Abstract
Consequences of prenatal alcohol exposure (AE) include motor hyperactivity, disrupted sleep and cognitive deficits. Hypothalamic orexin (ORX)-synthesizing neurons are important for the maintenance of vigilance and regulation of motor activity but their hyperactivity may contribute to anxiety disorders. Using a rat model, we tested whether ORX plays a role in behavioral consequences of prenatal AE. Male rat pups received 2.625 g/kg of alcohol (AE group) intragastrically twice daily on postnatal days (PD)4–9, a developmental period equivalent to the third trimester of human pregnancy. Control pups were sham-intubated (S group). On PD12–14, they received daily injections of either the ORX-1 receptor antagonist, SB-334867 (SB; 20 mg/kg, i.p.) or vehicle (V) during the lights-off period. On PD16, they were subjected to the homing response (HR) test. On PD17, their motor activity was monitored in a novel environment. The percentage of tests in which HR acquisition was not achieved and the number of trials needed to reach the shortest HR latency were higher, whereas the percentage of successful trials was lower, in AE-V than in S-V rats (p=0.0009–0.03). In contrast, these measures were not significantly different between AE-SB and either S-SB or S-V rats. Motor activity in AEV rats was significantly higher than in S-V (p=0.003), S-SB (p=0.007) or AE-SB (p=0.02) rats, with no difference between S-SB and AE-SB group. Our findings suggest that excessive activity of ORX neurons contributes to motor hyperactivity and impaired HR acquisition following perinatal AE and that these symptoms may be alleviated by systemic antagonism of ORX-1 receptors.
Keywords: ethanol, brain development, hypocretin, hypothalamus, learning, SB-334867
Alcohol exposure (AE) during prenatal development can lead to a range of adverse health consequences, such as the fetal alcohol syndrome and alcohol-related neurodevelopmental problems collectively referred to as the fetal alcohol spectrum disorders (FASD) (reviewed in [1–3]). The severity of FASD depends on the levels and timing of prenatal AE, but some cognitive and behavioral abnormalities consistently occur across the entire range of FASD. These include motor hyperactivity, disrupted sleep, and neuropsychological impairments, such as deficits in attention, memory and learning [1–5]. These features have been replicated in rodent models of prenatal AE [6–11], but their neurochemical mechanisms remain to be elucidated.
Orexin A and B (ORX) are two excitatory neuropeptides synthesized by hypothalamic cells that promote motor activation [12,13] and play a major role in the maintenance of wakefulness, alertness and motivated behaviors (reviewed in [14,15]). Activation of the ORX system is also involved in alcohol-seeking behavior [16], but its role in the consequences of perinatal AE has not been studied. Importantly, ORX are implicated in development of anxiety disorders [17], which are among the most commonly reported behavioral abnormalities in children and adults with FASD [3]. To date, most of the studies of pathologic conditions associated with the ORX system concentrated on ORX deficiency, as in narcolepsy/cataplexy (reviewed in [14]). However, it is plausible that hyperactivity of this system also occurs and may contribute to motor hyperactivity, sleep disruption, cognitive deficits and anxiety that are common in FASD patients. The goal of the present study was to explore such a role of the ORX system in the behavioral abnormalities caused by early developmental AE in a rat model.
