Abstract
Rationale
Recent evidence indicates that the hypocretin/orexin system participates in the regulation of reinforcement and addiction processes. For example, manipulations that decrease hypocretin neurotransmission result in disruptions of neurochemical and behavioral responses to cocaine.
Objectives
To further assess the relationship between the hypocretin system and cocaine reinforcement, the current studies used microdialysis and in vivo voltammetry to examine the effects of hypocretin 1 on cocaine-induced enhancement of dopamine signaling in the nucleus accumbens core. Fixed ratio, discrete trials, and progressive ratio self-administration procedures were also used to assess whether hypocretin 1 promotes cocaine self-administration behavior.
Results
Infusions of hypocretin 1 into the ventral tegmental area increased the effects of cocaine on tonic and phasic dopamine signaling and increased the motivation to self-administer cocaine on the discrete trials and progressive ratio schedules.
Conclusions
Together with previous observations demonstrating that a hypocretin 1 receptor antagonist disrupts dopamine signaling and reduces self-administration of cocaine, the current observations further indicate that the hypocretin system participates in reinforcement processes likely through modulation of the mesolimbic dopamine system.
Keywords: Hypocretin 1, Orexin A, Addiction, Reward, Microdialysis, Fast-scan cyclic voltammetry, Rat, Cocaine, Progressive ratio, Discrete trials, Self-administration, Hypocretin, Orexin
Introduction
The hypocretin/orexin (HCRT) peptides (HCRT-1 and HCRT-2) regulate arousal-related processes including sleep/wake function and locomotor activity (Bourgin et al. 2000; España et al. 2001, 2002; Hagan et al. 1999; Piper et al. 2000). Amassing evidence also indicates that the HCRT system regulates the reinforcing effects of various drugs of abuse including cocaine. For example, the HCRT1 receptor antagonist, SB-334867, decreases the motivation to self-administer cocaine across multiple self-administration procedures (Borgland et al. 2009; España et al. 2010), blocks reinstatement of cocaine-seeking (Aston-Jones et al. 2009; Boutrel et al. 2005; Harris et al. 2005; Smith et al. 2009, 2010), and decreases behavioral sensitization to cocaine (Borgland et al. 2006). It is likely that HCRT regulation of cocaine reinforcement processes involves actions on mesolimbic dopamine (DA) systems. Indeed, HCRT neurons provide moderate innervation of the ventral tegmental area (VTA), where both HCRT 1 and 2 receptors are found on DA neurons (Fadel and Deutch 2002; Marcus et al. 2001; Narita et al. 2006; Peyron et al. 1998). Furthermore, HCRT induces burst and/or tonic firing of DA neurons in the VTA and regulates cocaine-induced changes in glutamate-mediated excitatory drive of DA neurons (Borgland et al. 2009; Korotkova et al. 2003). Consistent with this, SB-334867 attenuates cocaine-induced increases in both tonic and phasic DA activity and reduces the effects of cocaine on DA uptake inhibition (España et al. 2010). Similar results are observed in HCRT knockout mice, in which reduced baseline DA uptake rates and reduced DA responses to morphine and cocaine are observed (España et al. 2010; Narita et al. 2006).
To further explore the involvement of HCRT systems in the regulation of tonic and phasic DA signaling, the current studies used microdialysis and in vivo voltammetry to examine whether HCRT-1 infusions into the VTA augment the effects of cocaine on DA signaling within the nucleus accumbens (NAc) core. Rats were also tested under a fixed ratio (FR), discrete trials (DT), or progressive ratio (PR) self-administration procedure to assess whether central HCRT-1 infusions augment cocaine self-administration.
Materials and methods
Animals
Male Sprague–Dawley rats (375–450 g, Charles River, Wilmington, MA) had ad libitum access to food and water and were kept on a reverse 12:12 h light/dark cycle (lights on at 3:00 pm). All protocols and animal care procedures were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), and approved by the Institutional Animal Care and Use Committee at Wake Forest University Health Sciences.
Surgery
Rats used for microdialysis experiments were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotaxic apparatus. Guide cannulae for microdialysis probes (CMA/Microdialysis, Stockholm, Sweden) were aimed at the NAc core (+1.6 A, ±1.6 L, −6.0 V) and 26-ga guide cannulae (Plastics One, Roanoke, VA) for HCRT-1 infusions were inserted 4-mm dorsal to the VTA (−5.3 P, ± 2.0 L, −3.5 V; 8° from vertical). Rats received post-surgical antibiotic (Neo-Predef, Pharmacia and Upjohn Co., New York, NY) and analgesic (Ketoprofen, Webster Veterinary, Sterling, MA) and recovered for 48 h prior to testing. Microdialysis probes (membrane length 2 mm; CMA/Microdialysis) were inserted approximately 16 h prior to the beginning of sample collection and extended 2 mm beyond the cannula tip.
Rats used for voltammetry experiments were anesthetized with 1.5 g/kg i.p. urethane and implanted with an intravenous (i.v.) catheter. Rats were placed into a stereotaxic apparatus and implanted with a bipolar stimulating electrode affixed to a 26-ga guide cannula (Plastics One) aimed at the VTA (−5.3 P, +1.0 L, −7.2 to −7.6 V). Stimulator leads were separated by 1.0 mm and the cannula tip ended 2.0 mm dorsal to the leads. A carbon fiber microelectrode was implanted within the core of the NAc (+1.3 A, +1.3 L, −6.5 to −7.0 V) and a reference electrode was implanted in contralateral cortex (+2.5 A, −2.5 L, −2.0 V).
For both the microdialysis and voltammetry studies, DA changes were measured in the NAc core based on extensive data indicating that this area of the NAc is involved in cocaine seeking behavior (Ito et al. 2000; Ito et al. 2004; Knackstedt et al. 2010; McFarland and Kalivas 2001; Mogenson and Yang 1991) and that blockade of HCRT 1 receptors reduces cocaine-induced changes in DA signaling in this region (España et al. 2010).
Rats used for self-administration experiments were anesthetized using ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and implanted with an i.v. catheter and guide cannula aimed 2 mm dorsal to the lateral ventricle for the FR and DT experiments (+0.8 A, +1.2 L, −1.3 V), or 4 mm dorsal to the VTA for the PR experiments. Rats received post-surgical antibiotic and analgesic and recovered for 3 days prior to training.
