Abstract
Introduction
Obesity-mediated changes in plasma adipokines have been associated with increased systemic inflammation and oxidative stress. However, it is unknown whether obesity induces similar changes in airway levels of these adipokines and whether these changes are associated with increased airway biomarkers of inflammation and oxidative stress.
Methods
Lean and obese asthmatics and controls underwent bronchoscopy with bronchoealveaolar lavage (BAL), spirometry, and provided fasting plasma leptin and adiponectin. Biomarkers of oxidation and inflammation in the BAL included exhaled nitric oxide, 8-isoprostanes, pH and nitrogen oxide products (NOx).
Results
Out of a total of 48 subjects 44% had asthma and 56% were healthy controls. Among subjects with asthma 66% were obese, 10% overweight, and 24% were lean; in the controls these proportions were respectively 63%, 11% and 26%. After adjusting for age, sex, smoking history, ethnicity, pre-bronchodilator forced exhalation in one second (FEV1), obesity was associated with higher BAL and plasma leptin levels in asthmatics and controls. Increasing BMI was associated with increased BAL leptin and was marginally and inversely associated with BAL adiponectin. Significant associations between BAL and plasma levels were only observed for leptin. No significant associations were observed between BAL or plasma adipokines with the airway biomarkers of oxidation and inflammation.
Conclusion
Increasing BMI is associated with changes in the concentrations of airway adipokines in asthmatics and healthy controls; however, these associations are not related with biomarkers of airway oxidation or inflammation.
Keywords: Asthma, obesity, leptin adiponectin, oxidative stress
Introduction
In healthy adults obesity has been associated with increased risk of asthma incidence and greater bronchial hyperresponsiveness (BHR) whereas in subjects with asthma, it has been associated with increased symptom severity, higher rates of healthcare utilization, and reduced inhaled corticosteroid effectiveness1–4. These associations may be partly explained by obesity-mediated changes in adipokines, such as increased leptin and/or reduced adiponectin5. Leptin increases satiety yet as a pleitropic adipokine it can also promote inflammation and oxidative stress6, 7. Leptin receptors are involved in the regulation of innate and adaptive immune responses8 and are present in the respiratory epithelium, submucosal space and activated lymphocytes in the lung9. Specific to asthma, leptin infusion has been shown to increase bronchial hyperresponsiveness (BHR) and systemic IgE in ovalbumin (OVA) sensitized mice10. In contrast, Adiponectin, which also has receptors in the respiratory epithelium and airway smooth muscle cells11, 12, has been shown to have predominant antinflammatory and antioxidative effects13 and to prevent OVA-mediated BHR, and airway inflammation14.
In the absence of allergic sensitization obese leptin-receptor deficient mice (db-/db-), have increased leptin and reduced adiponectin in the bronchioalveolar lavage (BAL) when compared to lean wild-type mice15. This suggests that the effect of obesity on these adipokines is similar in the plasma and in the airways. However, it is unknown whether obesity-mediated changes in the BAL concentration of leptin and adiponectin translate into changes in airway inflammation and/or airway oxidative stress. In addition, it is unknown whether any relationship exists between airway and plasma levels of leptin and adiponectin. This information may be important as inferences on the pathogenesis of obesity and asthma are being made using plasma levels of these adipokines, which may not necessarily reflect their corresponding airway concentrations16. We hypothesized that obesity-mediated changes in BAL leptin and adiponectin would be associated with increased airway inflammation and or airway oxidative stress. Additionally, the objectives of the study were to determine how BMI and plasma levels influence the concentration of these BAL adipokines in asthmatics and controls.
Methods
Study population
This study was conducted at Grady Memorial Hospital in Atlanta, Georgia and the Clinical Research Center of Crawford Long Hospital of Emory University with the approval of the institutional review board. A convenience sample of study subjects and controls were recruited through local media advertisement, hospital clinics and university websites.
Inclusion criteria for asthmatics included having the following: physician-diagnosed asthma at least 1 year previous to study enrollment, being older than 18 years of age, meeting criteria for moderate to severe persistent asthma (Global Initiative for Asthma (GINA) class III – IV)[10] and having a post bronchodilator FEV1/FVC (Forced exhalation volume in one second / Forced vital capacity) ratio greater than 0.7. Exclusion criteria included: current smokers, having a positive urine test for cotinine, ex-smokers who stopped smoking at least one year prior to study enrolment, or total-life smoking history > 10 pack-year, and evidence of other lung diseases or any other significant non-pulmonary co-morbidities such as congestive heart failure with ejection fraction < 50%, stable angina and disorders requiring systemic steroid treatment. Subjects were also excluded if they had an asthma exacerbation or were receiving a steroid taper for at least 1 month prior to enrolment. In addition, healthy obese subjects without any history of underlying lung disease or known allergies and meeting the same exclusion for tobacco use were recruited as the obese control group.