We used an established model of prenatal AE in which alcohol is administered to rats during early postnatal period of brain development that corresponds to the third trimester of human pregnancy [9,18–22]. The procedures for animal handling followed the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Neonatal treatments were administered to male Sprague-Dawley rats. The animals were housed on a 12:12 light/dark schedule with lights on at 7 am and ad lib access to food and water. Thirteen litters from timed-pregnant rats were culled and cross-fostered among lactating dams to obtain 8 experimental litters. Each experimental litter contained 10–11 male pups obtained from 6 or 7 different mothers (mean litter size: 10.13±0.13 (SE)). On PD4, each litter was randomly assigned to either alcohol (AE)- or sham-treated (S) group (non-mixed design [18]). The AE group received alcohol on PD4 through PD9 administered as two intragastric intubations per day (2.625 g/kg per intubation, 11.9% v/v in a custom milk formula, 2 h apart), as described previously [20,22]. Two hours after the second alcohol administration, an additional intubation with milk only was given to the AE group to compensate for reduced maternal milk consumption in intoxicated pups. The S group underwent the same daily routine of intubations, but no fluid was infused [21]. Pups were weighed daily and stayed with the dam between the treatments. To estimate blood alcohol concentration (BAC), blood was collected on PD4 from a separate group of AE rats (n=8) sacrificed 2 h after the second alcohol intubation. BAC was determined using NAD-ADH reagent (Sigma, St. Louis, MO, USA), and was 341±23 (SE) mg/dl, consistent with previously published results obtained from a similar experimental protocol [9,22].
On PD12–14, rats from both neonatal treatment groups received daily intraperitoneal injection of either the ORX-1 receptor antagonist, SB-334867 (N-(2-Methyl-6-benzoxazolyl)-N’-1,5-naphthyridin-4-yl urea; Tocris Bioscience, Ellisville, MO, USA; 20 mg/kg) or vehicle (10% w/v of (2-Hydroxypropyl)-β-cyclodextrin and 10% v/v dimethyl sulfoxide in sterile saline). The treatment started on PD12, three days after the end of AE, in order to allow for elimination of any withdrawal effects [10]. The dose and method of administration were selected based on previous studies [16,23,24]. We expected that the magnitude of the hypothesized effect of neonatal AE on the ORX system would vary with the circadian time because, in rats, activity of the ORX neurons and brain ORX levels peak during the lights-off (active) period and are low during the lights-on period [25,26]. In addition, we earlier determined that adult rats perinatally exposed to alcohol have reduced sleep during the lights-off period [11]. Based on these considerations, rats were subjected to the antagonist treatment and subsequent behavioral tests during the first half of the lights-off phase (8:00–11:30 PM).
On PD 16, rats were subjected to the homing response (HR) test, an established measure of spatial learning and memory in immature rats based on the quantification of their nest-seeking behavior [27–29]. The apparatus and the test protocol were adapted from a previous study [27]. Briefly, the apparatus included two transparent plexiglass boxes connected through an opening (4 cm diameter) located 1.5 cm above the floor. The “home box” comprised two compartments of the same size separated by a transparent wall and filled with bedding material from the home cage. One of these compartments was connected to a clean “starting box,” whereas the other compartment was used for habituation only. Pups were placed in the habituation compartment and left undisturbed for 10 min. Following habituation, they were placed, one at a time, in the middle of the starting box facing away from the home box. The time necessary for the pup to enter the home box (HR latency) was recorded. If the rat entered the home box with all four paws within 60 s, the trial was recorded as successful. If a trial was not successful, the pup was directed through the opening using gentle manual assistance. The test was repeated up to 10 times at 60–80 s intervals, or until 4 successful returns “home” were achieved in 5 consecutive trials (HR acquisition [29]). The starting box was cleaned and dried between the trials, and the entire apparatus was disassembled and cleaned between tests with different litters. The percentage of tests in which HR acquisition was not achieved, the percentage of successful trials within the last 5 trials, the shortest HR latency, and the number of trials needed to reach the shortest latency were compared among the treatment groups. After the tests, the pups were returned to their dams.
On PD17, motor activity of the rats was recorded in a novel environment using a system of infrared light beams (MicroMax, AccuScan Instruments, Columbus, OH, USA). During each session, two rats, one treated with SB-334867 and one treated with vehicle, were tested simultaneously in separate clean cages placed in a sound-dampened recording chamber to which they were not habituated. The total number of beam interruptions (total motor activity) and the number of stereotypy bouts (episodes during which animal breaks the same beam, or a set of beams, repeatedly with breaks longer than 1 s) were determined during recording sessions lasting 10 min using the software supplied by the manufacturer.