Microdialysis
Microdialysis probes were perfused (0.8 µL/min) with sterile artificial cerebrospinal fluid (148 mM NaCl; 2.7 mM KCl; 1.2 mM CaCl2; 0.85 mM MgCl2; pH 7.4). Samples were collected every 20 min and analyzed for DA by high-performance liquid chromatography with electrochemical detection (BAS, West Lafayette, IN). At least six baseline samples were collected, followed by an intra-VTA infusion of vehicle or 0.5 nmol HCRT-1 (250 nl over 2 min). Forty minutes after the infusion, rats received a single i.p. injection of 10 mg/kg cocaine. These studies used i.p cocaine injections to compare to an extensive microdialysis literature using i.p. cocaine delivery as the standard administration route. Dialysate samples were subsequently collected every 20 min for 2 h. To account for the time required for analytes to exit sampling lines, data was shifted by 20 min.
High-performance liquid chromatography
The HPLC (Bionalytical Systems, Mt. Vernon, IN) consisted of a syringe pump, a glassy carbon working electrode, a reference electrode, and an electrochemical detector. A 2 × 50 mm (3 µm particle) reverse-phase column (Luna, Phenomenex, Torrance, CA) was used to separate compounds. The applied potential was +650 mV as referenced to an Ag/AgCl electrode. The mobile phase (75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid sodium salt, 100 µL/L triethylamine, 25 µM EDTA, 10% acetonitrile v/v, pH=3.0) was pumped at a rate of 170 µL/min, with a detection limit for DA of 10 pM. DA quantification was achieved by comparing dialysate samples with DA standards of known concentration.
In vivo voltammetry
Voltammetry studies were conducted to provide a detailed examination of pharmacologically induced changes in DA release and uptake following intra-VTA HCRT-1 treatment. Urethane-anesthetized rats were used to avoid potential interference from behavioral factors and to avoid alterations in DA uptake kinetics that can occur when using other anesthetics (Greco and Garris 2003). Importantly, the effects of DA uptake inhibitors, such as cocaine, are similar in both freely moving and urethane-anesthetized rats (Greco and Garris 2003; Wightman et al. 2007). Following surgery, a stimulating electrode was lowered into the VTA and the carbon fiber electrode was lowered into the caudate putamen (+1.3 A, +1.3 L, −4.5 V), until a 1 s, 60 Hz, 4 ms monophasic (120 µA) stimulation train elicited a robust DA signal. The caudate putamen exhibits higher levels of DA release and faster uptake (~4 µM/s) than the NAc core (Jones et al. 1995; Kuczenski et al. 1991; Wu et al. 2001) and thus it is a useful region to maximize recording conditions. Once stimulator and carbon fiber electrode locations achieved adequate levels of release in the caudate putamen, the carbon fiber electrode was lowered 2–2.5 mm further into the area of the NAc core, which yields lower DA release levels and slower DA uptake (~2.5 µM/s). Once stable baselines were established in the NAc core, a 33-ga infusion needle (Plastics One), containing vehicle (artificial extracellular fluid—AECF; 147 mM NaCl, 1.3 mM CaCl2, 0.9 mM MgCl2, 2.5 mM KCl, 5.0 mM NaH2PO4; pH 7.4) or 0.5 nmol HCRT-1 (in vehicle) was inserted into the guide cannula. Infusion needles extended 2.0 mm beyond the cannula tip to the same dorsal/ventral location as the stimulator leads. After a minimum of 5 min, a 250 nl infusion was made over 2 min (125 nl/min) using an infusion pump (Harvard Apparatus, Holliston, MA). Cocaine injections (1.5 mg/kg) were administered as an experimenter-delivered, 2 s, ~200 µl i.v. bolus, 20 min after infusions of vehicle or HCRT-1.
Given the high temporal resolution obtained with voltammetry techniques, the current studies used i.v. cocaine injections to examine the rapid temporal profile of HCRT-1 effects on baseline and cocaine-induced alterations in DA signaling. Cocaine was delivered 20 min following HCRT-1 based on preliminary data indicating that HCRT-1 consistently increased levels of stimulated DA release within this time frame. Electrically stimulated DA response parameters were acquired 30-s post-cocaine injection, a time in which i.v. cocaine effects on DA signaling have peaked (España et al. 2008), and every 5 min thereafter.
Data acquisition
The electrode potential was linearly scanned as a triangular waveform from −0.4 to 1.2 V and back to −0.4 V vs Ag/AgCl during an 8 ms period. Cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms using a scan rate of 400 V/s by means of a voltammeter/amperometer (Chem-Clamp, Dagan Corporation, MN). The magnitude of electrically evoked DA release and transporter-mediated uptake kinetics, including maximal uptake rate (Vmax) and apparent affinity of endogenous DA (Km) for the DA transporter were monitored. During electrical stimulation, the ascending phase of the DA overflow curve represents both release and uptake while the descending portion of the overflow curves represents primarily uptake. DA overflow curves were fitted to a Michaelis–Menten-based kinetic model (Wu et al. 2001) using locally written Labview (National Instruments, Austin, TX)-based software (Demon Voltammetry and Analysis Software, Wake Forest University Health Sciences 2010) to obtain independent measures for DA release and uptake. Changes in release and uptake were obtained by setting baseline Km values (prior to any drug treatment) to 0.16 µM and establishing a baseline Vmax individually for each subject. Following cocaine injection, Vmax was held constant for the remainder of the experiment and thus cocaine-induced changes in uptake are attributable to changes in apparent Km. Extracellular concentrations of DA were assessed by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations of known concentrations of DA (1–10 µM).
Self-administration
Cocaine self-administration procedures have been described previously (McGregor et al. 1996; Roberts et al. 2002; Roberts and Goeders 1989). Rats were individually housed and trained to self-administer cocaine on a FR schedule in which single lever responses resulted in single cocaine injections. Lever responses resulted in delivery of 0.75 mg/kg cocaine (in saline; National Institute on Drug Abuse Bethesda, MD) over a 5-s period followed by a 20-s time-out period. FR training sessions were terminated after 20 injections. After establishment of stable cocaine intake (defined as 20 injections taken within 3 h with no inter-infusion intervals exceeding 20 min, for three to four consecutive sessions), rats were switched to a DT or PR self-administration schedule for additional training or tested with HCRT-1 on a 3 h unlimited access FR schedule.
