Abstract
Background
Prenatal exposure to moderate ethanol doses during late gestation modifies postnatal ethanol palatability and ingestion. The use of Pavlovian associative procedures, has indicated that these prenatal experiences broaden the range of ethanol doses capable of supporting appetitive conditioning. Recently, a novel operant technique aimed at analyzing neonatal predisposition to gain access to ethanol has been developed. Experiment 1 tested the operant conditioning technique for developing rats described by Arias et al. (2007) and Bordner et al. (2008). In Experiment 2 we analyzed changes in the disposition to gain access to ethanol as a result of moderate prenatal exposure to the drug.
Methods
In Experiment 1 newborn pups were intraorally cannulated and placed in a supine position that allowed access to a touch-sensitive sensor. Paired pups received an intraoral administration of a given reinforcer (milk or quinine) contingent upon physical contact with the sensor. Yoked controls received similar reinforcers only when Paired pups activated the circuit. In Experiment 2, natural reinforcers (water or milk) as well as ethanol (3% or 6 % v/v) or an ethanol-related reinforcer (sucrose compounded with quinine) were tested. In this Experiment pups had been exposed to water or ethanol (1 or 2 g/kg) during gestational days 17–20.
Results
Experiment 1 confirmed previous results showing that 1-day-old pups rapidly learn an operant task to gain access to milk, but not to gain access to a bitter tastant. Experiment 2 showed that water and milk were highly reinforcing across prenatal treatments. Furthermore, general activity during training was not affected by prenatal exposure to ethanol. Most importantly, prenatal ethanol exposure facilitated conditioning when the reinforcer was 3% v/v ethanol or a psychophysical equivalent of ethanol’s gustatory properties (sucrose-quinine).
Conclusions
The present results suggest that late prenatal experience with ethanol changes the predisposition of the newborn to gain access to ethanol-related stimuli. In conjunction with prior literature, this study emphasizes the fact that intrauterine experience with ethanol not only augments ethanol’s palatability and ingestion, but also facilitates the acquisition of response-stimulus associations where the drug acts as an intraoral reinforcer.
Keywords: fetus, ethanol, neonate, operant learning, extinction
Introduction
During early ontogeny, genetically heterogeneous rats show high affinity towards ethanol consumption and high sensitivity to the drug’s positive and negative (anxyolitic) reinforcing effects. For example, infant rats ingest large amounts of ethanol without initiation procedures (Lee et al., 1998; Sanders and Spear, 2007; Truxel and Spear, 2004); a phenomenon not observed in genetically heterogeneous adults. During adulthood, high voluntary ethanol ingestion is achieved only through long pre-exposure procedures or via techniques that initially serve to overshadow the drug’s orosensory cues (Cunningham et al., 2000; Samson, 1986). Furthermore, ethanol doses that are found to be aversive during adulthood promote conditioned preferences in preweanlings (Molina et al., 2007a; Molina et al., 2006; Pautassi et al., 2008). At this age relatively low ethanol doses (e.g. 0.125 – 0.5 g/kg, intragastric administration) have been found to inhibit the aversive consequences of social isolation and of hedonically negative tastants (Pautassi et al., 2007; Pautassi et al., 2006) and to exert positive reinforcing effects (Petrov, Varlinskaya & Spear, 2003; Nizhnikov et al, 2006a; Nizhnikov et al, 2006b; Nizhnikov, Varlinskaya & Spear, 2006; Nizhnikov, Molina & Spear, 2007).
Infantile disposition to consume varying ethanol concentrations as well as to encode ethanol’s postabsorptive reinforcing effects are augmented through prenatal or early postnatal experiences with the drug (intake studies: Arias & Chotro, 2005a; Arias & Chotro, 2005b; Domínguez et al., 1998; Youngentob et al., 2007; reinforcing studies: Nizhnikov et al., 2006; Petrov et al., 2003). Based on these studies it appears that ethanol use and abuse can be strongly determined or modulated through early ethanol exposure. This hypothesis has received further preclinical and epidemiological support when considering the effect of moderate or excessive maternal ethanol consumption during pregnancy or lactation on subsequent affinity for ethanol (animal studies: Pueta et al., 2008; Pueta et al., 2005; human studies: Alati et al., 2006; Baer et al., .1998; Baer et al., 2003; Yates et al., 1998). Recent studies support the hypothesis that early exposure to ethanol implies familiarization with the drug’s chemosensory properties as well as associative learning mediated by ethanol’s reinforcing postabsorptive effects (for recent revisions see: Abate et al., 2008; Molina et al., 2007b; Spear and Molina, 2005).
Animal studies focusing on the association between early ethanol experiences and subsequent ethanol intake or sensitivity to the drug’s reinforcing effects have been conducted using forced ethanol administration procedures. For example, ethanol palatability following maternal or infantile forced intragastric administration of the drug has been assessed through ingestive techniques in which a given ethanol concentration is directly infused into the mouth. Under this experimental framework, infants prenatally exposed to ethanol show an increase in mouthing responsiveness and a decrease in aversive motor activity (passive drips and wall climbing). This behavioral profile seems to be positively correlated with heightened ethanol consumption (Arias and Chotro, 2005a). Recently, Nizhnikov et al. (2006) have also found that maternal-fetal ethanol intoxication during late pregnancy (gestational days 17–20) is sufficient to broaden the range of intraperitoneal ethanol doses that act as appetitive unconditioned stimuli in neonatal rats.
Until recently, operant techniques utilized in adult animals to analyze ethanol seeking and ingestive behaviours were not available for the infant rat. The principal obstacle in developing operant techniques resides in the limited behavioral repertoire of the infant and the short span of infancy. In adults, long-lasting training procedures are required to modify the probability of execution of specific behaviors when ethanol acts as an intraoral or intracerebral reinforcer (Gatto et al., 1994; McBride et al., 1993; Samson and Czachowski, 2003). In infants, long-training procedures imply maternal deprivation arrangements that unfortunately are often sufficient to affect affinity for ethanol ingestion or sensitivity to the drug’s reinforcing effects (Huot et al., 2001; Pepino et al., 2004; Pepino et al., 1999; Ponce et al., 2004). An additional problem related to long-term training with ethanol is the vulnerability of the infant central nervous system to the teratogenic effects of the drug (Bonthius et al., 1992; Chen et al., 1999; Savage et al., 2002; Young and Olney, 2006).