Study measures
All participants underwent height and body weight measurements and BMI was calculated as (body weight in Kg/ (height in cm)2. BMI was divided into lean (18 < BMI ≤ 25), overweight (25 < BMI < 30) and obese (BMI ≥ 30) categories. In addition, participants answered a general questionnaire regarding past medical history, use of asthma medications and asthma morbidity. We used the Juniper asthma control questionnaire (ACQ) to determine the degree of asthma control in the week prior to study enrollment[11]. An ACQ score ≤ 0.75 is considered well-controlled asthma whereas an ACQ > 1.5 is compatible with poor asthma control[12]. Spirometry was done following the American Thoracic Society (ATS) recommendations17. Exhaled NO was measured with in-line sampling chemyluminescence (Eco-Medics CLD 88 sp, Michigan Ann Arbor) following ATS recommendations18.
Fiberoptic bronchoscopy and bronchoalveolar lavage
All participants underwent bronchoscopy in the morning after an overnight fasting at the Endoscopy Unit of Crawford Long Hospital and followed establish hospital protocols for sedation and recovery. Venipuncture was performed in all participants prior to bronchoscopy. Topical lidocaine was applied to the nostrils and sprayed into the throat. After an adequate level of conscious sedation was achieved with Fentanyl and Versed, a Fiberoptic bronchoscope was inserted nasally or orally and a 150 cc of normal saline was instilled after wedging in the right middle lobe bronchus. BAL aliquots were immediately placed on ice and transported to the laboratory for immediate analysis.
BAL and plasma measures
BAL fluid was processed within 1 hour of collection. BAL was centrifuged at 1200 rpm for 7 minutes at 4° C to separate the supernatant and cellular fractions. The supernatant was divided into 250 μl aliquots and frozen at −80°C prior to analysis. Total cell counts were performed manually with a hemocytometer and cellular differentials were determined from 300 consecutive cells after Diff Quick staining (Sigma Aldrich, St. Louis, MO).
Leptin and adiponectin levels were determined in the plasma and the BAL supernatant using commercially available ELISA kits (Invitrogen, Camarillo, CA) with sensitivities of 3.5 pg/mL for leptin and 100 pg/mL for adiponectin. 8-Isoprostane concentrations in the BAL supernatant were obtained by ELISA (Cayman Chemical, Ann Arbor, MI) with a detection limit of 2.7 pg/mL. H2O2 concentrations in the BAL supernatant were determined spectrophotometrically from a microplate assay kit (Amplex® Red, Molecular Probes, Eugene, OR) with a sensitivity of 50 nM.
Nitrite and total nitrite + nitrate concentrations were determined from the BAL supernatant using the Griess method (Cayman Chemical, Ann Arbor, MI) with a lower detection limit of 0.1 uM. Nitrate concentrations were determined by subtracting the concentration of nitrite from total nitrite + nitrate. The pH was also measured in the BAL supernatant using a standard Orion pH probe and meter (Thermo Scientific, Waltham, MA). All samples were measured in duplicate with a coefficient of variation of less than 10%.
Statistical analyses
For non-parametrically distributed data, the Kruskall Wallis test was used to compare variables between BMI categories in asthmatics and controls. Distribution of proportion between groups where compared using Fisher's exact test or the Chi squared test. Non-parametric trend analysis was done to determine trends for continuous data across BMI strata. Plasma and BAL adipokines were log-transformed to perform ANOVA tests and multivariable regression analysis. Regression models were adjusted for age, gender, ethnicity, smoking, pre-bronchodilator FEV1 and case vs. control status to evaluate the association between BMI categories and BMI as a continuous variable. All linear regressions were checked for collinearity, influential points and model fitness. The exponential of the log-transformed coefficients from the regression models was used to present the results in their original units. The Spearman and Pearson's tests were used to determine the correlation coefficients of non-parametric and normally distributed data, respectively. Non parametric results are presented as median and interquartile range (IQR), whereas normally distributed data is displayed as mean and 95% confidence interval (CI). A p value of < 0.05 was considered statistically significant. All statistics were performed with Stata 11.0, College Tx.