The FR schedule is known to engender stable rates of cocaine-reinforced responding and thus it is often the initial self-administration approach to investigate the effects of a pharmacological treatment (LeSage et al. 1999). Rats were given 3-h access to cocaine-associated levers (0.75 mg/kg, i.v.). On experimental days, rats were treated with vehicle or 0.5 nmol HCRT-1 into the lateral ventricle 15 min prior to the beginning of the FR session (9:30 a.m.). Rats were randomly assigned to receive either vehicle or HCRT-1 on the first experimental session and then received the opposite treatment on the following testing day, with a minimum of 3 days between treatments.
The DT procedure used herein is an extension of an FR schedule that allows 24 h access to cocaine but limits the number of injections an animal can receive each hour. During the DT session, rats had the opportunity to self-administer 1.5 mg/kg cocaine during 10-min trials initiated at 20-min intervals (three trials per hour, 24 h/day). Trials began with the presentation of a cocaine-associated lever and were terminated once the rat depressed the lever, or after 10 min. On experimental days, rats were treated with vehicle or 0.5-nmol HCRT-1 at lights on (3:00 p.m.). These dosing, time, and limited access conditions were chosen because they produce a robust diurnal pattern of self-administration that allows for optimal sensitivity for measuring the effectiveness of agents that increase cocaine self-administration (Brebner et al. 1999; Roberts et al. 2002). Rats were randomly assigned to receive either vehicle or HCRT-1 on the first experimental session and then received the opposite treatment on the following testing day, with a minimum of 3 days between treatments.
The PR schedule is useful for assessing the reinforcing effects of cocaine. Rats were given access to a response lever at 10:00 a.m. and single cocaine injections were contingent upon an increasing number of responses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603 (Richardson and Roberts 1996). When the required number of responses was made, a single 0.75 mg/kg cocaine injection was delivered and the session was terminated after 6 h. This dose of cocaine was chosen for the PR studies because it is relatively sensitive to pharmacological manipulations that either decrease or increase cocaine self-administration, thus it is a useful dose for testing the effects of HCRT-1. The number of injections taken before a 1-h time period elapsed with no further injections taken was defined as the “break-point”. Rats were treated either unilaterally or bilaterally with 250 nl (125 nl/min) of vehicle or 0.5-nmol HCRT into the VTA 20 min prior to the beginning of the PR session (9:30 a.m.). Rats were randomly assigned to receive either vehicle or HCRT-1 on the first experimental session and then received the opposite treatment on the following testing day, with a minimum of 3 days between treatments.
For the FR, DT, and PR experiments 33-ga infusion needles were loaded with vehicle or 0.5-nmol HCRT-1 and then inserted into the guide cannulae. Infusions were conducted using a microprocessor-controlled infusion pump (Harvard Apparatus), and following completion of the infusion, needles were removed. Needles used for the FR and DT infusions into the lateral ventricles extended 2 mm beyond the cannula tip, whereas intra-VTA infusions in the PR studies extended 4 mm beyond the cannula.
Histology
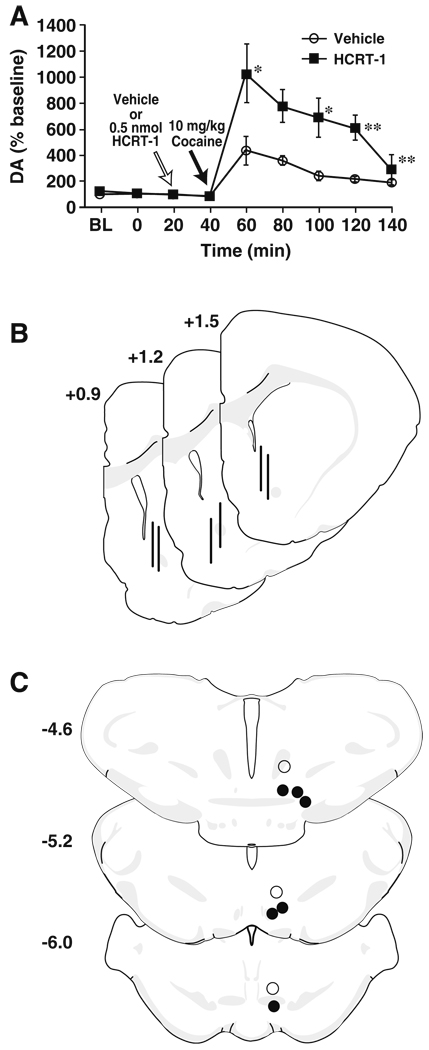
Rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and 0.5% Chicago Blue Dye (Sigma-Aldrich Inc, St. Louis, MO) was microinjected (1 µl) into the lateral ventricle or (250 nl) into the VTA. Rats were perfused transcardially with 60 ml 0.9% saline followed by 60 ml of 10% formalin (pH 7.0; Sigma-Aldrich Inc), and brains were removed and cryoprotected in 30% sucrose solution (in 0.01 M phosphate buffer, pH 7.4). Microdialysis probe (Fig. 1b) and carbon fiber microelectrode (Fig. 2e) locations within the NAc core, as well as microinfusion needle entry/locations within the lateral ventricle or the VTA (Figs. 1c and 2f for infusions into the VTA) were verified from 40 µm thick sections examined with a Nikon Eclipse C1 microscope (Nikon Instruments Inc., Melville, NY). Carbon fiber locations were reconstructed by combining the visual verification of electrode locations obtained using standard histology techniques, the known coordinates to which the electrode was lowered into the brain, and the unique profile of DA dynamics from the final recording location (España et al. 2010). A total of seven animals were removed from the microdialysis (n=3) and voltammetry studies (n=4) and all analyses due to incorrect placements of infusion needles.
Fig. 1.