Despite the difficulties associated with the development of adequate operant techniques for developing animals, there have been a few sets of studies that have dealt successfully with them. For example, one-day old pups rapidly learn to perform side head movements when rewarded with warmth (Flory et al., 1997) and exhibit significant changes in the rate of head and forelimb movements that result in paddle pressing when intraorally reinforced with milk (Johanson and Hall, 1979). These movements constitute a behavioral segment of the fixed-action pattern leading to nipple attachment and suckling. Nevertheless, the study conducted by Johanson & Hall (1979) and latter replicated by Dominguez et al. (1993) involved potentially stressful conditions such as prolonged maternal deprivation and undernourishment. Recently, two studies (Arias et al., 2007; Bordner et al., 2008) have shown that neonatal forepaw and head movements, rapidly and significantly increase when milk acts as a reinforcer and the organism is held in a supine position. In both studies, only 15 minutes of training were sufficient to install a rate of sensor presses that was significantly higher than that of yoked controls (pups given reinforcement only when the experimental counterpart touched the sensor).
To our knowledge there are no previous studies aimed at analyzing changes in the disposition to gain access to ethanol as a result of moderate prenatal exposure to the drug. As mentioned, moderate ethanol exposure during late gestation modulates later responsiveness to the drug (Abate et al., 2008; Molina et al., 2007b; Spear and Molina, 2005). Little is known about the acquisition and maintenance of responses aimed at gaining access to ethanol in newborn mammals, an ontogenetic stage where the organism seems to be particularly predisposed to learn about chemosensory cues that will determine or modulate later seeking behaviours as well as preference and consumption patterns of particular diets or specific substances (e.g. see (Delauney-El Allam et al., 2006; Mennella et al., 2001; Nizhnikov et al., 2002; Petrov et al., 2001; Petrov et al., 2003; Roth and Sullivan, 2006; Sullivan, 2003). It is similarly unclear whether prenatal ethanol treatments that clearly affect ethanol ingestion and preferences are capable of modifying early postnatal dispositions to gain access to the drug itself or to drug-related stimuli.
Taking these considerations into account, the main goal of the present study was to analyze the impact of late prenatal exposure to ethanol upon the capability of the neonate to learn an operant task where ethanol serves as an intraoral reinforcer. As will be explained in detail later, a first experiment was executed to verify if the operant conditioning technique described by Arias et al. (2007) and Bordner et al. (2008) in Sprague-Dawley rats is also effective in neonates of a different heterogenous rat strain (Wistar-derived). Positive results in this validation study allowed the execution of a second experiment in which we examined how prenatal ethanol exposure affects operant conditioning sustained by natural reinforcers (water or milk) or ethanol-related stimuli (3 or 6% v/v ethanol or a sucrose-quinine solution that has been found to mimic ethanol’s gustatory properties; Arias & Chotro, 2005b; Bachmanov et al., 2003; Domínguez et al., 1998).
Experiment 1
As mentioned, previous studies have shown that rapid and effective operant training is achieved in the neonatal and the infant rat (Arias et al.2007; Bordner et al., 2008). The aim of the present experiment was to replicate and extend these findings in a different laboratory using Wistar rather than Sprague Dawley-derived animals. In both strains we have observed similar early learning and retention capabilities, but also some differences in their behavioral performance. For example Wistar-derived pups appear to exhibit lower behavioral activation when initially stimulated with a surrogate nipple that delivers milk (Abate et al., 2002). Certain levels of behavioral activation are needed to ensure neonatal perception of an effective contingency leading to operant acquisition processes (see Arias et al., 2007). Given the relatively low levels of activation observed in Wistar pups we decided to increase the volume of reinforcement to maximize the probability of physical activity leading to target response under analysis (head and forelimb movements that result in physical contact with a sensor).
The preceding studies based on neonatal operant conditioning have employed only liquid solutions that neonates tend to accept rather than reject (e.g. milk and water; Blass, 1990; Cheslock et al., 2000; Johanson and Hall, 1979; Nizhnikov et al., 2002). In this experiment, physical contacts with a touch-sensitive sensor resulted in intraoral delivery of milk or quinine. Various experimental procedures have indicated that milk serves as a primary reinforcer in neonates (Johanson and Hall, 1979; Sullivan and Hall, 1988). Quinine, a bitter tastant, is known however to promote aversive orofacial reactions (Berridge, 2000; Grill and Norgren, 1978) even in 1-day old pups (Nizhnikov et al., 2002) without necessarily affecting intake patterns (Kozlov et al., 2008). Hence, we expected that head and forepaw contacts with a touch sensitive sensor would increase when resulting in milk but not in quinine intraoral delivery. Since intraoral stimulation with fluids can by itself promote behavioral activation (Hall, 1979; Robinson and Smotherman, 1992a; Robinson and Smotherman, 1992b) we also included yoked controls. This control procedure should allow exclusion of the possibility that an increase in target behaviors is merely due to general activation rather than a response-stimulus contingency.
Materials and Methods
Subjects
A total of 14 pairs of Wistar-derived pups representatives of 7 litters were used in this experiment. Same sex littermates composed each experimental pair. All animals were born and reared at the vivarium of the Instituto de Investigaciones Médicas Mercedes y Martín Ferreyra. Temperature was kept at 22–24° C with a 12-hr light/12-hr dark cycle (light onset at 0800 hr). Vaginal smears of female adult rats (pre-pregnancy weight: 230–300 gr) were microscopically analyzed daily. On the day of Proestrus, females were housed during the dark cycle with males (three females per male). Pregnant females were individually housed in standard maternity cages partially filled with wood shavings and continuous access to rat chow (Cargill, Buenos Aires, Argentina) and tap water delivered through automatic dispenser valves. Cages were examined daily and the day of parturition was considered as postnatal day 0. All litters were culled to 10 pups (5 males and 5 females whenever possible). Animals used in Experiment 1 and 2 were maintained and treated in compliance with guidelines for animal care established by the Institute of Laboratory Animal Resources, National Research Council, U.S.A. (1996).
Neonatal conditioning sessions
On postnatal day 1, neonates were intraorally cannulated to allow delivery of fluids directly into the intraoral cavity. This procedure has been extensively described in previous studies (e.g. Abate et al., 2004; Chotro et al., 1991; Domínguez et al., 1998). Briefly, 5-cm sections of polyethylene tubing (Clay Adams, PE10) were heated at one end to form a small flange (external diameter, 1.2 mm). The nonflanged end of the cannula was attached to a dental needle (30GA C-KJET, CK Dental, Buenos Aires, Argentina) shaped as a hook. The needle was positioned on the medial internal surface of the right cheek of the pup and pushed until the flanged end rested on the mouth mucosae. The cannulation procedure lasted less than 20 sec per pup and resulted in minimal pain and stress (Hoffmann et al., 1987; Spear et al., 1989). Following cannulation, pups were placed in a heated incubator (32–34 C; Fábrica Eléctrica Delver, La Plata, Argentina) with at least one other littermate for one hour, until the time of conditioning.