Results
Study population
A total of 48 adult subjects participated in the study. Of them, 44% had asthma and 56% were healthy controls. The characteristics of the study population are shown in Table 1. The majority of subjects in both the asthmatics and the controls were obese. In both groups obese subjects were older and predominantly Caucasian, when compared to their lean or overweight counterparts. There was a marginal significant difference in the distribution of ACQ scores across BMI categories in asthmatics, and no significant differences in lung function or BAL cell counts between the BMI categories in either subjects with asthma or controls.
Table 1.
Demographic and clinical characteristics of the study population
| Variables | Asthma | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Lean | Overweight | Obese | p | Lean | Overweight | Obese | p | |
| N (%) | 5 (24%) | 2 (10%) | 14 (66%) | 7 (26%) | 3 (11%) | 17 (63%) | ||
| Gender (% female) | 60 | 0 | 79 | 0.3 | 35 | 12 | 53 | 0.3 |
| Age (median, range) years | 28 (18 – 60) | 25 (20 – 30) | 42 (32 – 58) | .09 | 30 (22 – 39) | 49 (42 – 50) | 42 (21 – 63) | 0.02 |
| BMI | 22 (21 – 23) | 27 (26 – 28) | 37 (34 – 42) | 22 (23 – 24) | 28 (27 – 29) | 33 (32 – 37) | ||
| Ever smoked (%) | 40 | 0 | 35 | 0.3 | 0 | 33 | 23 | 0.5 |
| Caucasian n (%) | 20 | 0 | 78 | 0.01 | 28 | 100 | 71 | 0.07 |
| African American n (%) | 80 | 100 | 22 | 42 | 0 | 29 | ||
| Other n(%) | 0 | 0 | 0 | 28 | 0 | 0 | ||
| Inhaled steroids n (%) | 100 | 100 | 100 | |||||
| Long acting beta agonists (%) | 0 | 0 | 7 | 0.7 | ||||
| Leukotriene antagonists (%) | 0 | 50 | 35 | 0.3 | ||||
| ACQ | 1.3 (0.6 – 1.4) | 0.9 (0.6 – 1.2) | 2.1 (1.3 – 2.6) | 0.06 | ||||
| FEV1% | 81 (80 – 97) | 86 (85 – 87) | 84 (79 – 92) | 0.8 | 102 (86 n– 109) | 98 (90 – 101) | 98 (93 – 105) | 0.7 |
| FEV1 (L) | 2.9 (1.8 – 3.7) | 4 (4.1 – 4.2) | 2.2 (1.9 – 2.6) | 0.1 | 3.3 (2.4 – 3.9) | 2.3 (2 – 3.2) | 3.2 (2.4 – 3.5) | 0.1 |
| FVC% | 88 (85 – 105) | 90 (87 – 94) | 86 (74 – 91) | 0.4 | 102 (87 – 107) | 89 (76 – 95) | 96 (83 – 101) | 0.2 |
| FVC (L) | 3.4 (2.3 – 4.8) | 5.1 (4.9 – 5.4) | 2.3 (2 – 3.3) | 0.09 | 4.3 (4 – 6) | 2.3 (2.2 – 3.7) | 3.6 (3.2 – 3.9) | 0.1 |
| FEV1/FVC | 0.83 (0.8 – 0.87) | 0.8 (0.76 – 0.86) | 0.83 (0.78 – 0.86) | 0.6 | 0.87 (0.85 – 0.89) | 0.88 (0.87 – 0.97) | 0.86 (0.83 – 0.91) | 0.5 |
| BAL Eosinophils% | 0.3 (0.2 – 2.3) | 0.8 (0.67 – 0.97) | 1.1 (0.3 – 1.6) | 0.9 | 0.6 (0 – 1) | 0 (0 – 2) | 1 (0 – 1.6) | 0.6 |
| BAL Macrophages% | 91 (91 – 93) | 90 (89 – 91) | 92 (89 – 93) | 0.6 | 90 (86 – 93) | 94 (80 – 95) | 92 (87 – 92) | 0.6 |
| BAL Neutrophils% | 2.6 (2.3 – 3.3) | 2.4 (1.6 – 3.3) | 1.7 (1.3 – 2.2) | 0.2 | 2 (1.9 – 3.2) | 1.3 (1 – 14) | 2 (1.8 – 4.5) | 0.6 |
| BAL Lymphocytes% | 4 (2.6 – 4.2) | 5.9 (5.8 – 6) | 4.4 (3 – 7.3) | 0.2 | 6 (3 – 7.6) | 3 (2.5 – 4.2) | 4.8 (3.2 – 6.2) | 0.2 |
BMI: Body mass index; ACQ: Asthma control questionnaire; FEV1: Forced exhalation volume in one second; FVC: Forced vital capacity; BAL: Broncheoalveolar lavage
p= Kruskall Wallis.