The significance of differences in proportions derived from the categorical data between the groups was examined using Pearson’s Chi-square test. All datasets with continuous variables were tested for normality and equal variance. The significance of differences between the groups was then examined using one-way ANOVA with Bonferroni’s contrast. When normality criteria were not fulfilled, nonparametric analysis was performed with Kruskal-Wallis ANOVA with Bonferroni’s contrast. All differences were considered significant at p<0.05 (Analyse-It software, Leeds, UK).
Body weight was lower in pups of the AE than S group starting from PD5 (22.3g±0.4 (SE) vs. 28.1±0.3 on PD12; p<0.0001) reflecting a growth lag similar to that described in earlier studies utilizing this model [9,18,20,22]. Since we used a non-mixed litter design, we can exclude the possibility of a growth disadvantage of AE pups caused by a selectively altered maternal interaction. The slower body weight gain during the treatment was likely caused by reduced caloric intake due to inhibited suckling [18]. Body weight data collected on the day of HR testing (PD16) indicate that prior treatment with SB-334867 did not alter the significant body weight deficit caused by the earlier AE (30.8g±0.9 (SE) vs. 36.6g±0.5 in rats treated with SB-334867, and 32.9±0.5 vs. 36.6±0.7 in vehicle-treated rats).
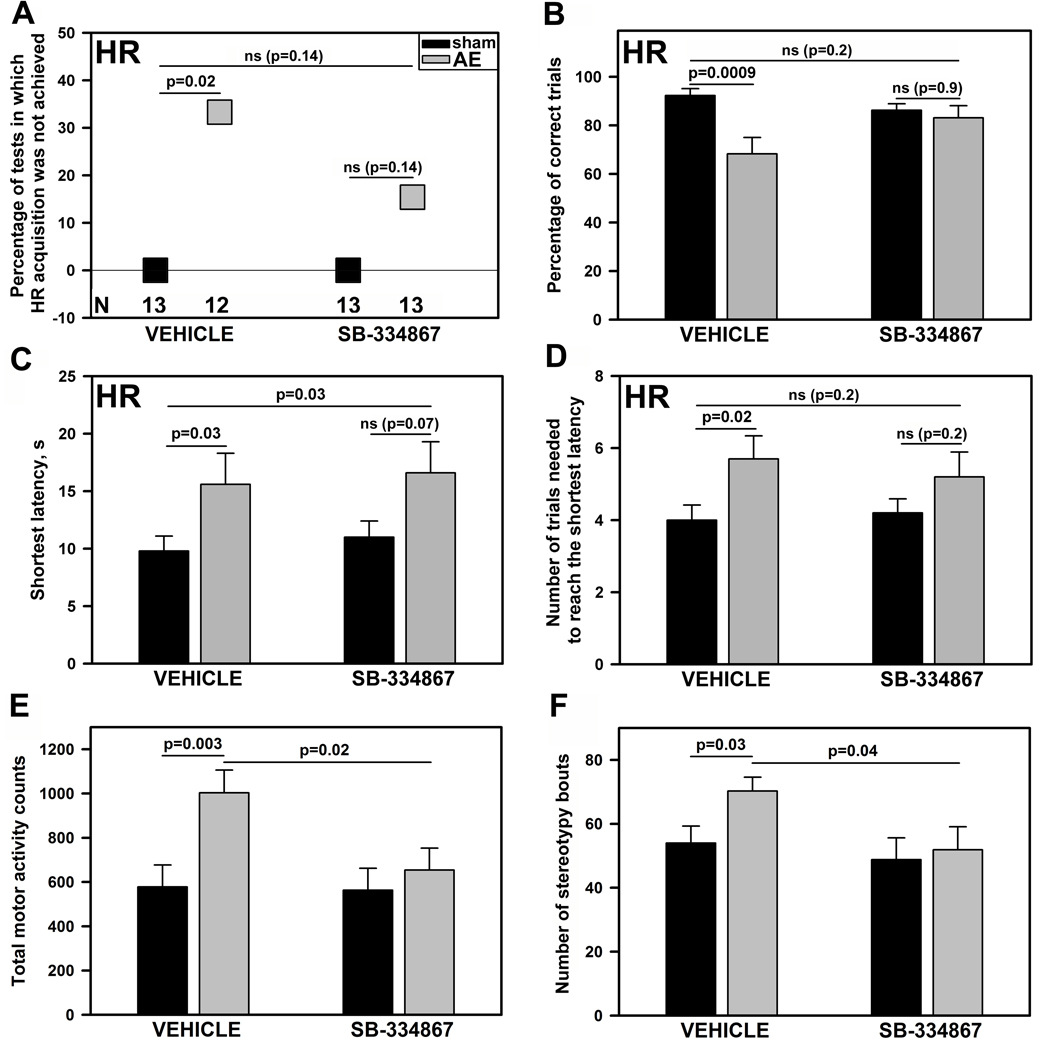
On PD16, 7 days after the last AE, the AE rats treated with vehicle (AE-V) exhibited a significant impairment of HR acquisition when compared to the S group treated with vehicle (S-V) (Fig. 1A–D; left side of the graphs). The percentage of tests in which HR acquisition was not achieved (Fig. 1A), the shortest HR latency (Fig. 1C), and the number of trials needed to reach the shortest latency (Fig. 1D) were all higher, whereas the percentage of successful trials was lower (Fig 1B), in AE-V than in S-V rats (p=0.0009–0.03; N=12 or 13 per group). In contrast, these measures were not significantly different between the