has the potential to mediate chromosome segregation simply by tethering CENP-C and CENP-T to a chromatin locus, for example using a Lac operator / LacI system such as the one described here, or a tetO / tetR system as used by Nakano and co-workers (Nakano et al., 2008) where such synthetic kinetochores could be turned on by addition of tetracycline.
However, although ectopic targeting of CENP-T and CENP-C directs formation of kinetochore-like structures, there are still critical differences between the LacO-based kinetochore foci in U2OS cells and endogenous kinetochores. First, there are several proteins that we did not observe at the induced CENP-T/CENP-C foci under the tested conditions in human cells. This may reflect technical issues (such as the nature of the available antibodies), as some proteins such as CENP-E were observed at ectopic foci in chicken DT40 cells, but not human cells. Alternatively, this may suggest that either CENP-A or the C-termini of CENP-T or CENP-C are required to recruit some kinetochore proteins. Second, although endogenous kinetochores are of a homogenous size in vertebrate cells based on fluorescent intensity, the ectopic kinetochore-like structures are typically 5–50 fold larger. This is due at least in part to the extended array of LacO repeats that directs formation of these foci. Endogenous kinetochore size is homogeneous, even though the size of alpha-satellite centromeric chromatin can vary, suggesting epigenetic regulation of kinetochore size is critical. Testing different size LacO repeats or modulating the expression level of CENP-T and CENP-C may provide a way to generate structures more similar in size to endogenous kinetochores. Third, although we have bypassed the requirement for CENP-A in directing the recruitment of outer kinetochore proteins, it is important to note that centromeric chromatin may be critical to proper kinetochore function. Centromeric chromatin structure may reflect a role for CENP-A, or the specialized histone modifications that are found at centromeres, neither of which are likely to be present at the ectopic foci. This chromosome structure may be required to resist spindle forces or prevent chromosomal twisting that would result in merotelic attachments. Indeed, we found that some ectopic kinetochore-like structures were highly deformed and showed apparent merotelic attachments to the spindle.
In sum, although CENP-A is essential for specifying the site of kinetochore formation, our studies reveal that the CCAN proteins CENP-T and CENP-C act as key components to drive assembly of a functional kinetochore capable of binding microtubules to allow accurate chromosome segregation during mitosis.
Experimental procedures
Cell culture and transfection
Human cell lines were maintained as described previously (Kline et al., 2006). The U2OS LacO cell line (a generous gift from S. Janicki) was maintained in 200 μg/ml Hygromycin B. Stable clonal cells lines expressing GFPLAP fusions were generated in HeLa cells as described previously (Cheeseman et al., 2004). Codon optimized and RNAi resistant CENP-T (Mr. Gene) was used in all experiments. Transient transfections were carried out using Effectene (Qiagen) according to the manufacturer's instructions. Pools of siRNAs against CENP-T (CAAGAGAGCAGUUGCGGCA, GACGAUAGCCAGAGGGCGU, AAGUAGAGCCCUUACACGA, CGGAGAGCCCUGCUUGAAA) and CENP-C (GCGAAUAGAUUAUCAAGGA, GAACAGAAUCCAUCACAAA, CGAAGUUGAUAGAGGAUGA, UCAGGAGGAUUCGTGAUUA), and a non-targeting control were obtained from Dharmacon. RNAi experiments were conducted as described previously (Kline et al., 2006).
Immunofluorescence and Microscopy
Immunofluorescence in human cells was conducted as described previously (Kline et al., 2006), using antibodies described in Table S1. Cy2, Cy3, and Cy5-conjugated secondary antibodies were obtained from Jackson Laboratories. DNA was visualized using 10 μg/ml Hoechst. Where indicated, cells were incubated for 15 hrs with 3.3 μM nocodazole, for 1 h with 20 μM MG132 or for 1 h with 2 μM ZM447439.