Before commencement of each conditioning session, pups were stimulated in the anogenital area with a cotton swab to induce miction and defecation. Pups were subsequently placed in a restrictor vest fashioned out of spandex, allowing head and limb movements along with maintenance of the appropriate supine position during conditioning procedures. The supine position is commonly observed during nursing from a biological dam. Total time invested in the placement procedure took less than 2 minutes per newborn. Conditioning took place in a transparent Plexiglas glove box (63 × 50 × 25 cm) equipped with a fan system and two holes in the front section that allowed the experimenter to access the neonate. A 40 to 50-cm section of PE-50 polyethylene tubing was connected to the end of the oral cannula of each subject and to a 5 cc disposable syringe fitted in an infusion pump (Apema, PC11U, Bs.As., Argentina).
For conditioning, 2 same-sex littermates with roughly equivalent body weight were placed in the conditioning surface below a circular touch-sensitive sensor (diameter: 1 cm; Model E-11x Evaluation Board. Quantum Research Group; Pittsburg, Pennsylvania). Within a litter, one pair of animals (paired and its corresponding yoked control) received milk while a second pair received quinine. These grouping procedures were meant to avoid possible confounding effects of litter or sex upon behavioral performance (Holson and Pearce, 1992).
Head and forepaw movements of the paired subject that resulted in physical contact with the sensor led to activation of the infusion pump and therefore to intraoral delivery of a given reinforcer. The sensor was equipped with a red light and a tone that were also activated whenever a pup touched the device and signaled the occurrence of an effective physical contact. These audiovisual signals served as feedback cues for the experimenter. The activation of the tone and the light set the opportunity for recording a positive contact with the sensor. These cues were also present in the case of Yoked controls and during extinctions sessions. It is important to note that eye opening and onset of auditory detection in the infant rat occurs during the second week of life (Hooks and Chen, 2007; Hyson and Rudy, 1984; Geal-Dor, Freeman, Li and Sohmer, 1993). Hence, these stimuli probably do not act as discriminative cues or conditioned reinforcers in the case of 1-day-old pups. An articulated iron stand equipped with alligator clips was used to position the sensor 5 mm above the subject’s nose and forelimbs. In paired pups, each physical contact with the sensor resulted in the delivery of 10 µl of fluid in 1 second directly into the intraoral cavity (fixed ratio schedule: 1). In this Experiment, newborns received either milk (Milk –San Regim milk, SanCor, Santa Fe, Argentina, 1.5% fat content with supplement of vitamins A and B) or a quinine solution (0.0001 M). Yoked pups received the corresponding reinforcer only when the paired pup touched the sensor. No attempts were made to shape behavioral responding in the paired neonates. All subjects received 3 priming pulses of milk or quinine delivered at seconds 1, 60 and 120 within the conditioning session. As stated, each pulse lasted 1 second and resulted in the delivery of 10 µl of fluid. These pulses were administered independently of the initial rate of motor activity and were intended to familiarize the newborn with the corresponding fluid and to minimally stimulate head and body movements.
The conditioning session duration was 15 minutes. Physical contacts with the sensor were recorded using 30s bins. Total number of sensor contacts during the conditioning session served as one of the dependent variables under consideration. Maximum contacts with the sensor attained during one bin also served as a dependent variable. Immediately following conditioning, pups’ body weights were again recorded. Consumption of milk or of the quinine solution was calculated according to the following equation: Percent body weight gain (BWG%): 100 × [(postconditioning weight - preconditioning weight)/ preconditioning weight].
Experimental Design and Statistical Analysis
The experimental analysis was defined by a between and a within factor: Intraoral Reinforcer (Milk or Quinine) and Conditioning treatment (Paired or Yoked), respectively.
The Analysis of Variance (ANOVA) assumes that each observation of a between-factor design must be completely independent. This is not the case for the data derived from the paired and yoked conditions. Consequently, in this Experiment and the following one, whenever conditioning treatment (paired or yoked) was included in an ANOVA it was regarded as a within-measure factor. As a function of these considerations, data were analyzed through a two-way mixed analysis of variance (reinforcer x conditioning). In this study as well as in Experiment 2, the loci of significant main effects or 2-way interactions were further analyzed with pair-wise comparisons (Newman Keuls post hoc tests with an alpha value set at 0.05). In the case of a 3-way significant interaction (see Experiment 2), follow-up ANOVAs were employed before the use of post-hoc tests. This procedure served to minimize the probability of Type I errors arising from multiple group comparisons.
Results and Discussion
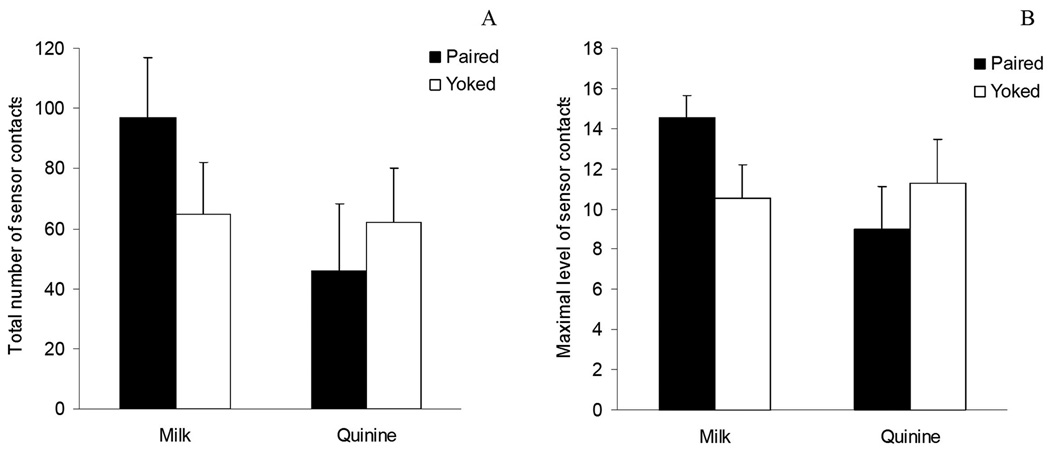
The 2 (reinforcer: milk or quinine) x 2 (conditioning treatment: paired or yoked) ANOVA indicated that physical contacts with the sensor were significantly affected by the interaction of the factors under consideration (F(1,12)=7.64; p<.025). Pair-wise comparisons showed that paired pups reinforced with milk exhibited significantly higher responding when compared with any of the remaining groups. In the case of pups reinforced with quinine, paired pups did not differ from pertinent yoked controls. Yoked pups reinforced with milk were also found not to differ from paired or yoked animals stimulated with quinine. Similar results were obtained when maximum sensor contacts in a given 30 sec bin served as the dependent variable. In this case, the ANOVA also indicated a significant interaction between the factors under consideration (F(1,12) = 18.56; p < 0.001). Post-hoc tests not only showed that paired pups reinforced with milk exhibited significantly higher scores than all the remaining groups but also that paired pups exposed to quinine had significantly lower scores when compared with their pertinent yoked controls. These results have been depicted in Figure 1.