AE and S rats treated with the ORX-1 receptor antagonist (AE-SB and S-SB rats; N=13 per group; Fig. 1A–D; right side of the graphs) and between the S-V and S-SB rats (Fig. 1A–D; left vs. right side of the graphs). Importantly, all these measures except the shortest HR latency were also not different between the AE-SB and S-V rats (Fig. 1A–D; left vs. right side of the graphs).
Figure 1.
Homing response (HR) performance (A–D) and motor activity (E, F) in sham-intubated control (S) and alcohol-exposed (AE) rats with and without subsequent treatment with the orexin-1 receptor antagonist, SB-334867 (mean ± SE). The percentage of tests in which HR acquisition was not achieved (A), the shortest HR latency (C), and the number of trials needed to reach the shortest latency (D) were all higher, whereas the percentage of successful trials was lower (B), in AE than S group. Among the rats treated with SB-334867, these measures were not significantly different between AE and S rats, and all measures except the shortest HR latency were also not different between the AE group treated with SB-334867 and S group treated with vehicle. The total number of movements during 10 min period spent in a novel environment (E) and the number of bouts of stereotypic motor behaviors (F) were higher in AE rats subsequently treated with vehicle than in either the S group treated with vehicle or the AE group treated with SB-334867. There was no difference between the S groups treated with either SB-334867 or vehicle. The numbers of animals per group (N) and the legend for group coding shown in A apply to all panels.
On PD17, total motor activity and the number of stereotypy bouts were significantly higher in AE-V than S-V rats (p=0.003 and 0.03, respectively; Fig. 1E,F). Subsequent treatment with the ORX-1 receptor antagonist effectively abolished this difference. Both measures were significantly lower in AE-SB than AE-V rats (p=0.02 and 0.04, respectively) and did not differ from those in either S-V or S-SB groups (Fig. 1E,F; p>0.5).