BAL and plasma adipokine levels in asthmatics and controls by BMI categories and in relation to BMI as a continuous variable
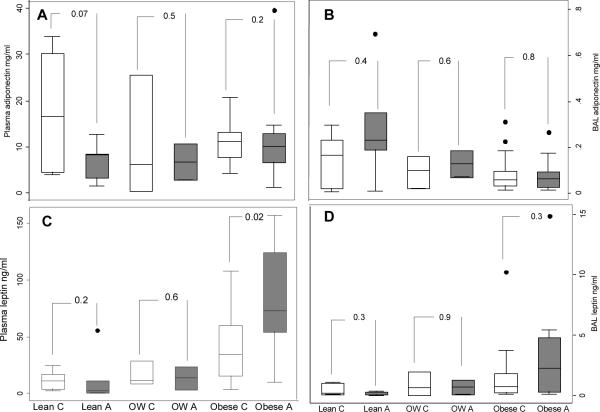
The levels of plasma leptin increased in relation to the BMI categories in both asthmatics (p trend=0.002) and controls (p trend=0.007); in contrast, BAL leptin only increased significantly in relation with the BMI categories in asthmatics (p trend=0.01). In the multiple group comparison analysis using ANOVA, there were significant differences between lean and obese asthmatics and controls. For BAL leptin levels, there were significant differences between lean and obese asthmatics only. There were no differences in the distribution of plasma or BAL adiponectin in relation to BMI categories in either group (See Table 2). Within each BMI category, comparisons of plasma and BAL levels across asthmatics and controls were largely not significant with the exception of plasma leptin levels which were higher in obese asthmatics vs. obese controls. (See figure 1).
Table 2.
Plasma and BAL leptin and adiponectin by weight categories in subjects with asthma and healthy controls.
| Asthma | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Lean | Overweight | Obese | Lean | Overweight | Obese | |||
| n | 5 | 2 | 14 | Anova | 7 | 3 | 17 | Anova |
| Plasma leptin* ng/ml | 2 (.6 – 11) | 14 (4 – 23) | 72 (55 – 124) | <0.01 | 11 (4 – 17) | 12 (9 – 29) | 34 (16 – 60) | 0.01 |
| BAL Leptin* ng/ml | 0.3 (0.15 – 0.27) | 0.7 (0.3 – 1.3) | 2 (0.3 – 5) | 0.02 | 0.2 (.1 – 1) | 0.6 (0.05 – 2) | 0.8 (0.3 – 1.8) | 0.1 |
| Plasma adiponectin mg/ml | 8 (3 – 9) | 7 (3 –11) | 10 (7 –13) | 0.5 | 16 (4 – 30) | 6 (0.5 – 26) | 11 (8 – 13) | 0.2 |
| BAL adiponectin mg/ml | 0.2 (0.2 –0.3) | 0.1 (0.07 – 0.2) | 0.06 (0.03 – 0.2) | 0.3 | 0.16 (0.02 – 0.2) | 0.09 (0.02 – 0.15) | 0.06 (0.03 – 0.1) | 0.8 |
Results are presented as median and inter quartile range.
= In all the significant p ANOVA tests, the between group comparisons were different (p <0.05) between lean vs. obese categories. Values were log-transformed to achieve normal distribution for the ANOVA analysis
BAL: Broncheoalveolar lavage
Figure 1.
Comparison of the BAL and plasma leptin and adiponectin distribution between asthmatics and controls within each weight category
Footnote: A: Plasma adiponectin, B: BAL adiponectin, C: Plasma leptin, d: BAL leptin. C: Control; A: asthma; OW: Overweight.
In multivariable linear regression analysis, obesity and overweight (marginally) categories where associated with reduced plasma and BAL leptin, after adjusting for age, gender, race, and previous smoking history. Models were also adjusted by case status (asthma vs. control), which showed no evidence of confounding, suggesting that the association between body weight categories and adipokine levels, after adjusting for these covariates, was not different across these two groups (See Table 3). No significant associations were observed with adiponectin and BMI categories in the multivariable analysis (Table 3).
Table 3.