FIGURE 1.
A: Total number of operant responses (physical contacts with the sensor). B: Maximum level of operant responses in a 30 sec. bin as a function of conditioning treatment (Paired or Yoked) and the solution that was intraorally infused (Milk or Quinine). Vertical lines represent standard errors of the means.
In terms of fluid consumption evaluated through relative body weight gains (%BWG) it was observed that pups subjected to intraoral delivery of milk exhibited higher body weight gain relative to newborns receiving quinine (mean +/− standard error values collapsed across conditioning training: milk, 0.74 +/− 0.13 and quinine, 0.49 +/− 0.13). Nevertheless, the corresponding ANOVA did not show significant main effects or a significant interaction between reinforcer and conditioning.
These results confirm prior experimental outcomes indicating that milk rapidly acts as a primary reinforcer in the neonate rat (Arias et al., 2007; Bordner et al., 2008). Under the present experimental conditions, paired pups reinforced with milk activated the sensor significantly more than did any other group. Quinine, an aversive tastant, was not observed to significantly decrease total number of sensor contacts of the paired relative to the yoked condition. Nevertheless, in terms of the maximum level of responding exhibited during a given 30 sec bin, it was evident that paired pups stimulated with quinine exhibited significantly lower scores than those observed in quinine-yoked controls. It is possible that the present procedure is not sensitive to detection of behavioral inhibition caused by aversive stimuli when considering the total number of responses exhibited in a 15-min conditioning session. This limitation may be due to a floor effect in terms of overall baseline patterns of motility caused by the posture of the animal or the delivery of a relatively high amount of fluid (10 µl) into the mouth during a limited amount of time. This baseline limitation seems to disappear when operant profiles are temporally dissected and the maximum response achieved in a given 30 sec bin is the focus of the analysis.
While sensor touches clearly differentiated the groups explicitly reinforced with milk or quinine, consumption patterns of these fluids did not reveal changes in accordance with the hedonic values of the fluids. This null result in terms of intake regulated by stimuli that otherwise seems to differ in their motivational value has been observed when using alternative techniques. For example, in some cases the nature of a tastant affects duration of attachment to a surrogate nipple but has no effect on amount ingested by the newborn (Abate et al., 2002; Smotherman et al., 1997; but also see Nizhnikov et al., 2002).
Experiment 2
According to the preceding experiment Wistar-derived pups are capable of rapidly changing their motor activity patterns as a function of the contingency existing between these patterns and intraoral delivery of certain fluids. As reported by Arias et al. (2007) and Bordner et al. (2008), neonates exhibited high numbers of physical contacts with a sensor when reinforced with milk. The explicit intention of the present experiment was to evaluate the impact of moderate ethanol exposure during late gestation upon neonatal operant conditioning sustained by natural reinforcers such as milk and water or by ethanol-related stimuli. Based on a series of previous studies indicating that prenatal ethanol exposure increases the palatability and intake patterns of the drug (Abate et al., 2008; Molina et al., 2007; Spear & Molina, 2005), we hypothesized that pups with such an exposure would more likely operate to gain access to ethanol-related reinforcers than pups without a prenatal history with ethanol. Ethanol-related reinforcement was operationalized through the use of 3 or 6% v/v ethanol solutions or through a sucrose-quinine compound known to mimic alcohol’s taste of low to moderate ethanol concentrations in young and adult rats (Arias & Chotro, 2005b; DiLorenzo et al., 1986; Domínguez et al., 1998; Kiefer, 1995). It has been observed that early memories related with the chemosensory attributes of ethanol have a strong impact upon the palatability of a sweet-bitter compound operationalized through the mixture of sucrose and quinine. Prenatal exposure to ethanol increases the ingestion of this compound without affecting intake patterns of either sucrose or quinine presented as single stimuli (Arias et al., 2005b; Dominguez et al, 1998). This equivalence between ethanol’s taste and the gustatory properties of the compound has also been detected in adult rats. Conditioned taste aversions to relatively low ethanol concentrations (3, 6 or 9 % v/v EtOH) generalize to a sucrose-quinine compound without affecting responsiveness to any of the 4 basic tastants (DiLorenzo et al., 1986; Kiefer, 1995; Kiefer et al 1990; Kiefer et al., 1988).
To test this general hypothesis we included yoked control groups representative of each prenatal treatment and each reinforcement condition. These yoked groups are critical not only in terms of the analysis of the contingency regulating behavioral performance but are also necessary to determine whether the history of ethanol exposure may exert motor activity changes that can eventually modify the probability of physical contacts with the sensor. In this regard, it is necessary to observe that near term pups treated with ethanol are likely to display hyper-reactivity in response to handling procedures or when stimulated with salient chemosensory cues (Arias et al., 2008; Chotro & Spear, 1997; Domínguez et al., 1996). To strengthen the internal validity of the results we also videotaped each conditioning session. This procedure allowed careful examination of parameters linked with the target behaviors under consideration (head and forelimb movements that resulted in physical contacts with the sensor) as well as the analysis of bursts of overall movements that did not necessarily result in activation of the sensor or delivery of a given reinforcer.
In the present experiment we also included an extinction session that took place one hour after conditioning. During this session, reinforcers were withheld. This strategy represents an experimental approach meant to examine the memory of the contingency between responses and reinforcers, which in turn should allow evaluation of the persistency of seeking behaviors as a function of the different experimental conditions.
Materials and Methods
Subjects and Prenatal Treatments
One hundred and seventeen pairs of Wistar rat neonates derived from 27 dams were tested. Pups were representative of one of three different prenatal conditions. During gestational days 17–20 (GDs 17-20) pregnant females received a daily intragastric administration of either 0, 1 or 2 g/kg ethanol. Each prenatal condition included 9 pregnant females. Administration procedures took place between 1100 and 1300 h. The 1g/kg dose was achieved by administering 0.015 ml/g of an 8.4% v/v ethanol solution (190 proof alcohol, Porta Hnos.). The 2 g/kg ethanol dose was achieved using a similar volume of a 16.8% v/v ethanol solution. Control dams (0 g/kg group) received 0.015 ml/g of tap water (vehicle employed for the above mentioned ethanol doses). Following the last i.g. administration (GD 20) dams remained undisturbed until delivery. Maternity cages were checked on a daily basis (1000–1600 h). Date of birth was considered as postnatal day 0 (PD 0). Each litter was culled to 10 pups (5 males and 5 females whenever possible). Maternal body weight gain during GDs 17-20, litter size at birth and average pup’s body weights per litter were recorded on PD1. In prior studies we have systematically observed that this regimen of ethanol administration does not affect maternal body and placental weights during late gestation, maternal behaviors during lactation or a variety of morphological and functional parameters in the offspring (Abate et al. 2000; 2001, Chotro et al., 1996; 2003, Domínguez et al 1996; 1998; 1999).