Our data show that pre-weaning rats exposed to alcohol during the developmental period equivalent to the third trimester of human gestation exhibit significant impairment of HR acquisition. We found that, among the vehicle-treated rats, the proportion of those that were unable to reach HR within the assigned number of trials (HR acquisition) was significantly higher, whereas the percentage of trials generating HR was lower, in AE than S group. In addition, AE resulted in a significant increase in the shortest HR latency and in the number of trials necessary to reach HR with the shortest latency. The HR test quantifies the nest-seeking behavior of pre-weaning rats, which may depend on the sensory and motor functions, motivation and learning abilities [28]. The test does not allow one to clearly differentiate among these impairments. However, a contribution of sensory or motor deficits is unlikely, because an earlier study did not reveal abnormalities in sensory development or locomotion in pre-weaning rats following even more severe AE [6]. HR test is also considered to reflect olfactory orientation of pups and functioning of the working memory [27,28]. Results from a previous study utilizing the same model of AE show that pre-weaning AE rats exhibit unaltered sensory component of the orienting response to olfactory stimuli but may fail to form a working memory of the olfactory stimulus [30]. It is, therefore, plausible that impaired working memory in AE rats in the present study contributed to delayed HR acquisition and increased HR latency because they likely kept responding to each subsequent HR trial with a novelty reaction. There is also evidence that certain types of developmental malnutrition lead to increased HR latency in rats [31]. Therefore, reduced intake of nutrients due to inhibited suckling during AE may have contributed to the observed increase in HR latency in AE rats which was not diminished by treatment with the ORX-1 receptor antagonist.
The results of the present study demonstrate that perinatal AE leads to significant increase in both total motor activity and number of stereotypy bouts. Motor hyperactivity is a common symptom of FASD, even in children with minimally impaired intelligence [2,4,32], and in animal models of prenatal AE [6,10]. Motor hyperactivity can result from AE-associated dysfunction of various brain regions and neurotransmitter systems [2,6,10]. Hyperactivity and impaired spatial learning are consistent with a dysfunction of the hippocampus which is particularly vulnerable to AE during the period equivalent to the third trimester of human gestation [33].
Our main new finding is that three days of treatment with the ORX-1 receptor antagonist, SB-334867, administered a few days after AE improved HR performance and eliminated motor hyperactivity typical of rats exposed to alcohol. Since SB-334867 was administered during a developmental period that is equivalent to human infancy [34], this finding is of clinical relevance because it suggests that pharmacological intervention after birth may reduce severity of FASD symptoms.
In this study, we used systemic administration of SB-334867, which was previously shown to modulate various behavioral responses dependent on ORX-1 receptors, including food intake and locomotion [35], rapid eye movement (REM) sleep [36], motivated behaviors [16,23,24], and panic anxiety [17]. However, one limitation of this approach is that it may result in antagonizing ORX-1 receptors in multiple brain regions, thus making it difficult to identify the key brain regions where SB-334867 exerted its beneficial effects in our study. SB-334867 is a selective antagonist of ORX-1 receptors with at least 50-fold selectivity over ORX-2 receptors [37]. ORX-1 is the predominant type of ORX receptors expressed in the hippocampal formation [38] which appears to be vulnerable to AE during early development [33]. Importantly, ORX impairs rat performance in the Morris water spatial learning task when administered i.c.v. prior to the test, and suppresses hippocampal long-term potentiation (LTP) in vitro [39]. Since earlier data show that prenatal AE can lead to life-long suppression of hippocampal LTP [40], one possible mechanism of action of SB-334867 in our experiments may be that it facilitated restoration of hippocampal LTP that was impaired by perinatal AE.
ORX cell activity is strongly associated with motor activation [12,13]. Systemic administration of SB-334867 decreases grooming and food intake stimulated by i.c.v. pre-treatment with ORX [35]. Infusions of ORX into the ventral tegmental area increase dopamine release in the prefrontal cortex [41], whereas systemic treatment with SB-334867 reduces amphetamine-induced dopamine release in the nucleus accumbens [42]. Excessive brain levels of dopamine may lead to development of motor stereotypies which are also present in some neurologic and psychiatric disorders [43]. Our present data suggest that treatment with SB-334867 can eliminate both hyperactivity and increased motor stereotypy caused by perinatal AE without affecting motor activity in control rats.
Prenatal AE may cause persistent activation of the hypothalamic-pituitary-adrenal axis, thus increasing vulnerability to depression and anxiety disorders, the most commonly diagnosed mental disorders in FASD patients [3]. Recent data from a rat panic model demonstrate that activation of ORX-1 receptors can elevate anxiety and the associated increase in locomotion [17]. Based on these data, it is possible that one of the potential mechanisms by which SB-334867 improved HR performance in AE rats was by alleviating their anxiety-like state.