Images were acquired on a DeltaVision Core deconvolution microscope (Applied Precision) equipped with a CoolSnap HQ2 CCD camera. ~40 Z-sections were acquired at 0.2 μm steps using a 100×, 1.3 NA Olympus U-PlanApo objective. Images were deconvolved using the DeltaVision software. For time-lapse imaging, cells were maintained in CO2 independent media (Invitrogen) at 37 °C, and imaged every 4 min using 8 Z-sections at 0.8 μm intervals using a 40× Olympus UApo/340 objective. To quantify fluorescent intensity, individual kinetochores and ectopic foci were analyzed as described previously (Kline et al., 2006).
Protein purification and biochemical assays
For the expression and purification of the recombinant proteins in E. coli, 6×His-CENP-T (full length or amino acids 288–561) and untagged CENP-W were co-expressed from pST39. 6×His-Mis12 complex and Ndc80Bonsai complex were expressed as described previously (Kline et al., 2006; Ciferri et al., 2008). GST-CENP-C N-terminus (amino acids 1–235), GST-CENP-T, and GST-CENP-K were expressed from pGEX-6P-1. Proteins were purified using Glutathione agarose (Sigma) or Ni-NTA Agarose (Qiagen) according to the manufacturer's guidelines and then exchanged into 25 mM HEPES pH 7.5, 200 mM NaCl, 1 mM EDTA, 1 mM β-mercaptoethanol, followed by size exclusion chromatography. For in vitro binding assays, proteins were bound to Glutathione agarose or Ni-NTA agarose, incubated with test proteins, washed 3× with PBS, 250 mM Nacl, and 0.1% Tween, 1 mM β-mercaptoethanol, or 50 mM HEPES pH 7.5, 300mM NaCl, 0.1 % Tween, 25 mM Imidazole, and resuspended in SDS-PAGE sample buffer.
GFPLAP tagged Mis12 complex and endogenous CENP-C were isolated from HeLa cells as described previously (Cheeseman, 2005). CENP-C was isolated using rabbit anti-CENP-C antibodies. Proteins were identified by mass spectrometry using an LTQ XL Ion trap mass spectrometer (Thermo) using MudPIT and SEQUEST software as described previously (Washburn et al., 2001).
Chicken DT40 experiments
DT40 cells were cultured and transfected as described previously (Hori et al., 2008). Mutant or full length CENP-C and CENP-T constructs under control of tetracycline repressive promoter were transfected into DT40 cells with a tet-repressible transactivator in the presence of tetracycline.
Immunofluorescent staining of chicken cells was performed as described previously (Fukagawa et al., 2001), using antibodies described in Table. S1. Images were collected with a cooled EM CCD camera (QuantEM, Roper Scientific Japan) mounted on an Olympus IX71 inverted microscope with a 100× objective lens together with a filter wheel and a DSU confocal unit. ~20 Z-sections were acquired at 0.3 μm steps.
DT40 cells in which the centromere of chromosome Z can be conditionally removed were used for in centromere replacement assays (Shang et al., 2010). A LacO repeat was inserted at a ~50kb region adjacent to the centromere by homologous recombination. GFP-CENP-TΔC-LacI or GFP-LacI constructs were introduced into these cells. Using a Mer-Cre-Mer construct integrated in these cells, Cre recombinase was activated upon hydroxytamoxifen (OHT) addition. Removal of the centromere was confirmed by Southern hybridization (Fig. S5). Cells with lagging chromosomes during anaphase were counted 18 h after addition of OHT.