Neonatal conditioning and extinction sessions
On PD1, pups from a given litter were quasirandomly assigned to a group defined by contingency (paired or yoked) and by reinforcer (water, milk, sucrose-quinine compound, 3 or 6% v/v ethanol). No more than one pup per litter was assigned to a given postnatal treatment condition. Whenever possible, precautions were taken to equate sex and body weight across each pair of neonates assigned to a given reinforcer. Of the 117 pairs of pups employed for this study, only 3 pairs of subjects (one from the 0g/kg, one for the 1g/kg and one for the 2g/kg of prenatal group) were not sex-paired. Hence, when analyzing sex as an independent variable, data derived from these subjects were not included. Intraoral cannulation and conditioning sessions were executed according to the procedures described in Experiment 1. In the present study 10 µl of either natural reinforcers (water or milk) or ethanol-related reinforcers (sucrose-quinine, 3 or 6% v/v ethanol) were intraorally delivered whenever a paired pup touched the sensor. The sucrose-quinine solution was generated by mixing, in equal proportion (50%), a 0.1 M sucrose solution with a 0.0001 M quinine solution. Following conditioning, pups were returned to the incubator where they remained undisturbed during 60 minutes. Pups were then tested in a 15-min extinction session where reinforcers were completely withheld. This session took place in the same experimental setting employed for training purposes.
As mentioned, during conditioning various dependent measures were recorded. For the target behaviors under analysis we scored the overall number of contacts with the sensor throughout the conditioning and extinction sessions. Maximum number of operant responses attained within a 30 sec bin was also recorded for each session. In addition, during extinction, latency to perform the first contact with the sensor was registered. Frequency and duration of overall activity was calculated through the use of real-time-computer based software while scoring the videotapes. The analysis of the videotapes was performed by an experimenter blind to prenatal treatment, conditioning procedure and nature of the reinforcer. In previous studies we have observed that a positive and significant correlation exists between independent researchers working in our lab (r > 0.90). Activity included stretching, probing, kicking, hindlimb and forelimb movements.
Experimental Design and Data Analysis
A 3 (prenatal treatment: 0, 1, and 2g/kg) x 5 (reinforcer: water, milk, sucrose-quinine compound, 3 or 6% v/v ethanol) x 2 (conditioning treatment: Paired or Yoked) factorial design defined this experiment. As in Experiment 1, conditioning treatment was considered a within factor.
Three-way mixed ANOVAs (prenatal treatment x conditioning treatment x reinforcer) served to analyze number of operant responses during acquisition and extinction sessions. In these ANOVAs the dependent measures were: total number of sensor contacts, maximum operant frequency in a 30-sec bin. During extinction, latency to exhibit the first physical contact with the sensor also served as a dependent variable. Similar ANOVAs were utilized to analyze overall activity of the organisms during acquisition.
Whenever an ANOVA indicated statistically significant interactions comprising two or more factors, follow-up ANOVAs were utilized. These ANOVAs were performed considering the nature of the reinforcers. The first follow-up ANOVA considered natural reinforcers (non-ethanol-related solutions such as water and milk). The second ANOVA served to analyze responsiveness modulated by ethanol-related reinforcers (sucrose-quinine compound, 3 or 6% v/v ethanol). The results of these follow-up ANOVAs, whenever necessary, were further examined through the use of post-hoc comparisons (Newman Keuls post hoc test; p < 0.05). Table 1 depicts the basic design of this experiment as well as the number of pups per group.
Table 1.
Experimental Design and Number of pups per group
| Prenatal Treatment |
Conditioning Treatment |
Water | Milk | S+Q | EtOH 3% |
EtOH 6% |
|---|---|---|---|---|---|---|
| 0g/kg | Paired | 6 | 8 | 8 | 7 | 7 |
| Yoked | 6 | 8 | 8 | 7 | 7 | |
| 1g/kg | Paired | 8 | 9 | 6 | 7 | 9 |
| Yoked | 8 | 9 | 6 | 7 | 9 | |
| 2g/kg | Paired | 8 | 8 | 8 | 9 | 9 |
| Yoked | 8 | 8 | 8 | 9 | 9 | |
Table 1 represents the experimental design resulting from prenatal treatment, conditioning treatment at PD 1, and fluid solution given as a reinforcer. The table also shows the number of pups evaluated per group.
Results
Maternal body weight gain during gestational days 17–20, litter size and pup’s weight on PD1
Percentage of dam’s body weight gain during late pregnancy were calculated as follows: {[(Maternal Body Weight at GD20 - Maternal Body Weight at GD17)/ Maternal Body Weight at GD17] *100}. A one-way ANOVA showed that prenatal treatments had no significant effects upon this weight index. Similarly, prenatal treatment did not affect number of pups per litter or pups’ body weights on PD 1 (averaged within each litter) These results are illustrated in Table 2.
Table 2.
Prenatal treatment did not affect either of the following dependent measures:
| Table 2. | Prenatal Treatment (g/kg) | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Percentage of Dam’s BWG during GD17-20 (Means ± SEM) |
9.08 ± 0.51 | 9.60 ± 0.98 | 7.95 ± 1.01 |
| Litter weight at PD1 (Means ± SEM) |
6.78 ± 0.28 | 6.83 ± 0.28 | 6.96 ± 0.19 |
| Number of Pups per litter (Means ± SEM) |
12.44 ± 0.87 | 13.22 ± 0.81 | 13.00 ± 0.56 |
Table 2 shows percentage of maternal body weight gains during late gestation (DG17-20), litter weight averaged at postnatal day 1 and number of pups born alive per litter, as a function of prenatal treatment. Data are presented as means ± standar error of the mean.
Preliminary examination of the behavioral data indicated that sex consistently failed to exert significant main effects on any of the dependent variables under examination or to significantly interact with prenatal and/or postnatal treatments. For this reason, additional inferential processing of the data was performed by collapsing sex across prenatal and neonatal treatment conditions.
Ingestion during training
Percent body weight gains were calculated as an index of fluid ingestion, as described above. Two main effects were encountered: conditioning treatment; F(1,101)=5,38; p<,025, and solution given as a reinforcer; F(4,101)=2,61; p< 0.05. Newman-Keuls pos-hoc test revealed that Yoked animals exhibited significantly higher %BWGs than Paired pups. As mentioned, nature of the reinforcer also affected fluid consumption, post-hoc tests revealed that animals receiving 6% v/v ethanol exhibited significantly less body weight gain than pups receiving water (water: 0.72 +/− 0.07 %BWG; milk: 0.65 +/− 0.06 %BWG; sucrose-quinine: 0.64 +/− 0.07 %BWG; 3% v/v ethanol: 0.64+/− 0.076 %BWG; 6% v/v ethanol: 0.43 +/− 0.07 %BWG). Prenatal treatment did not affect fluid ingestion; there were no interactions between the factors under analysis.