Intra-VTA HCRT-1 augments cocaine-induced elevations in tonic DA in the NAc core. Shown are the mean ± SEM level of DA, expressed as a percent of baseline, within the NAc core following (a) intra-VTA infusion of vehicle (n=6) or 0.5 nmol HCRT-1 (n=6). White arrows indicate the time of vehicle or HCRT-1 infusion and black arrows indicate the time of 10 mg/kg i.p. cocaine injection. b Shown are schematic depictions of representative microdialysis probe placements (vertical bars) in the NAc core. c Shown are schematic depictions of effective (solid circles) and ineffective (open circles) infusion locations in the VTA. Distance from bregma is shown beside each coronal section. *P<0.05; **P<0.01 relative to vehicle
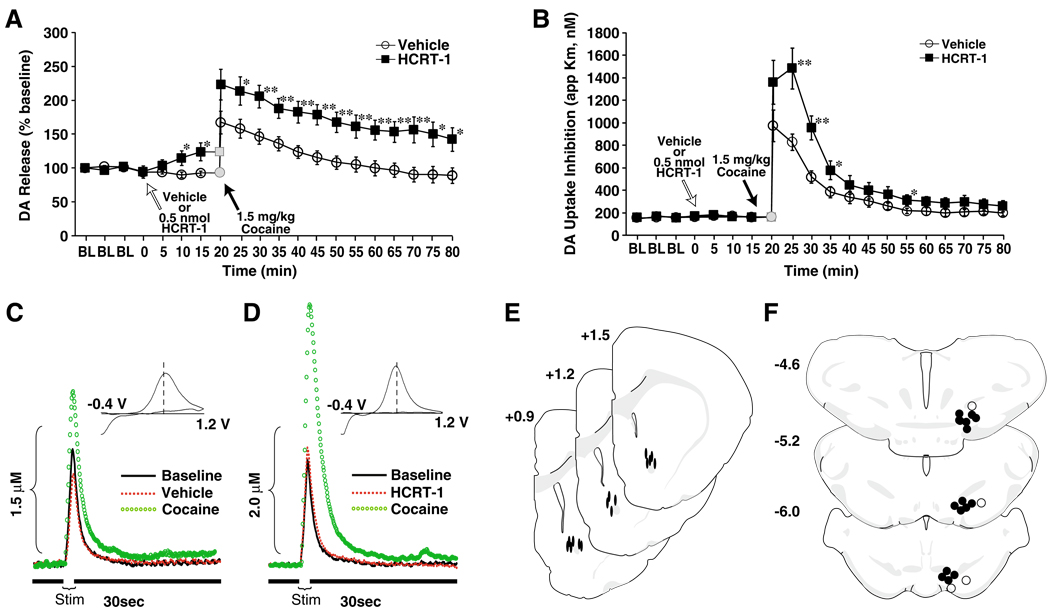
Fig. 2.
Intra-VTA HCRT-1 enhances phasic DA signaling and augments DA responses to cocaine. Shown are the mean ± SEM of a stimulated DA release expressed as a percent of baseline and b DA uptake inhibition (apparent Km) following intra-VTA infusions of vehicle (n=10) or 0.5 nmol HCRT-1 (n=7). Gray symbols shown at time 0 represent a non-collection time-point during which 1.5 mg/kg cocaine was injected i.v. over a 2-s period. Shown are representative concentration-time plots of DA responses from rats that received pretreatment infusions of c vehicle or d 0.5 nmol HCRT-1 into the VTA. Stim represents the time of electrical stimulation. (Insets in c and d) Cyclic voltammograms depict two current peaks, one at 600 mV (positive deflection) for DA oxidation and one at −400 mV (negative deflection) for reduction of DA-o-quinone. The position of the peaks identifies the substance oxidized as DA. Shown are schematic depictions of e carbon fiber electrode locations within the NAc core (solid ovals) and f effective (solid circles) and ineffective (open circles) infusion locations in the VTA. Distance from bregma is shown beside each coronal section. *P<0.05; **P<0.01 relative to vehicle
Data analysis and statistics
Microdialysis
Data was calculated as the percent change from baseline, with baseline (100%) defined as the average of three samples that occurred prior to the injection of drug or vehicle. The effects of HCRT-1 on cocaine-induced increases in extracellular DA within the NAc core were assessed using a two-way mixed design analysis of variance (ANOVA) with drug (vehicle vs. HCRT-1) as the between subjects variable and time as the repeated measures variable. Simple effect analyses were conducted to compare extracellular DA levels between groups using t tests.
In vivo voltammetry
Stimulated DA release was calculated as the percent change from baseline, with baseline (100%) defined as the average of three samples that occurred prior to the injection of cocaine. Changes in maximal uptake rate following HCRT-1 were expressed as Vmax and changes in uptake inhibition following cocaine were expressed as apparent Km. DA release and uptake measures were derived from a Michaelis–Menten-based model (Wu et al. 2001). The effects of HCRT-1 on Vmax were assessed using a t test comparing Vmax prior to infusion and Vmax 20 min later, immediately prior to cocaine injection (BL vs. pre cocaine). The effects of HCRT-1 on DA release and cocaine-induced uptake inhibition (apparent Km) were assessed using a two-way mixed design ANOVA with drug (vehicle vs. HCRT-1) as the between subjects variable and time as the repeated measures variable. Where appropriate, simple effect analyses were conducted using t tests.
Cocaine self-administration
Self-administration data for the FR studies FR studies was expressed as the rate of cocaine injections taken per hour. For the DT studies, data was expressed as the total number of injections taken during the 6 h following vehicle or HCRT-1. For the PR studies, data were expressed as break-points (total number of cocaine injections taken) and total number of lever responses made during the session. The number of lever responses was log transformed to account for non-normality and unequal variance of scores. No statistical differences were observed between unilateral and bilateral intra-VTA of vehicle, thus these data were pooled for figure presentation (Fig. 4a) but not for statistical analyses. Vehicle and HCRT-1 data were compared with the previous 3 days of baseline responding. The effects obtained from the DT studies were assessed using a t test. The PR studies were analyzed using a one-way repeated measures ANOVA for the bilateral experiments and using a one-way between subjects ANOVA for the unilateral studies (vehicle vs HCRT-1). All statistical analyses were conducted using SPSS (SPSS Inc, Chicago, IL).