Multivariable analysis of the association between obesity and plasma and BAL levels of leptin and adiponectin with BMI categories.
| Plasma leptin (ng/ml) | BAL leptin (ng/ml) | Plasma Adiponectin (mg/ml) | BAL adipoenctin (mg/ml) | |
|---|---|---|---|---|
| β (95% C.I.) n=48 | β (95% C.I.) n=48 | β (95% C.I.) n=48 | β (95% C.I.) n=48 | |
| Overweight (univariate) | 2 (0.7 – 6.6) | 2 (0.4 – 7.4) | 0.53 (0.22 – 1.3) | 0.22 (0.3 – 2.7) |
| Obese (Univariate) | 6.7 (3 – 13.4)* | 5.4 (1.08 – 13.4)* | 1.06 (0.95 – 1.9) | 0.57 (0.3 – 1.8) |
| Overweight (adjusted) | 4.5 (1.7 – 11)* | 3.7 (0.94 – 14.8)§ | 1.05 (0.22 – 1.5) | 0.90 (0.3 – 2.7) |
| Obese (Adjusted) | 11 (5.4 – 20)* | 7.4 (2.7 – 20)* | 0.12 (−0.5 – .82) | 0.52 (0.22 – 1.2) |
| BMI (univariate) | 1.9 (1.05 – 1.12)* | 1.08 (1.04 – 1.12)* | 1 (0.8 – 1.3) | 0.98 (0.94 – 1)† |
| BMI (Adjusted) | 1.08 (1.05 – 1.11)* | 1.08 (1.04 – 1.12)* | 1 (0.8 – 1.3) | 0.97 (0.94 – 1)± |
Models adjusted for: age, gender, ethnicity, case status (asthma vs. control), previous smoking (yes/no), and pre-bronchodilator FEV1. Lean category is the reference comparison
p < 0.05
p=0.06
p=0.09
p=0.058
Linear models were also fitted to determine the association between adipokines and BMI as a continuous variable (Table 3 lower end). In univariate analysis leptin was associated with BMI in both subjects with asthma and controls. These associations remained significant after adjusting for the previously described confounders. BAL adiponectin was marginally and inversely associated with BMI in the univariate and multivariable analysis.
Among subjects with asthma only, the addition of average Juniper ACQ scores did not significantly change any of the model estimates (Results not shown).
BAL and plasma adipokines
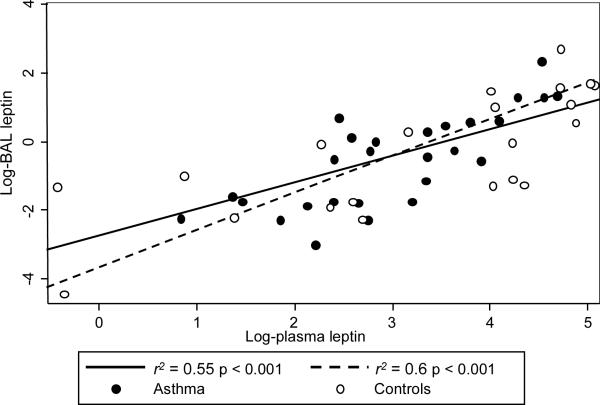
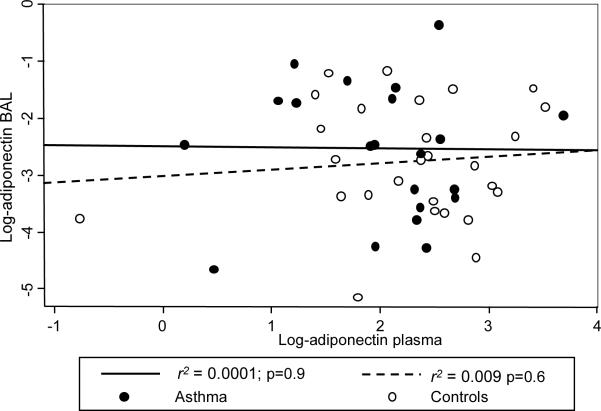
To determine the association between BAL and plasma adipokine levels, linear models were fitted and adjusted using the previously described covariates. BAL leptin levels were significantly associated with plasma levels; adjustment for case status did not confound the model estimate, suggesting that the association between BAL and plasma leptin, after adjusting for covariates, is similar in asthmatics and in controls (See Figure 2). No significant associations were found between BAL and plasma levels of adiponectin (See Figure 3).
Figure 2.
Association between BAL and plasma leptin
Figure 3.
Association between BAL and plasma adiponectin
Among subjects with asthma only, the addition of the Juniper average ACQ scores did not significantly change these associations (Results not shown).
