SPEED KILLS
Lynch Syndrome is the hereditary disease caused by a germline mutation in a DNA mismatch repair (MMR) gene.1 This disease predisposes to cancers of the colon and other organs, early onset of cancer, multiple primary colorectal cancers (CRC), and a variety of other unique clinical features2. It is widely accepted that CRC in Lynch Syndrome begins as an adenoma which progresses to an advanced neoplasm at a more rapid rate than with sporadic CRCs. However, this conclusion has been reached mainly by the expert opinion of those managing this disease, and no one has left small polyps in place to observe the growth rate – and almost certainly, this will never be done. The recommended colonoscopic surveillance interval for patients with Lynch Syndrome is ideally one year, since intervals of 2 or 3 years are accompanied by the disturbing occurrence of “interval cancers”, in which symptomatic cancers are found prior to the next scheduled exam3–5. Such intense surveillance is neither required nor recommended in CRC predispositions other than Lynch Syndrome. The disease is unique in this regard.
In this issue of Clinical Gastroenterology and Hepatology, Edelstein and colleagues report their experience with 54 patients who have mutation-documented Lynch Syndrome, were followed colonoscopically for a mean of 9.3 years, and had colonoscopic surveillance at a mean interval of 1.7±1.2 years. They found a total of 112 adenomas, 31 CRCs, 32 hyperplastic polyps, and 1 sessile serrated polyp. The cumulative risk of colorectal neoplasia (i.e., adenomas or carcinomas) was 43% in the 4th decade of life, and by the 8th decade, neoplasia had developed in 68% of women and 80% in men. A number of important findings were made in this non-controlled, observational report. First, the mean age for the first adenoma was 46.5±9.7 years, and for the first CRC, 46.8±9.9 years. Although the standard deviations are rather wide, one is struck that they found adenomas and cancers at essentially the same age. By ages 30, 40, 50, 60, 70 and 80, the mean numbers of neoplastic lesions was 1.3, 1.8, 2.2, 3.5, 5.3, and 7.6 respectively. This is not a lot of polyps (compared with a polyposis syndrome for example), but represents a gentle upward exponential curve for polyp number versus age (polyp number = 0.856e0.358x, where x=age).
The investigators used serial colonoscopy to estimate the “polyp dwell time” - the amount of time between a negative colonoscopy and the detection of a colorectal neoplasm. The polyp dwell times were 33.0±16.2 and 35.2±22.3 months for advanced adenomas and CRCs respectively; again, although the standard deviations are large, these are very similar numbers. The polyp dwell times probably reflect 2 different variables: the rapid growth of the adenomas (which is what had been assumed), or, more ominously, that some polyps may have been missed at colonoscopy. Literature reports suggest that colonoscopic protection against CRC mortality has a location bias in the colon. A substantial reduction in mortality exists for distal CRCs, but no reduction in mortality occurs for cancers in the proximal colon6–8. If colonoscopy in Lynch Syndrome patients was associated with substantially more missed polyps in the proximal than distal colon, one might anticipate a shorter polyp dwell time in the proximal colon, because of the additive effect of two factors. In fact, the polyp dwell time in the proximal colon was 28.7±16.6 months versus 43.6±28.5 months in the distal colon. The number of neoplasms involved here is very small and the differences are not significant; however, it is likely that rapid tumor growth and missed small adenomas are both at work. At the very least, the trend suggests redoubling our diligence when examining the proximal colons in patients with Lynch Syndrome, as this is where most of the neoplasms are found. An essential point is that all of these patients were known to have Lynch Syndrome, the clinicians were alert to the risk of proximal lesions, and still, the amount of time between a negative exam and a dangerous lesion was unsettlingly short. Of note, they only found one serrated adenoma in this setting, so those lesions would not appear to be an important premalignant lesion in this disease. We assume that most hyperplastic polyps are not premalignant lesions; it is likely that these are not clinically important, but the location of those lesions was not reported. It would be reassuring if these were all diminutive and located in the rectum or distal sigmoid colon. Perhaps the most encouraging finding in the paper by Edelstein is that the 5 year survival after the detection of CRC was 96%.
The authors reached other conclusions from their data. The investigators noted that men had predominantly proximal colonic neoplasms, whereas in women, left sided lesions were more common. This has not been previously noted, and may be a random consequence of the small study size. Also, based upon the mean numbers of adenomatous polyps found by ages 30 and 50 (1.3 and 2.2 respectively), they suggest that finding ≥3 colorectal neoplasms by 30, or ≥6 by 50 would favor the diagnosis of a polyposis syndrome over Lynch Syndrome. However, the small size of this study mandates additional confirmation before this suggestion is accepted. It would be reassuring if this rule of thumb were to withstand the test of time, as it is complicated to accurately characterize some familial clusters of colorectal neoplasia.