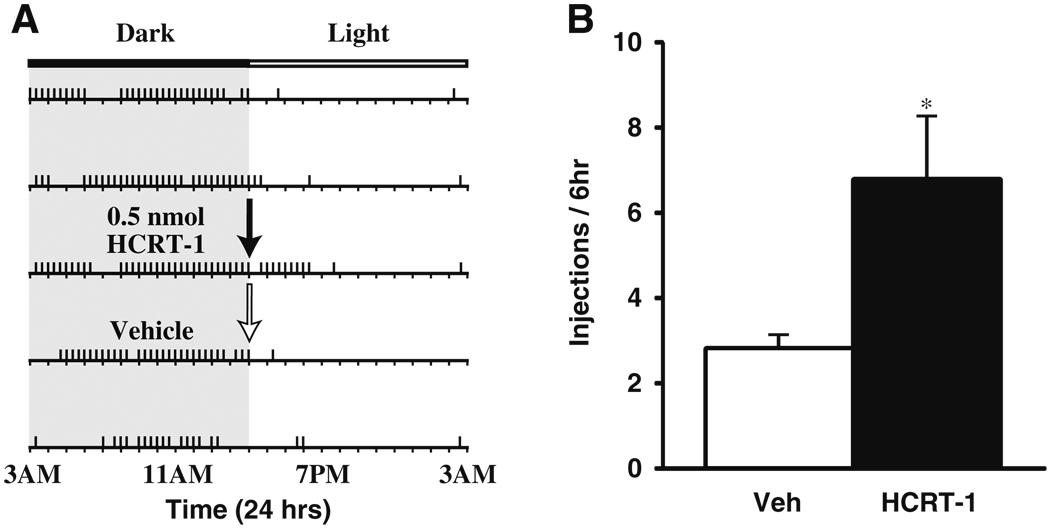
Fig. 4.
HCRT-1 increases cocaine self-administration on a DT schedule. a Shown is a response pattern from an individual rat across 5 days of testing. Horizontal rasters represent 24-h periods. Vertical tick marks represent trials in which a 1.5 mg/kg cocaine injection was taken. In this case, the rat received either a vehicle (white arrow) or 0.5 nmol HCRT-1 infusion (black arrow) into the lateral ventrical at 3:00 p.m., a time during which rats typically discontinue taking cocaine. b Shown are the mean ± SEM number of cocaine injections taken over the 6 h period following infusion of vehicle (n=6) or 0.5 nmol HCRT-1 (n=7). *P<0.05 relative to vehicle
Results
Microdialysis
The effects of HCRT-1 on tonic DA signaling in the NAc core were assessed using microdialysis. Rats were pretreated with intra-VTA infusions of vehicle (n=6) or HCRT-1 (0.5 nmol; n=6) 20 min prior to receiving an i.p. injection of 10 mg/kg cocaine. Similar to that reported previously (Vittoz and Berridge 2006) neither vehicle nor HCRT-1 had a significant effect on baseline DA levels (Fig. 1a). Following cocaine injection, vehicle-treated rats displayed typical increases in DA, however, HCRT-1 (0.5 nmol) augmented cocaine-induced increases in DA (Treatment F(1, 12)=5.3, P<0.05; Time F(8, 96)=17.9, P< 0.001; treatment×time F(8, 96)=3.8, P<0.001). Figures 1b, c show schematic representations of microdialysis probe locations in the NAc core and infusion needle locations in the VTA, respectively.
Voltammetry
The effects of intra-VTA HCRT-1 on phasic DA signaling were examined using in vivo voltammetry. Rats received vehicle (n=10) or 0.5-nmol HCRT-1 (n=7) directly into the VTA 20 min prior to receiving a single i.v. injection of 1.5 mg/kg cocaine. As shown in Fig. 2, electrically evoked DA responses were stable prior to pharmacological manipulation and following vehicle infusions (250 nl) into VTA, remained at baseline levels. HCRT-1 alone had no effect on Vmax or Km, however, over the 20 min following infusion, HCRT-1 produced a significant increase in stimulated DA release (~111–135% of vehicle control; treatment×time F(1, 15)=12.1, P<0.01; Fig. 2a).
HCRT-1 also increased the effects of i.v. cocaine on stimulated DA release (treatment F(1, 15)=11.3, P<0.01; time F(17, 255)=28.9, P<0.001; treatment×time F(17, 255)= 3.6, P<0.001). Following vehicle pretreatment, 1.5 mg/kg i.v. cocaine increased DA levels to 167±16% within 5 min of injection (Fig. 2a, c). In contrast, following HCRT-1 infusions, cocaine increased levels to 223±22% during this time frame (Fig. 2a, d).
A similar effect of HCRT-1 was also observed on cocaine-induced inhibition of DA uptake (apparent Km, treatment F(1, 15)=7.7, P<0.05; time F(17, 255)=66.1, P< 0.001; treatment×time F(17, 255)=5.4, P<0.001). Prior to cocaine administration, neither vehicle nor HCRT-1 infusions into the VTA had any effect on DA uptake (Fig. 2b). Although i.v. cocaine (1.5 mg/kg) inhibited DA uptake in animals pretreated with either vehicle or HCRT-1, the magnitude of inhibition was significantly higher in animals treated with HCRT-1 (vehicle, 975±138%; HCRT-1, 1,360±193%; Fig. 2b–d). Figures 2e, f show schematic representations of carbon fiber microelectrode tip locations in the NAc core and infusion needle locations in the VTA, respectively.