We did not investigate the dose-response relationship of SB-334867. Rather, we used a single dose selected from a range of doses previously found effective in modulating various behavioral outcomes in adult rats [16,17,35,36]. In 2-week old rats, ORX neurons and ORX-containing fibers appear to be relatively well developed [44] but their density and immunoreactivity are lower than in adult rats [45]. Thus, it is possible that a lower dose of SB-334867 than the one that we used might be sufficient to produce the behavioral effects that we found in the present study.
Collectively, our findings suggest that endogenous activation of ORX-1 receptors contributes to motor hyperactivity and impaired HR acquisition following perinatal AE, and that these symptoms may be alleviated by systemic antagonism of ORX-1 receptors.
Acknowledgements
The study was partially supported by grant HL-071097 (LK) and DFG Research Fellowship Ste1899/1-1 (GMS).
Abbreviations
- AE
alcohol exposure
- BAC
blood alcohol concentration
- FASD
fetal alcohol spectrum disorders
- HR
homing response
- LTP
long-term potentiation
- ORX
orexin
- PD
postnatal day
- S
sham-treated
- V
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rasmussen C, Horne K, Witol A. Neurobehavioral functioning in children with fetal alcohol spectrum disorder. Child Neuropsychol. 2006;12:453–468. doi: 10.1080/09297040600646854. [DOI] [PubMed] [Google Scholar]
- 2.Niccols A. Fetal alcohol syndrome and the developing socio-emotional brain. Brain Cogn. 2007;65:135–142. doi: 10.1016/j.bandc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson S, Riley E. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 5.D'Angiulli A, Grunau P, Maggi S, Herdman A. Electroencephalographic correlates of prenatal exposure to alcohol in infants and children: A review of findings and implications for neurocognitive development. Alcohol. 2006;40:127–133. doi: 10.1016/j.alcohol.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Kelly SJ, Hulsether SA, West JR. Alterations in sensorimotor development: Relationship to postnatal alcohol exposure. Neurotoxicol Teratol. 1987;9:243–251. doi: 10.1016/0892-0362(87)90009-2. [DOI] [PubMed] [Google Scholar]
- 7.Stone W, Altman H, Hall J, Arankowsky-Sandoval G, Parekh P, Gold P. Prenatal exposure to alcohol in adult rats: Relationships between sleep and memory deficits, and effects of glucose administration on memory. Brain Res. 1996;742:98–106. doi: 10.1016/s0006-8993(96)00976-6. [DOI] [PubMed] [Google Scholar]
- 8.Hausknecht KA, Acheson A, Farrar AM, Kieres AK, Shen RY, Richards JB, et al. Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci. 2005;119:302–310. doi: 10.1037/0735-7044.119.1.302. [DOI] [PubMed] [Google Scholar]
- 9.O'Leary-Moore S, McMechan A, Mathison S, Berman R, Hannigan J. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38:99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 11.Volgin D, Kubin L. Adult rats exposed to alcohol during perinatal period have altered REM sleep amounts and sleep initiation. SLEEP. 2009;32:A26. [Google Scholar]
- 12.Torterolo P, Yamuy J, Sampogna S, Morales F, Chase M. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;26:25–28. [PubMed] [Google Scholar]
- 13.Mileykovskiy B, Kiyashchenko L, Siegel J. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno K, Sakurai T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence A, Cowen M, Yang H, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson P, Truitt W, Fitz S, Minick P, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Light K, Kane C, Pierce D, Jenkins D, Ge Y, Brown G, et al. Intragastric intubation: Important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcohol Clin Exp Res. 1998;22:1600–1606. doi: 10.1111/j.1530-0277.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 19.Goodlett C, Pearlman A, Lundahl K. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- 20.Volgin D. Perinatal alcohol exposure leads to prolonged upregulation of hypothalamic GABAA receptors and increases behavioral sensitivity to gaboxadol. Neurosci Lett. 2008;439:182–186. doi: 10.1016/j.neulet.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods Mol Biol. 2008;447:101–110. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGough NN, Thomas JD, Dominguez HD, Riley EP. Insulin-like growth factor-I mitigates motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2009;31:40–48. doi: 10.1016/j.ntt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris G, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 24.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, et al. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett. 2000;279:109–112. doi: 10.1016/s0304-3940(99)00955-6. [DOI] [PubMed] [Google Scholar]