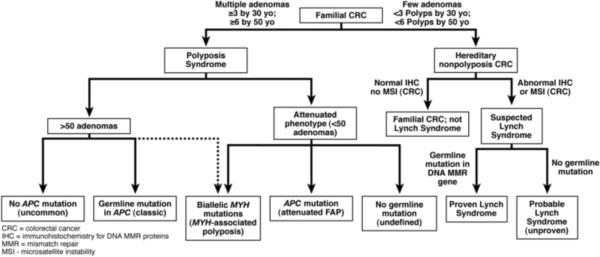
Let's assume that Edelstein et al. have reached a robust conclusion regarding the ability of the number of adenomatous polyps by ages 30 and 50 to make a diagnosis of Lynch Syndrome unlikely. A consequent management algorithm using this approach is illustrated in Figure 1. Patients with family histories of CRC can usually be separated into those with multiple polyps (raising the likelihood of familial adenomatous polyposis [FAP] or MYH-associated polyposis [MAP]), and those with fewer polyps. The genes tested for polyposis syndromes (APC and MYH) are completely different from those tested for Lynch Syndromes (MSH2, MLH1, MSH6 and PMS2). We know that some polyposis patients may have only a few adenomas at the time of evaluation, especially if they are young, and particularly in those with attenuated FAP and MAP, which then overlaps with Lynch Syndrome9. The clinical point is whether some critical number of polyps can reliably exclude Lynch Syndrome from consideration, as this would help direct the most efficient use of genetic testing.
Figure 1.
What is not yet certain is whether the use of ≥3 and ≥6 adenomatous polyps by ages 30 and 50 represents reliable benchmarks to exclude Lynch Syndrome; this requires additional confirmation, perhaps in a multicenter trial. The number of polyps used to direct the decision tree was derived statistically (> 2 standard deviations from the mean) in a small study, and outliers will certainly occur with small numbers of polyps in some FAP or MAP patients. Moreover, the algorithm proposed leaves a very wide zone of uncertainty for patients who have between 6 and 50 polyps, a group that provides the greatest amount of difficulty clinically. When there are >100 polyps, there is almost always a germline mutation in APC (or rarely, MYH). We need a firm estimate of what number of polyps will permit us to comfortably leave the diagnosis of Lynch Syndrome. More importantly, we need to know quite a bit more about what causes people to have between 6 and 50 (or perhaps 100) polyps. We cannot link many of these patients to specific genetic causes at this time, and therefore, cannot advise their relatives about their risk.
Finally, we must be very careful colonoscopists and use all of our technology and expertise to detect small polyps in the proximal colon of this group of patients. Proper surveillance will reduce mortality by at least 70% in Lynch Syndrome10, but small adenomas are frequently missed in these patients11. Various surveillance regimes have been proposed over the years, starting with colonoscopies every 3 years3, moving to every 1–2 years12, 13, and finally to recommendations for annual colonoscopy2, 14 because of the “interval cancer” problem. Patients from familial CRC clusters that are not Lynch Syndrome do not need such intense surveillance13, 15. It is now possible to substantially individualize our care of patients at high risk for CRC on a familial basis. It will require us to use clinical histories, some laboratory diagnostics, and our very best colonoscopic techniques to achieve the greatest reductions in mortality.
Acknowledgments
Supported in part by NIH Grant R01 CA72851, The Sammons Cancer Center,and the Baylor Research Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no conflicts of interest to report.
Reference List
- 1.Boland CR. Evolution of the nomenclature for the hereditary colorectal cancer syndromes. Fam Cancer. 2005;4:211–218. doi: 10.1007/s10689-004-4489-x. [DOI] [PubMed] [Google Scholar]
- 2.Boland CR, Shike M. Report from the Jerusalem workshop on Lynch syndrome-hereditary nonpolyposis colorectal cancer. Gastroenterology. 2010;138:2197. doi: 10.1053/j.gastro.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 4.Mecklin JP, Aarnio M, Laara E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology. 2007;133:1093–1098. doi: 10.1053/j.gastro.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, et al. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Nugent Z, Demers AA, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–1137. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130:665–671. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Stoffel EM, Turgeon DK, Stockwell DH, et al. Missed adenomas during colonoscopic surveillance in individuals with Lynch Syndrome (hereditary nonpolyposis colorectal cancer) Cancer Prev Res (Phila) 2008;1:470–475. doi: 10.1158/1940-6207.CAPR-08-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasen HF, Abdirahman M, Brohet R, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology. 2010;138:2300–2306. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 14.Engel C, Rahner N, Schulmann K, et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol. 2010;8:174–182. doi: 10.1016/j.cgh.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Dove-Edwin I, de Jong AE, Adams J, et al. Prospective results of surveillance colonoscopy in dominant familial colorectal cancer with and without Lynch syndrome. Gastroenterology. 2006;130:1995–2000. doi: 10.1053/j.gastro.2006.03.018. [DOI] [PubMed] [Google Scholar]