BAL adipokines and BAL biomarkers of oxidation and inflammation
As shown in Table 4, there were no significant differences in the concentration of BAL biomarkers across lean and obese asthmatics and controls (In table 4E in the online supplement, results are presented corrected for dilution using urea). BAL or plasma leptin and adiponectin were not correlated with any of the oxidative or inflammatory biomarkers after Bonferroni adjustment for multiple comparisons.
Table 4.
Airway inflammatory and oxidative biomarkers in subjects with asthma and controls by BMI categories.
| Asthma | Controls | p* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lean | Overweight | Obese | Lean | Overweight | Obese | ||||
| p | p | ||||||||
| FeNO ppb | 10 (8.7 – 14) | 26 (8 – 45) | 13 (9 – 27) | 17 (10 – 20) | 14 (7.5 – 17) | 11 (8 – 14) | 0.8 | ||
| 8-Isoprostanes (pg/ml) | 4.2 (3 – 4.5) | 3.5 (1.4 – 6) | 6 (3 – 6) | .1 | 9.4 (3.2 – 11) | 1.2 (0 – 1.6) | 6.2 (4.2 – 7) | .05 | 0.06 |
| NOx (μM/L) | 0.3 (0.22 – 0.24) | 0.10 (0 – 0.21) | 0.36 (0.24 – 0.52) | .1 | 0.46 (0.21 – 0.51) | 0.27 (0.21 – 0.28) | 0.26 (0.21 – 0.56) | .7 | 0.4 |
| Nitrites (μM/L) | 0.10 (0.07 – 0.13) | 0.13 (0.13 – 0.14) | 0.12 (0.07 – 0.14) | .7 | 0.12 (0.08 – 0.18) | 0.12 (0.12 – 0.13) | 0.13 (0.11 – 0.18) | .9 | 0.9 |
| Nitrates (μM/L) | 0.27 (0.1 – 0.28) | 0.04 (0 – 0.08) | 0.3 (0.12 – 0.46) | .1 | 0.3 (0.09 – 0.51) | 0.11 (0.09 – 0.14) | 0.27 (0.1 – 0.43) | .7 | 0.6 |
| H2O2 (μM/L) | 0.05 (0.04 – 0.06) | 0.09 (0.04 – 0.14) | 0.07 (0.02 – 0.1) | .9 | 0.09 (0.04 – 0.13) | 0.11 (0.08 – 0.17) | 0.10 (0.04 – 0.16) | .7 | 0.5 |
| pH | 6.5 (6.4 – 6.9) | 6.2 (5.8 – 6.6) | 6.6 (6.5 – 6.8) | .6 | 6.8 (6.6 – 7) | 6.2 (4.4 – 6.2) | 6.6 (6.2 – 6.8) | .07 | 0.1 |
FENO: Fractional exhaled nitric oxide; NOx: Nitrogen oxide products. MDA: Malondialdehyde
Results are shown as median and interquartile range values
P= Kruskal Wallis for between BMI categories for asthmatics and controls
p= Kruskal Wallis for overall difference between groups.
Table 4E.
Urea corrected levels of airway inflammatory and oxidative biomarkers in subjects with asthma and controls by BMI categories, corrected for dilution using urea.
| Asthma | Controls | p* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lean | Overweight | Obese | Lean | Overweight | Obese | ||||
| 8-Isoprostanes (pg/ml) | 261 (62 – 348) | 302 (119 – 485) | 194 (111 – 273) | .9 | 242 (63 – 349) | 159 (58 – 261) | 264 (136 – 443) | .3 | .7 |
| NOx (μM/L) | 29 (28.8 – 37.6) | 36 (.07 – 72) | 19 (7 – 41.9) | .9 | 8.17 (6 – 22) | 12.1 (9.3 – 16.2) | 16.6 (9.7 – 33.6) | .4 | .7 |
| Nitrites (μM/L) | 15.6 (3.8 – 18) | 23.4 (2.87 – 44.08) | 5.4 (2 – 18) | .6 | 3 (2.4 – 4.88) | 5.7 (5.5 – 8.5) | 6.9 (3.2 – 9.1) | .2 | .6 |
| Nitrates (μM/L) | 13.2 (11 – 23.6) | 14 (0 – 28) | 10 (5.6 – 24) | .9 | 5.2 (3.2 – 13) | 6.4 (3.8 – 7.7) | 11.8 (4.5 – 24.8) | .4 | .6 |
| H2O2 (μM/L) | 5.7 (2.7 – 7) | 8.5 (3 – 13) | 2.4 (1.3 – 5) | .2 | 1.7 (0.6 – 5.3) | 4.9 (3.8 – 12.8) | 4.9 (1.3 – 4.9) | .4 | .3 |
| pH | 6.49 (6.44 – 6.96) | 6.25 (5.88 – 6.63) | 6.63 (6.46 – 6.88) | .6 | 6.76 (6.58 – 6.99) | 6.21 (4.36 – 6.44) | 6.48 (6.34 – 6.44) | .07 | .6 |
FENO: Fractional exhaled nitric oxide; NOx: Nitrogen oxide products. MDA: Malondialdehyde
Results are shown as median and interquartile range values
p= Kruskal Wallis for overall difference between groups.