Electron Microscopy
U2OS cells were treated with 500 ng/ml nocodazole or 10 μM MG132 for 2 h to prepare mitotic cells. Mitotic cells were cyto-centrifugated onto a glass slide, permeabilized by 1% Triton, and fixed in 3% paraformaldehyde/1.5% glutaraldehyde for 15 min. Samples were rinsed in 0.5% BSA / 0.1% triton in PBS, incubated for 1 h at 37 °C with primary antibodies (anti-CENP-T or anti-Hec1 (9G3)), washed three times in 0.5% BSA / 0.1% triton in PBS, and incubated with FITC-conjugated secondary antibodies and a 1.4 gold-labeled secondary antibodies (Nanoprobes), simultaneously for 2 h at 37 °C. Samples were observed by fluorescence microscopy to identify CENP-T-Lac-I-GFP containing foci. The position of the chromosome with CENP-T-Lac-I-GFP was marked and samples were fixed with 2.5% glutaraldehyde / 3 % PFA in 0.1 M cacodylate buffer, pH 7.2, at 4 °C for 20 h. Samples were silver enhanced using a HQ-silver kit (Nanoprobes) according to the manufacture's protocol. Post-fixation performed in 0.5% OsO4 on ice. The cells were dehydrated in ethanol and then infiltrated with Epon812. Polymerization was performed at 60°C for 48 h. Serial sections were cut with an ultramicrotome equipped with a diamond knife (170 nm). Samples were stained by uranylacetate and lead citrate and imaged at room temperature using a JEM1010 TEM (JEOL) at 100 kV.
Supplementary Material
Acknowledgments
We thank Mike Lampson, Rick Young, Terry Orr-Weaver, David Page and members of the Cheeseman and Hochwagen labs for helpful discussions and critical reading of the manuscript, and the anonymous reviewers for constructive suggestions. This work was supported by awards to IMC from the Massachusetts Life Sciences Center and the Searle Scholars Program, and a grant from the NIH/National Institute of General Medical Sciences to IMC (GM088313). IMC is a Thomas D. and Virginia W. Cabot Career Development Professor of Biology. KEG is supported by an EMBO long-term fellowship. Work in the Fukagawa Lab was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to TF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano M, Suzuki A, Hori T, Backer C, Okawa K, Cheeseman IM, Fukagawa T. The CENP-S complex is essential for the stable assembly of outer kinetochore structure. The Journal of Cell Biology. 2009:1–10. doi: 10.1083/jcb.200903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Milks KJ, Straight AF. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol. 2010;189:1143–1155. doi: 10.1083/jcb.201001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Silva MC, Godek KM, Jansen LE, Straight AF. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11:896–902. doi: 10.1038/ncb1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM. A Combined Approach for the Localization and Tandem Affinity Purification of Protein Complexes from Metazoans. Science's STKE. 2005;2005:pl1–pl1. doi: 10.1126/stke.2662005pl1. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore- microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Rybina S, Muller-Reichert T, Shevchenko A, Hyman A, Oegema K. KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev. 2003;17:2421–2435. doi: 10.1101/gad.1126303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LET, Black BE, Bailey AO, Yates JR, Cleveland DW. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8:458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, Yanagida M. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell. 2007;12:17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Fukagawa T, Mikami Y, Nishihashi A, Regnier V, Haraguchi T, Hiraoka Y, Sugata N, Todokoro K, Brown W, Ikemura T. CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. EMBO J. 2001;20:4603–4617. doi: 10.1093/emboj/20.16.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne KE, Cheeseman IM. Kinetochore assembly: if you build it, they will come. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.07.007. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet. 1997;15:345–355. doi: 10.1038/ng0497-345. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fujita Y, Iwasaki O, Adachi Y, Takahashi K, Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Heun P, Erhardt S, Blower MD, Weiss S, Skora AD, Karpen GH. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell. 2006;10:303–315. doi: 10.1016/j.devcel.