FR cocaine self-administration
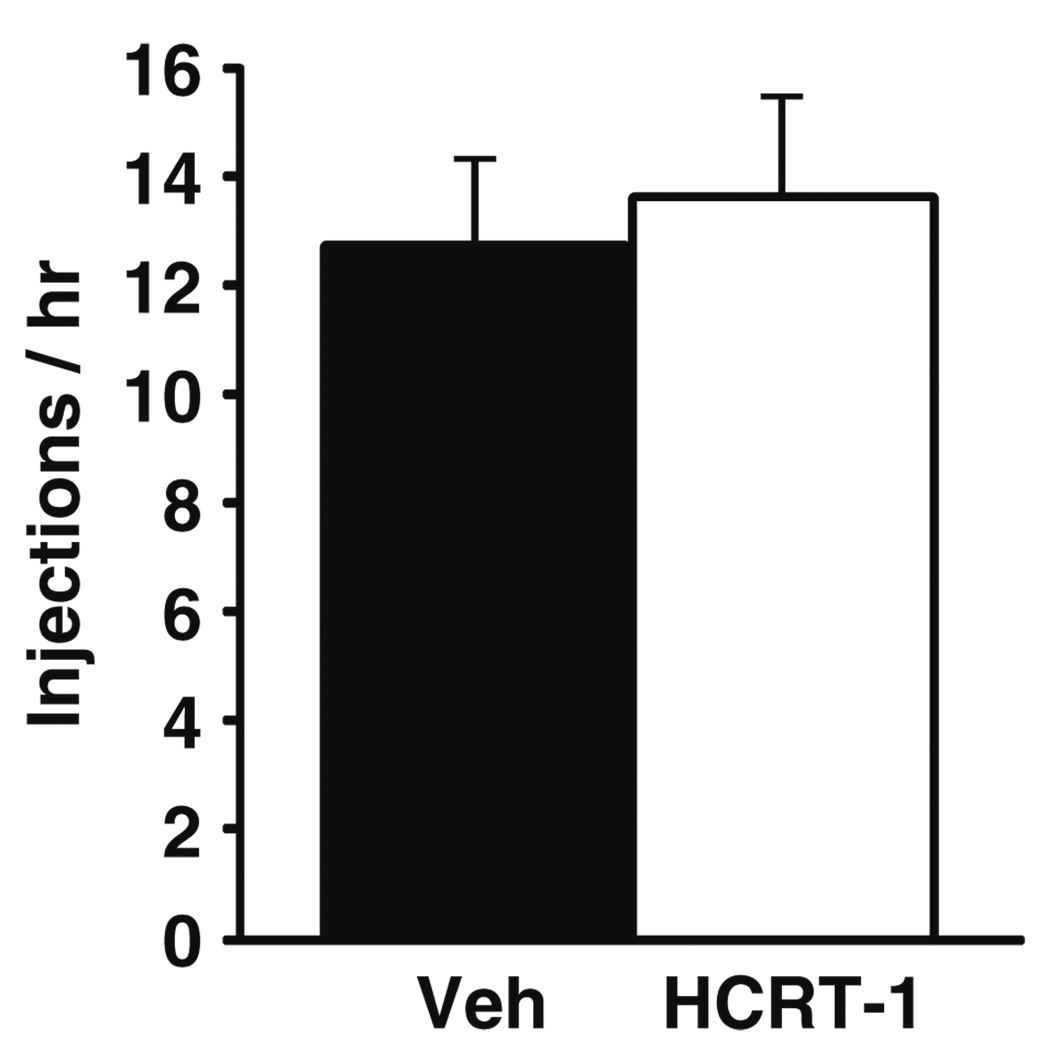
To examine the extent to which HCRT-1 alters responding for unrestricted access to 0.75 mg/kg cocaine, animals were treated with vehicle (n=5) or HCRT-1 (0.5 nmol, n=5) into the lateral ventricles 15 min prior to the beginning of the FR session. Infusions were made into the lateral ventricles to obtain a global effect of HCRT-1 to better compare with previous reports (España et al. 2010). Similar to that described previously (Boutrel et al. 2005), no differences in rates of drug responding were observed between vehicle and HCRT-1 treated animals (Fig. 3).
Fig. 3.
HCRT-1 does not affect cocaine consumption on an FR schedule. Shown are the mean ± SEM number of 0.75 mg/kg cocaine injections taken per hour following i.c.v. injection of vehicle (n=5) or 0.5 nmol HCRT-1 (n=5) on an FR schedule
DT cocaine self-administration
To examine whether HCRT-1 alters responding for cocaine (1.5 mg/kg) under a restricted access condition, rats were tested under a DT schedule of reinforcement. Rats were treated with vehicle (n=6) or HCRT-1 (0.5 nmol; n=7) into the lateral ventricles during a time in which rats show low probability of responding for cocaine (3:00 p.m.). Infusions were made into the lateral ventricles to obtain a global effect of HCRT-1 to better compare with previous reports (España et al. 2010). Responding on the DT schedule was characterized by low rates of cocaine intake during the rest/light phase of the light/dark cycle (Fig. 4a). As shown in Figs. 4a, b, relative to vehicle (2.8±0.4), HCRT-1 produced a moderate, yet significant increase in cocaine intake (6.9±1.4) over the 6 h following treatment (F(1, 12)=6.4, P< 0.05).
PR cocaine self-administration
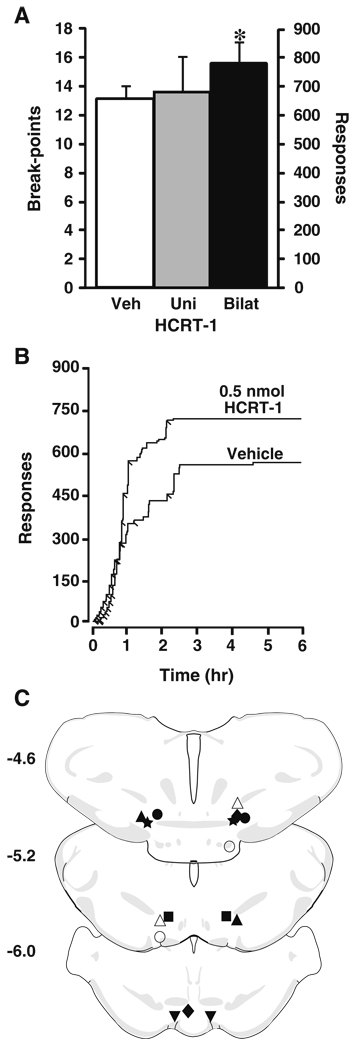
To further examine the extent to which HCRT-1 increases the reinforcing effects of cocaine (0.75 mg/kg), rats were treated with unilateral intra-VTA vehicle (n=5) or HCRT-1 (0.5 nmol; n=5) or bilateral intra-VTA vehicle (n=6) or HCRT-1 (0.5 nmol; n=6), 20 min prior to the beginning of a PR session (9:40 a.m.). Intra-VTA infusions we used in these studies to better compare with previous reports and to examine the importance of HCRT signaling within the VTA (España et al. 2010). As shown in Fig. 5a, unilateral infusions of HCRT-1 did not significantly increase break-points (13.6±1.9) or the number of lever responses (480.6±234.2) relative to vehicle (break-points—12.8±1.3; lever responses—297.0±95.6). In contrast, bilateral infusions of HCRT-1 produced a significant increase in break-points (15.5±1.4, F(1, 5)=12.3, P<0.05) and the number of lever responses (638.0±201.0, F(1, 5)=14.1, P<0.05) relative to vehicle (break-points—13.2±0.9; lever responses—323.8±68.96).
Fig. 5.