Discussion
This study describes the distribution of BAL leptin and adiponectin levels in obese and non-obese asthmatics and controls and their association with BMI, plasma levels, and biomarkers of airway inflammation and oxidation. Our results show that obese and overweight asthmatics and controls have significantly higher BAL leptin and that the concentration of plasma leptin is higher in obese asthmatics vs. obese controls. BAL leptin was significantly correlated with BMI and its corresponding plasma levels in both asthmatics and controls. In contrast, BAL adiponectin was marginally and inversely related to BMI but showed no correlation with its corresponding plasma level. There were no associations between BAL adipokines or plasma adipokines with biomarkers of oxidation or inflammation.
Animal studies have found that body weight strongly influences the concentration of adipokines in the airways. Obese leptin receptor deficient mice (db−/db−) have been shown to have significantly larger concentration of leptin in the BAL and reduced BAL adiponectin when compared to lean wild-type mice15. Changes in the airway concentration of these adipokines in relation to body weight may be relevant to our understanding of the mechanisms by which obesity affects asthma incidence and asthma severity. There are potentially several mechanisms by which leptin could lead to greater BHR and allergic inflammation10. Leptin is a pleiotropic hormone which can promote activation and production of oxidative burst of inflammatory cells19. Also, in murine alveolar macrophages, leptin has been shown to increase the production of arachidonic acid, PGE2 and leukotrienes in a dose dependent manner via activation of phospholipase A220. Further, activation of leptin receptors found in airway smooth muscle (ASM) have been shown to increase release of vascular endothelial growth factor (VEGF), which is implicated in asthma severity11, 21, 22. Epidemiologically, cross sectional studies in adults and children have shown that asthmatics have higher plasma leptin levels; however, airway leptin levels not been previously reported in subjects with asthma23, 24. There are several explanations for the lack of associations between leptin and airway biomarkers in our study, including lack of power and potentially due to the fact that increased airway leptin levels may potentially be relevant only during asthma exacerbations. The lack of significant differences in BAL 8-isoprostanes, contrasts our own previous results, which had shown that 8-isoprostanes increased in relation to BMI25. This inconsistency may be potentially explained by the differences in sampling methods (exhaled breath condensate vs. BAL). This study is the first one to show that in humans there is a significant association between plasma and airway leptin levels, which is highly suggestive of passive leptin diffusion between these compartments.
In contrast to leptin, adiponectin is a pleitrophic hormone with many anti-inflammatory properties, which may have a protective role in asthma. In a murine model, pre treatment with adiponectin reduced OVA-induced BHR and airway inflammation by decreasing the amount of inflammatory cells in the airway and Th-2 derived inflammatory cytokines14. Further, when compared to wild-type mice, adiponectin deficient mice (APN −/−) exposed to OVA had increased airway accumulation of eosinophils and monocyte/macrophages and increased Chemokine levels (CCL 2, 7, 11 & 12)26. Adiponectin receptors Adipo R1 and Adipo R2 are found in ASM and respiratory epithelium and are up-regulated by tobacco exposure and in the respiratory epithelium of smokers12, however, it remains unclear what physiological role do Adipo R1 and R2 have in lung biology. Epidemiologically, higher plasma adiponectin levels are associated with lower asthma prevalence rates in women16; however any protective effects from increased plasma adiponectin is likely to be independent from airway levels, given the lack of correlation found in our study. Lack of correlations between adiponectin and airway biomarkers of inflammation and oxidative stress may be subject to the same factors outlined for leptin. In contrast to leptin, we did not observe any correlation between airway and plasma levels of adiponectin, which may be explained by adiponectin's higher molecular weight. There is evidence that high molecular weight adiponectin is actively transported into the airways, which appears to be mediated by T-Cadherin, a cell-cell adhesion molecule and a known receptor for HMW adiponectin27.