Operant performance during training
The corresponding 3-way mixed ANOVA showed a significant main effect of conditioning [F(1,102)=41.73; p<.0001] tempered by a significant interaction between conditioning treatment and reinforcer (F(4,102)=3.36; p<.025) as well as a 3-way interaction (prenatal treatment x reinforcer x conditioning; F(8,102)=2.41; p<.025). To analyze the locus of this interaction, two follow-up ANOVAs were performed. The first ANOVA took into account the effects of prenatal treatment and contingency when employing natural reinforcers (i.e. water and milk). A second ANOVA was conducted to analyze operant performance derived from the use of ethanol-related reinforcers [Sucrose+Quinine (a psychophysical equivalent of ethanol flavor), 3% v/v or 6% v/v of EtOH]. This is a fairly conservative approach since it minimizes the number of multiple post-hoc comparisons required to dissect the locus of the 3-way interaction attained with the omnibus ANOVA. In other words, our intention was to avoid possible Type I errors derived from the multiple group comparisons related with the 3-way interaction. Despite this consideration, it is important to note that both inferential strategies, follow-up ANOVAs and the corresponding use of post-hoc comparisons or the direct use of multiple comparisons following the omnibus ANOVA, lead to similar conclusions.
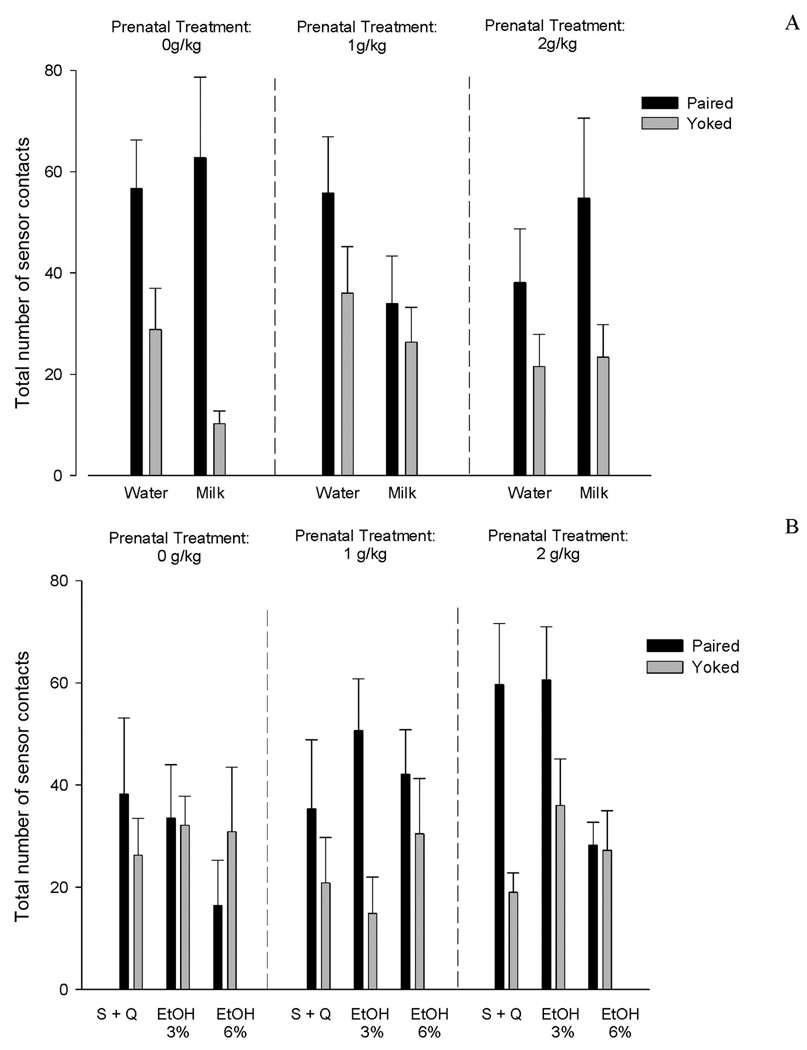
The follow-up ANOVA regarding natural reinforcers (water and milk) showed that independently of prenatal treatment, Paired pups, reinforced with either milk or water, exhibited higher number of operant responses than the corresponding yoked control pups (main effect of conditioning: F(1,41) = 24.49, p < 0.0001). No significant interactions were observed. In other words, when Paired pups, relative to Yoked controls, were trained with water or milk, they increased the number of physical contacts with the sensor regardless of the reinforcer and of the prenatal treatment (Figure 2A).
FIGURE 2.
Total number of operant responses (physical contacts with the sensor) during acquisition as a function of prenatal treatment, conditioning treatment and solution given as a reinforcer. A) Water and Milk reinforcers. B) Ethanol related solutions [sucrose and quinine (S+Q), 3% or 6% v/v ethanol]. Black bars represent data from Paired pups and white bars show data from Yoked control pups. Vertical lines represent standard errors of the means.
On the other hand, the follow-up ANOVA comprising ethanol-related reinforcers (sucrose-quinine, 3% v/v and 6% v/v of EtOH solution) indicated a significant main effect of conditioning (F(1,61)= 17.51; p < 0.0001) tempered by the following interactions: conditioning x solution, and conditioning x prenatal treatment (F(2,61) = 4.94; p < 0.025 and F(2,61) = 4.54, p < 0.025, respectively). When focusing on the interaction between conditioning treatment and reinforcer, subsequent post-hoc tests showed that Paired pups reinforced with sucrose-quinine or 3% v/v ethanol, were more responsive than Yoked controls. This was not the case when utilizing 6% v/v ethanol as a reinforcer. The frequencies of operant responding in Paired pups was similar to that exhibited by the corresponding Yoked condition and significantly lower than in Paired subjects reinforced with sucrose-quinine or 3% v/v ethanol. As previously mentioned, conditioning also significantly interacted with prenatal treatment. Post hoc-tests showed that when pups were trained with ethanol-related solutions, only paired pups prenatally treated with alcohol (1 or 2 g/kg) differed from its yoked controls. Paired pups prenatally exposed to water (0 g/kg) failed to acquire an instrumental response when ethanol-related solutions served as reinforcers. As can be observed in Figure 2B, the interaction between prenatal treatment and conditioning seems to be mainly driven by the differences attained when using sucrose-quinine or 3% v/v ethanol as reinforcers. Apparently, with 6% v/v ethanol, differences between Paired and Yoked pups were not so evident even when considering prenatal status.