Intra-VTA HCRT-1 increases cocaine self-administration on a PR schedule. a Shown are the mean ± SEM number of cocaine break-points and lever responses following unilateral or bilateral intra-VTA infusions of vehicle (unilateral, n=5; bilateral, n=6) or 0.5 nmol HCRT-1 (unilateral, n=5; bilateral, n=6). b Shown are event records from an individual rat that received an intra-VTA infusion of vehicle or 0.5 nmol HCRT-1. Cocaine injections taken are indicated by diagonal tick marks. c Shown are schematic depictions of bilateral infusion locations in the VTA from individual rats (matched symbols) open symbols denote ineffective infusions. Distance from bregma is shown beside each coronal section. *P<0.05 relative to vehicle
Analysis of cumulative records from individual rats tested under the PR schedule indicated that HCRT-1 increased break-points without altering the initial pattern (first 1 h) of cocaine intake. Rats treated with HCRT-1 showed initial response rates identical to vehicle-treated rats, yet completed more responses before reaching their final break-point (Fig. 5b). Figure 5c shows a schematic representation of infusion needle locations within the VTA.
Discussion
The current observations indicate that increased HCRT signaling in the VTA enhances baseline and cocaine-induced DA responses within the NAc core and promotes cocaine self-administration under conditions that require effortful responding or in which cocaine access is limited. Together with previous work indicating that compromised HCRT activity disrupts behavioral and neurochemical responses to cocaine, the present observations provide further support for the hypothesis that HCRT participates in reinforcement processes via modulation of the mesolimbic DA system.
Dopamine signaling
Previous observations indicate that peripheral and intra-VTA blockade of HCRT-1 receptors disrupts baseline DA signaling and reduces DA responses to cocaine (España et al. 2010). The current experiments extend these observations by demonstrating that enhanced HCRT neurotransmission facilitates DA signaling in response to cocaine. Microdialysis experiments indicate that intra-VTA infusions of HCRT-1 significantly increased tonic DA responses to cocaine although no effects on baseline DA levels were observed. The lack of effect on baseline DA activity is in line with previous work demonstrating that HCRT-1 increases DA levels in the prefrontal cortex and NAc shell, but not the core (Vittoz et al. 2008; Vittoz and Berridge 2006). Consistent with these observations, intra-VTA injections of HCRT-1 produced a twofold greater Fos activation in VTA DA neurons that project to the NAc shell, when compared with the core. While the functional consequences of these differences are unknown, future studies will need to compare the effects of HCRT agents on cocaine-induced DA responses across regions in the striatum.
Our previous work demonstrated that intact HCRT neurotransmission is necessary to support normal DA responses to cocaine (España et al. 2010). In those studies, intra-VTA infusions of SB-334867 reduced baseline DA activity and attenuated the effects cocaine on DA uptake and evoked DA release. Additionally, HCRT knockout mice showed disrupted DA signaling, with reduced DA uptake rates under baseline conditions and attenuated cocaine-induced increases in DA release and uptake. Consistent with these observations, the current in vivo voltammetry studies indicate an enhancement of DA responsivity following HCRT-1 administration. Intra-VTA HCRT-1 significantly increased electrically evoked DA release in the NAc core under baseline conditions, prior to cocaine administration, and also augmented the effects of cocaine on both evoked DA release and uptake inhibition.
The effects of enhanced HCRT neurotransmission on DA signaling and cocaine effects are likely due to increased DA neuron activity in the VTA. Previous work demonstrates that HCRTs increase both burst and tonic firing of DA neurons (Korotkova et al. 2003) and increase DA release in the brain (Narita et al. 2006; Vittoz and Berridge 2006). Cocaine-induced increases in DA are dependent upon impulse flow from the cell body regions, and thus, it is likely that alterations in the activity of DA neurons could result in augmented responses to cocaine. This hypothesis is strengthened by work indicating that HCRT promotes excitatory synaptic transmission in DA neurons via trafficking of NMDA receptors (Borgland et al. 2006), and potentiates the effects of glutamate in animals with a history of cocaine self-administration (Borgland et al. 2009). Furthermore, recent observations indicate that in addition to blocking DA reuptake, cocaine also increases the incidence or magnitude of DA release events in the NAc shell (Aragona et al. 2008), and stimulates glutamate release in the VTA of cocaine-experienced rats (Wise et al. 2008). These observations suggest that the enhanced effects of cocaine observed in rats treated with HCRT-1 could be associated with enhancement of DA signaling within the VTA and that these actions may involve HCRT-induced potentiation of glutamatergic synaptic transmission in the VTA. Alternatively, it is possible that HCRT-induced increases in DA firing rates could lead to changes in the state of DA terminals in the NAc, (e.g., via phosphorylation or glycosylation of the DA transporter) which could result in altered transporter function that ultimately increases cocaine’s effectiveness (Foster et al. 2008; Li et al. 2004; Mortensen et al. 2008).
Cocaine self-administration
In the current studies, rats were tested under three self-administration schedules that model different aspects of reinforcement processing. Under the FR schedule, which is useful for assessing changes in cocaine consumption, rats had unrestricted access to cocaine and thus were able to attain preferred blood levels of cocaine. Similar to a previous report (Boutrel et al. 2005), HCRT-1 had no effect on cocaine intake under this schedule, suggesting that the HCRT system may not influence cocaine self-administration when conditions allow for effortless access to cocaine.
In contrast to that seen under the FR studies, HCRT-1 increased cocaine intake when access to cocaine was restricted in the DT studies. Under the DT schedule used here, cocaine intake was limited by restricting cocaine access to three trials per hour. With this restricted access, rats are unable to maximize their blood levels of cocaine and are thus disinclined to self-administer cocaine during the light phase (Roberts et al. 2002). The interaction between cocaine availability and dose in the current DT paradigm renders this schedule more vulnerable to pharmacological and physiological influences than schedules with less restricted access to cocaine. Under these conditions, HCRT-1 infusions into the lateral ventricles significantly increased cocaine intake over the 6 h following infusion. These effects complement our previous work demonstrating that systemic blockade of HCRT 1 receptors significantly reduces cocaine intake under the DT paradigm (España et al. 2010).
The PR schedule used here is useful for assessing changes in the motivational influences of drugs. During the early portions of the PR session, single cocaine injections are obtained with relatively low effort, however, as the lever response requirement is increased, rats must exert progressively greater effort to obtain a single cocaine injection. Under these conditions, bilateral intra-VTA HCRT-1 infusions increased break-points without altering the initial pattern of behavior. These effects are consistent with previous observations indicating that both i.p. (Borgland et al. 2009), and intra-VTA (España et al. 2010) blockade of HCRT 1 receptors reduces PR responding for cocaine. However, a previous study demonstrated that 1.5-nmol HCRT-1 infusions into the lateral ventricle did not alter PR responding (Boutrel et al. 2005). The reasons for these discrepant findings are unclear, however, it is possible that differences in HCRT-1 doses (0.5 vs. 1.5 nmol) or site of infusion (intra-VTA vs. lateral ventricle) may affect behavioral responses to cocaine on the PR schedule.