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, Mcewen BF, Shang W-H, Suzuki E, Okawa K, et al. CCAN Makes Multiple Contacts with Centromeric DNA to Provide Distinct Pathways to the Outer Kinetochore. Cell. 2008;135:1039–1052. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KH. Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci U S A. 2000;97:1148–1153. doi: 10.1073/pnas.97.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, Ried T, Shav-Tal Y, Bertrand E, Singer RH, et al. From silencing to gene expression: real-time analysis in single cells. Cell. 2004;116:683–698. doi: 10.1016/s0092-8674(04)00171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline SL, Cheeseman IM, Hori T, Fukagawa T, Desai A. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. The Journal of Cell Biology. 2006;173:9–17. doi: 10.1083/jcb.200509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp JJ, Hirota T, Poser I, Peters JM. Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J Cell Sci. 2007;120:1245–1255. doi: 10.1242/jcs.03425. [DOI] [PubMed] [Google Scholar]
- Masumoto H, Ikeno M, Nakano M, Okazaki T, Grimes B, Cooke H, Suzuki N. Assay of centromere function using a human artificial chromosome. Chromosoma. 1998;107:406–416. doi: 10.1007/s004120050324. [DOI] [PubMed] [Google Scholar]
- Nakano M, Cardinale S, Noskov VN, Gassmann R, Vagnarelli P, Kandels-Lewis S, Larionov V, Earnshaw WC, Masumoto H. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev Cell. 2008;14:507–522. doi: 10.1016/j.devcel.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousiainen M, Sillje HH, Sauer G, Nigg EA, Korner R. Phosphoproteome analysis of the human mitotic spindle. Proc Natl Acad Sci U S A. 2006;103:5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohzeki J-I. CENP-B box is required for de novo centromere chromatin assembly on human alphoid DNA. The Journal of Cell Biology. 2002;159:765–775. doi: 10.1083/jcb.200207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Cheeseman IM, Hori T, Okawa K, Mcleod IX, Yates JR, Desai A, Fukagawa T. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nature Cell Biology. 2006;8:446–457. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- Przewloka M, Venkei Z, Bolanos-Garcia V, Debuski J, Dadlez M, Glover D. CENP-C is a Structural Platform for Kinetochore Assembly. Current Biology. 2011 doi: 10.1016/j.cub.2011.02.005. Advanced online publication. [DOI] [PubMed] [Google Scholar]
- Santamaria A, Wang B, Elowe S, Malik R, Zhang F, Bauer M, Schmidt A, Sillje HH, Koerner R, Nigg EA. The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti E, De Antoni A, Alushin G, Petrovic A, Melis T, Nogales E, Musacchio A. Direct Binding of Cenp-C to the Mis12 Complex Joins the Inner and Outer Kinetochore. Current Biology. 2011 doi: 10.1016/j.cub.2010.12.039. Advanced Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang WH, Hori T, Toyoda A, Kato J, Popendorf K, Sakakibara Y, Fujiyama A, Fukagawa T. Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res. 2010;20:1219–1228. doi: 10.1101/gr.106245.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Yata H, Muro Y, Himeno M. Human centromere protein C (CENP-C) is a DNA-binding protein which possesses a novel DNA-binding motif. J Biochem. 1994;116:877–881. doi: 10.1093/oxfordjournals.jbchem.a124610. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hori T, Nishino T, Usukura J, Miyagi A, Morikawa K, Fukagawa T. Spindle microtubules generate tension-dependent changes in the distribution of inner kinetochore proteins. J Cell Biol. 2011 doi: 10.1083/jcb.201012050. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser AA, Ouspenski II, Gregson HC, Starr DA, Yen TJ, Goldberg ML, Yokomori K, Earnshaw WC, Sullivan KF, Brinkley BR. Specification of kinetochore-forming chromatin by the histone H3 variant CENP-A. J Cell Sci. 2001;114:3529–3542. doi: 10.1242/jcs.114.19.3529. [DOI] [PubMed] [Google Scholar]
- Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Tomkiel J, Saitoh H, Johnson DH, Earnshaw WC. Identification of overlapping DNA-binding and centromere-targeting domains in the human kinetochore protein CENP-C. Mol Cell Biol. 1996;16:3576–3586. doi: 10.1128/mcb.16.7.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.