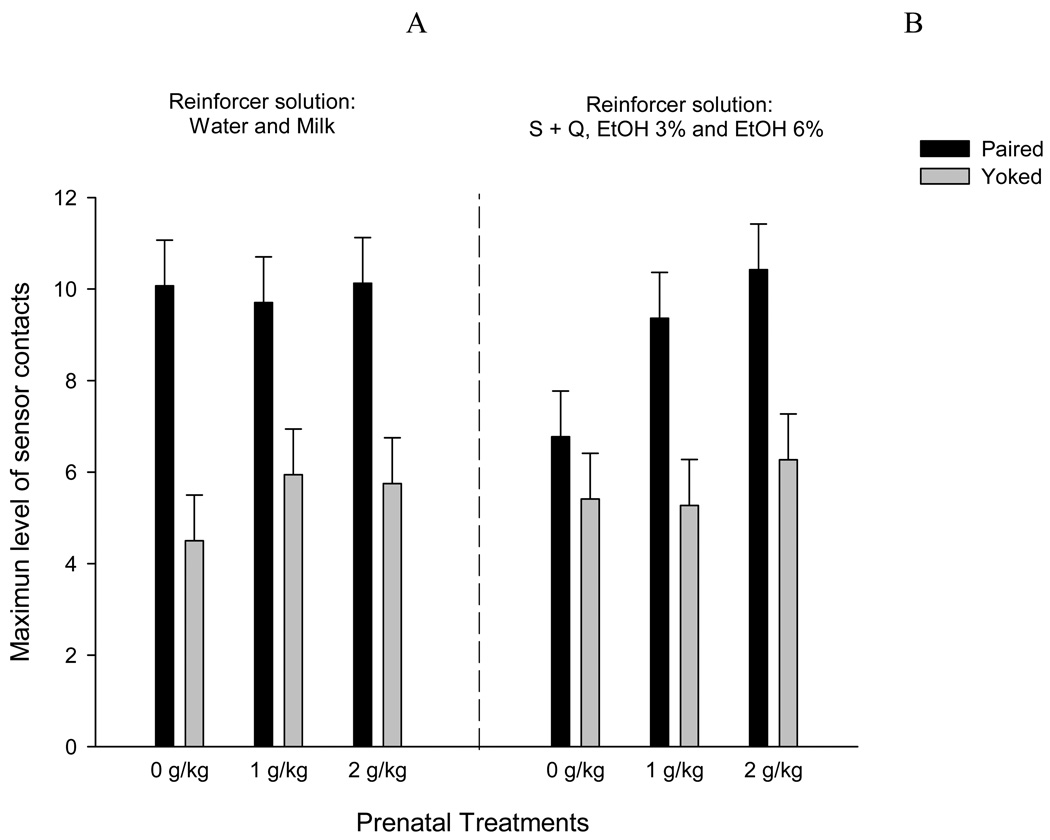
The above described inferential profile was similar to that obtained for maximum level of sensor contacts in a given 30-sec bin. In this case, natural reinforcers resulted in greater activity in Paired relative to Yoked pups; F(1,41) = 54.18, p < 0.0001 (Figure 3A). When ethanol-related reinforcers were utilized, the ANOVA showed a significant main effect of conditioning tempered by an interaction between prenatal and conditioning treatment; F(1,61) = 42.87 and F(2,61) = 3.73, both p’s < 0.05; respectively. Post-hoc tests showed that Paired pups prenatally treated with ethanol had a maximum number of sensor contacts that was significantly higher than that of Yoked subjects. Paired pups prenatally treated with water (0 g/kg) did not differ from Yoked controls, and their maximum level of responsiveness was significantly lower than that of Paired pups with prenatal ethanol experience (1 or 2 g/kg) (Figure 3B).
FIGURE 3.
Maximum level of operant responses (physical contacts with the sensor) in a given 30 sec bin registered in pups conditioned with ethanol-related reinforcers. Data is depicted as a function of prenatal treatment and conditioning treatment and has been collapsed across the following natural reinforcers (Water and Milk- FIGURE 3A) and across ethanol-related reinforcers (S+Q, 3 % or 6 % of v/v ethanol- FIGURE 3B). Black bars represent data from Paired pups and white bars represent data from Yoked control pups. Vertical lines represent standard errors of the means.
Latency to exhibit the first physical contact with the sensor was not significantly affected by prenatal treatment, conditioning procedure or reinforcer. No significant interactions were detected between these factors.
General activity during operant training
The 3-way mixed ANOVA for frequency of overall body movements did not reveal significant main effects or interactions. Similar null results were obtained when duration of overall activity served as the dependent variable. This was also the case when examining latency of the first body movement.
Operant performance during extinction
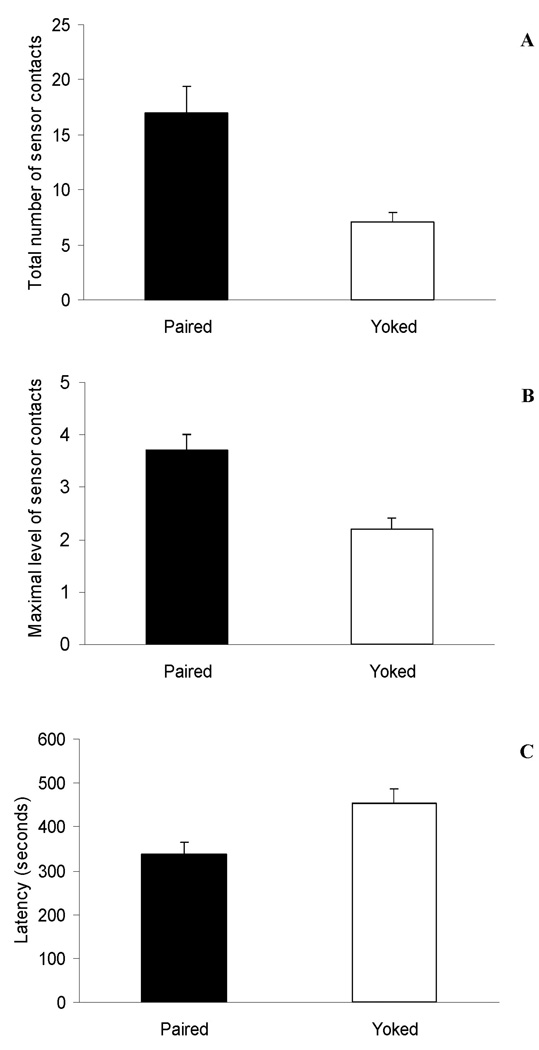
The omnibus 3-way ANOVA indicated that when the reinforcers were absent, the number of sensor contacts varied significantly as a function of conditioning treatment; F(1,99) = 17.55, p < 0.0001. It was clear that Paired pups independently from prenatal treatment and nature of the reinforcer during training, pressed the sensor significantly more than Yoked controls. A similar significant main effect of conditioning treatment was observed when taking into account the maximum level of sensor contacts in a given 30-sec. bin; F(1,99) = 20.66; p < 0.0001. Latency to contact the sensor for the first time during extinction was also significantly affected by conditioning; F(1,99) = 11.06; p < 0.001. Paired pups pressed the sensor sooner relative to Yoked controls. These results are illustrated in Figure 4.