Several limitations need to be considered in interpreting these results. This is a small convenience sample of subjects with asthma and healthy controls; it is therefore possible that several comparisons were not statistically significant given the high probability of a type II error. Also, given that all the patients had persistent asthma and were taking inhaled corticosteroids, it is impossible to determine whether this fact explained the lack of differences in 8-isoprostanes or exhaled NO between asthmatics and controls.
In summary, this study shows that overweight and obesity are associated with greater BAL leptin than lean asthmatics and controls, although plasma leptin levels in obese asthmatics are higher than in the obese controls. Also, increased BMI was marginally and inversely associated with BAL adiponectin. BAL and plasma leptin levels were significantly associated whereas the opposite was true for adiponectin. This result may imply that leptin and adiponectin have different transit mechanisms into the airways. Although no associations between BAL or plasma adipokines with biomarkers of oxidation and inflammation were found, this relationship may be more complex and could vary substantially depending on the degree of airway inflammation, asthma control or with exposure to triggering factors.
Acknowledgments
Funding: NIH/NCRR K12 RR017643, Emory Carlyle Fraser Heart Center & Emory University Research Committee.
Bibliography
- 1.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 3.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax. 2002;57:581–5. doi: 10.1136/thorax.57.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–93. doi: 10.1016/j.jaci.2008.03.004. quiz 94–5. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Romero C, Sanchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–6. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 8.Vernooy JH, Bracke KR, Drummen NE, et al. Leptin Modulates Innate and Adaptive Immune Cell Recruitment after Cigarette Smoke Exposure in Mice. J Immunol. doi: 10.4049/jimmunol.0900963. [DOI] [PubMed] [Google Scholar]
- 9.Bruno A, Chanez P, Chiappara G, et al. Does leptin play a cytokine-like role within the airways of COPD patients? Eur Respir J. 2005;26:398–405. doi: 10.1183/09031936.05.00092404. [DOI] [PubMed] [Google Scholar]
- 10.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–9. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Shin JH, Kim JH, Lee WY, Shim JY. The expression of adiponectin receptors and the effects of adiponectin and leptin on airway smooth muscle cells. Yonsei Med J. 2008;49:804–10. doi: 10.3349/ymj.2008.49.5.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182:684–91. doi: 10.4049/jimmunol.182.1.684. [DOI] [PubMed] [Google Scholar]
- 13.Kantartzis K, Rittig K, Balletshofer B, et al. The relationships of plasma adiponectin with a favorable lipid profile, decreased inflammation, and less ectopic fat accumulation depend on adiposity. Clin Chem. 2006;52:1934–42. doi: 10.1373/clinchem.2006.067397. [DOI] [PubMed] [Google Scholar]
- 14.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–95. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Holguin F, Rojas M, Hart CM. The peroxisome proliferator activated receptor gamma (PPARgamma) ligand rosiglitazone modulates bronchoalveolar lavage levels of leptin, adiponectin, and inflammatory cytokines in lean and obese mice. Lung. 2007;185:367–72. doi: 10.1007/s00408-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 16.Sood A, Cui X, Qualls C, et al. Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63:877–82. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.ATS Workshop Proceedings: Exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate: Executive summary. Am J Respir Crit Care Med. 2006;173:811–3. doi: 10.1164/rccm.2601014. [DOI] [PubMed] [Google Scholar]
- 19.Shirshev SV, Orlova EG. Molecular mechanisms of regulation of functional activity of mononuclear phagocytes by leptin. Biochemistry (Mosc) 2005;70:841–7. doi: 10.1007/s10541-005-0193-1. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004;287:L497–502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari V, Choo-Wing R, Chapoval SP, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci U S A. 2006;103:11021–6. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanazawa H, Hirata K, Yoshikawa J. Involvement of vascular endothelial growth factor in exercise induced bronchoconstriction in asthmatic patients. Thorax. 2002;57:885–8. doi: 10.1136/thorax.57.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sood A, Ford ES, Camargo CA., Jr. Association between leptin and asthma in adults. Thorax. 2006;61:300–5. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai XM, Bottcher MF, Leijon I. Leptin and asthma in overweight children at 12 years of age. Pediatr Allergy Immunol. 2004;15:523–30. doi: 10.1111/j.1399-3038.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 25.Komakula S, Khatri S, Mermis J, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medoff BD, Okamoto Y, Leyton P, et al. Adiponectin-deficiency Increases Allergic Airway Inflammation and Pulmonary Vascular Remodeling. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Hug C, Kasahara DI, et al. Impact of Adiponectin Deficiency on Pulmonary Responses to Acute Ozone Exposure in Mice. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]