Relation to previous studies
The current observations are consistent with an emerging literature indicating that the HCRT system regulates the reinforcing effects of cocaine. In particular, it has been shown that blockade of HCRT 1 receptors blocks reinstatement of cocaine seeking (Aston-Jones et al. 2009; Boutrel et al. 2005; Harris et al. 2005; Smith et al. 2009, 2010), decreases behavioral sensitization to cocaine (Borgland et al. 2006), and reduces the motivation to self-administer cocaine across multiple self-administration procedures (Borgland et al. 2009; España et al. 2010). Additionally, it appears that HCRT regulation of cocaine reinforcement processes involves actions within the VTA. For example, HCRT increases firing of DA neurons in the VTA and regulates cocaine-induced changes in glutamate-mediated excitatory drive of DA neurons (Borgland et al. 2009; Korotkova et al. 2003). Additionally, blockade of HCRT 1 receptors in the VTA attenuates cocaine-induced increases in both tonic and phasic DA activity in the NAc (España et al. 2010). While the current data extend these observations by demonstrating that HCRT-1 augments cocaine self-administration and enhances the effects of cocaine on DA signaling, it is important to note that HCRT-1 binds to both HCRT 1 and HCRT 2 receptors with equal affinity (Sakurai et al. 1998). Thus, although previous studies using SB-334867 indicate that signaling at HCRT 1 receptors in the VTA is important in regulating reinforcing properties of cocaine, the current studies do not distinguish between HCRT-1 actions at HCRT 1 or HCRT 2 receptors.
HCRT and arousal
Extensive evidence indicates that HCRT regulates arousal-related processes, including sleep/wake function and locomotor activity (Hagan et al. 1999; Bourgin et al. 2000; Piper et al. 2000; España et al. 2001, 2002). Thus, it is possible that the HCRT system influences cocaine self-administration, by producing generalized enhancements in arousal in addition to any direct actions on reinforcement mechanisms. This is particularly the case for the current DT experiments in which rats were treated with HCRT-1 at a time when rats normally reduce their cocaine intake (immediately before lights on). Under these conditions, HCRT-1 increased the number of cocaine injections taken over a 6 h period by prolonging self-administration behavior. While these effects may be associated with HCRT-1 induced increased in waking/locomotor activity, the time-course of these actions do not exactly match the time-course of increased cocaine intake. For example, HCRT-1 at doses higher than used here increases active waking and locomotor activity for ~1.5 h before animals return to baseline levels (España et al. 2001). However, in the DT studies, HCRT-induced increases in cocaine intake lasted for 3–6 h.
For the FR and PR studies, rats were treated with HCRT-1 during the dark-phase, a time in which control animals exhibit near-maximal waking. Thus, under these conditions, HCRT-1 effects on waking would be negligible and thus it is unlikely that increases in self-administration would be related entirely to alterations in waking per se. Nevertheless, HCRT-1 significantly increases locomotor activity during the dark-phase, and thus HCRT-1 could still alter cocaine self-administration via alterations in locomotor activity. However, in the FR studies and in the early portions of the PR experiments, conditions in which maintaining preferred blood levels of cocaine requires relatively low effort, HCRT-1 had no effect on the rate of cocaine intake, suggesting that rats did not display nonspecific locomotor-related responding. Further, in the PR experiments, rats were treated with HCRT-1 directly into the VTA, which produces only modest, short-lived (<1 h) increases in waking and locomotor activity (Vittoz and Berridge 2006). In the present studies the effects of HCRT-1 on PR self-administration continued into the second and third hours of the PR session, again suggesting that the time-course of increased cocaine intake does not exactly match the time-course of waking and locomotor effects. Given these observations, it is unlikely that the pharmacological effects of HCRT-1 on self-administration can be solely explained by generalized HCRT-1 effects on waking and locomotor activity. Nevertheless, given that the complex interaction between processes that govern reward, motivation, and reinforcement are likely to be sensitive to alterations in generalized arousal, the contribution of HCRT-1 enhancement of arousal, waking, and locomotor activity to changes in cocaine self-administration cannot be entirely ruled out (Berridge et al. 2010).
Conclusions
The current studies demonstrate that increased HCRT neurotransmission within the VTA enhances the effects of cocaine on DA signaling in the NAc. These studies also indicate that enhanced HCRT signaling augments the reinforcing effects of cocaine. These data are consistent with previous work demonstrating that disrupted HCRT signaling results in decreased DA activity in the NAc and reductions in cocaine self-administration behavior. When taken together, these observations provide strong evidence in support of the hypothesis that the HCRT system is involved in reward and reinforcement processes, particularly as it relates to cocaine, through actions on the VTA-mesolimbic DA system.
Acknowledgments
We would like to thank Joanne K. Konstantopoulos and Jason L. Locke for their expert technical assistance. These studies were supported by K01 DA025279 (R.A.E), R01 DA021325 (S.R.J.), P50 DA06634 (D.C.S.R., S.R.J.), R01 DA14030 (D.C.S.R).
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56 Suppl 1:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Froestl W, Andrews M, Phelan R, Roberts DCS. The GABA(B) agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38:1797–1804. doi: 10.1016/s0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- España RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- España RA, Roberts DCS, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J Neurochem. 1995;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine—relationship between locomotor and stereotypy response profiles and caudate and accumbens-dopamine dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Stafford D, Glowa JR. Preclinical research on cocaine self-administration: environmental determinants and their interaction with pharmacological treatment. Neurosci Biobehav Rev. 1999;23:717–741. doi: 10.1016/s0149-7634(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem. 2004;279:21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DCS. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Larsen MB, Prasad BM, Amara SG. Genetic complementation screen identifies a mitogen-activated protein kinase phosphatase, MKP3, as a regulator of dopamine transporter trafficking. Mol Biol Cell. 2008;19:2818–2829. doi: 10.1091/mbc.E07-09-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Goeders NE. Drug self-administration: experimental methods and determinants. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Neuromethods. Clifton: Humana Press; 1989. pp. 349–398. [Google Scholar]
- Roberts DCS, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS ONE. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]