- 26.Estabrooke I, McCarthy M, Ko E, Chou T, Chemelli R, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slamberová R, Pometlová M, Charousová P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:82–88. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Pometlová M, Hrubá L, Slamberová R, Rokyta R. Cross-fostering effect on postnatal development of rat pups exposed to methamphetamine during gestation and preweaning periods. Int J Dev Neurosci. 2009;27:149–155. doi: 10.1016/j.ijdevneu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Mikulecká A, Mares P. Effects of mGluR5 and mGluR1 antagonists on anxiety-like behavior and learning in developing rats. Behav Brain Res. 2009;204:133–139. doi: 10.1016/j.bbr.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Hunt PS, Phillips JS. Postnatal binge ethanol exposure affects habituation of the cardiac orienting response to an olfactory stimulus in preweanling rats. Alcohol Clin Exp Res. 2004;28:123–130. doi: 10.1097/01.ALC.0000108650.02216.1A. [DOI] [PubMed] [Google Scholar]
- 31.Galler JR. Home-orienting behavior in rat pups surviving postnatal or intergenerational malnutrition. Dev Psychobiol. 1980;13:563–572. doi: 10.1002/dev.420130602. [DOI] [PubMed] [Google Scholar]
- 32.Shaywitz SE, Cohen DJ, Shaywitz BA. Behavior and learning difficulties in children of normal intelligence born to alcoholic mothers. J Pediatr. 1980;96:978–982. doi: 10.1016/s0022-3476(80)80621-4. [DOI] [PubMed] [Google Scholar]
- 33.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 34.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers R, Halford J, Nunes de Souza R, Canto de Souza A, Piper D, Arch J, et al. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith M, Piper D, Duxon M, Upton N. Evidence implicating a role for orexin-1 receptor modulation of paradoxical sleep in the rat. Neurosci Lett. 2003;341:256–258. doi: 10.1016/s0304-3940(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 37.Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. SB-334867-A: The first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus J, Aschkenasi C, Lee C, Chemelli R, Saper C, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 39.Aou S, Li XL, Li AJ, Oomura Y, Shiraishi T, Sasaki K, et al. Orexin-A (hypocretin-1) impairs Morris water maze performance and CA1-Schaffer collateral long-term potentiation in rats. Neuroscience. 2003;119:1221–1228. doi: 10.1016/s0306-4522(02)00745-5. [DOI] [PubMed] [Google Scholar]
- 40.Swartzwelder HS, Farr KL, Wilson WA, Savage DD. Prenatal exposure to ethanol decreases physiological plasticity in the hippocampus of the adult rat. Alcohol. 1988;5:121–124. doi: 10.1016/0741-8329(88)90008-0. [DOI] [PubMed] [Google Scholar]
- 41.Vittoz N, Berridge C. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: Involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 42.Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2010;56:11–15. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Chartoff EH, Marck BT, Matsumoto AM, Dorsa DM, Palmiter RD. Induction of stereotypy in dopamine-deficient mice requires striatal D1 receptor activation. Proc Natl Acad Sci USA. 2001;98:10451–10456. doi: 10.1073/pnas.181356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoyanova II, Rutten WL, le Feber J. Orexin-A and orexin-B during the postnatal development of the rat brain. Cell Mol Neurobiol. 2010;30:81–89. doi: 10.1007/s10571-009-9433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawai N, Ueta Y, Nakazato M, Ozawa H. Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci Lett. 2010;468:51–55. doi: 10.1016/j.neulet.2009.10.061. [DOI] [PubMed] [Google Scholar]