FIGURE 4.
Main effects of conditioning treatment during extinction. Data has been collapsed across prenatal treatments and reinforcers. A: Total number of operant responses (physical contacts with the sensor). B: Maximun level of operant responses in a given 30 second bin. C: Latency to display the first operant response. Black bars represent data from Paired pups whereas white bars depict data from Yoked control pups. Vertical lines represent standard errors of the means.
General activity during extinction
Due to technical problems in the videotapes, the recordings of 9 pups were lost. Practically all groups remained with a number of pups that ranged between 6 and 9. The only group that had fewer animals (n = 4) due to the technical problem was prenatally exposed to 1 g/kg ethanol and postnatally trained with intraoral delivery of the sucrose-quinine solution.
The 3-way ANOVAs considering overall activity showed main significant effects of conditioning in terms of both frequency, F(1,93) = 8.96; p < 0.01, and duration, F(1,93) = 13.56; p < 0.001. Paired pups displayed higher levels of general motor activity than Yoked controls. This difference was observed independently from prenatal treatment and nature of the reinforcer.
Summary of Results
As in Experiment 1, milk was found to act as an effective reinforcer capable of modifying the frequency of neonatal behaviors such as head and forepaw movements. Water was also found to be an effective reinforcer when physical sensor contacts resulted in intraoral delivery of this fluid. Prenatal exposure to ethanol did not modify operant responsiveness when using either of these reinforcers.
When focusing on the behavioral repertoire of the neonate that resulted in intraoral delivery of ethanol-related stimuli, it was observed that conditioning was more effective in pups prenatally exposed to the drug, a result that seems to be primarily driven by the reinforcing capabilities of a sucrose-quinine solution and 3% v/v ethanol (see Figure 2). No empirical evidence supported the possibility that these behavioral changes during conditioning were modulated by hyperactivity or hyper-reactivity resulting from ethanol exposure during late gestation. In terms of fluid ingestion, Yoked pups had higher body weight gain than Paired neonates, independent of prenatal treatment or the nature of the reinforcers.
One hour after conditioning pups were again placed in the operant chamber, but this time reinforcers were explicitly withheld (extinction session). Independently of the reinforcers used during training and of the prenatal status of the animals, it was observed that Paired pups (reinforced during training) were more likely than Yoked controls to engage in physical contact with the sensor. During this session it was also observed that Paired pups exhibited heightened levels of overall motor activity relative to Yoked controls.
GENERAL DISCUSSION
One-day old pups rapidly changed the frequency of suckling-related movements when this motor activity was reinforced with milk. This result, obtained with Wistar-derived neonates, replicates the basic behavioral profile observed in Sprague-Dawley neonates (Arias et al., 2007; Bordner et al., 2008). The probability of execution of these behaviors also increased with water as a reinforcer (Experiment 2) and decreased when an aversive tastant such as quinine was contingent upon the operant response (in terms of maximal level of responsiveness attained in a 30-s bin, Experiment 1).
Of major importance for the aims of the present study, it was clear that prenatal exposure to ethanol facilitates neonatal operant learning supported by intraoral administration of a low ethanol concentration (3% v/v) or a psychophysical equivalent of the drug’s taste (sucrose-quinine solution). Pups prenatally exposed to 1 or 2 g/kg ethanol exhibited high levels of responding when explicitly reinforced with the above described stimuli, a phenomenon not observed in pups without prenatal exposure to ethanol. This difference across prenatal treatments could not be attributed to differences in associative learning capabilities: with natural reinforcers (milk or water; Figure 2), there was no effect of prenatal ethanol. The results of Experiment 2 are not better explained by arguing a hyperactivity or hyperreactivity effect. These effects have been encountered when high doses of ethanol have been chronically administered during gestation (Abel, 1980; Anandam et al., 1980; Bond, 1988) or even when low-to moderate ethanol exposure is restricted to late pregnancy (Arias et al., 2008; Chotro & Spear, 1997; Domínguez et al., 1996). These teratological consequences of the drug imply heightened levels of activity or increased reactivity to environmental stimulation. Both phenomena may in turn, increase the probability of body movements leading to physical contacts with the sensor. In other words, these phenomena may alter the probability of reinforcement due to changes in baseline or sensory-elicited patterns of activity. Yet, this possibility seems unlikely since during conditioning: a) Yoked pups across reinforcers exhibited similar numbers of physical contacts with the sensor regardless of prenatal treatment; b) general motor activity did not differ across experimental manipulations defined by prenatal, conditioning or reinforcement factors; and c) effective conditioning was observed regardless of prenatal treatment when water or milk were the reinforcers.
The literature on effects of prenatal exposure to low or moderate ethanol doses systemically indicates heightened postnatal acceptance of ethanol-related stimuli. An ethanol-related memory acquired during gestation that affects ethanol palatability and ingestion, as well as responsiveness to psychophysical equivalents of ethanol (sucrose-quinine compounds), appears to be generated through the following mechanisms that are not mutually exclusive. Mere pre-exposure to the sensory attributes of the drug is likely to exert these effects. Ethanol rapidly accumulates in the amniotic fluid and in fetal blood and hence has the possibility of stimulating chemosensory systems which are already functional during late gestation (Abate et al., 2008; Bachmanov et al., 2003; Domínguez et al., 1998; Schaal et al., 1998; Schaal et al., 2000). A second possibility that has received recent experimental support indicates that fetuses form an associative memory comprising ethanol chemosensory cues and its positive hedonic effects (Abate et al., 2002; Chotro and Arias, 2003; Nizhnikov et al., 2006). Both hypotheses predict similar postnatal outcomes, i.e., higher affinity for ethanol. The results of the present experiment do indicate this heightened affinity. In animals prenatally exposed to ethanol, conditioning was facilitated when intraoral delivery of 3% v/v ethanol or a solution that mimics ethanol gustatory properties (bitter-sweet) served as appetitive reinforcers.
One could argue that prenatally exposed pups exhibit operant conditioning without discriminating the sensory properties of stimuli intraorally infused. However, this seems not to be the case. In the present study, the highest ethanol concentration (6% v/v) failed to promote heightened levels of operant responsiveness in Paired pups representative of the different prenatal treatments.
Beyond these considerations it is important to note that the level of operant responsiveness when the reinforcers were removed (extinction) was very low and that the pattern of results did not resemble that attained during acquisition. It is possible that these low levels of responding (i.e., functional floor effect) do not allow the expression of prenatal or reinforcer effects during extinction. Furthermore, newborn rats show a considerable deficit in their capability to retrieve pertinent information in absence of reminder cues or when a long retention interval is in effect (Cheslock et al., 2000; Spear and Riccio, 1994). During the extinction session conducted in this study (60 min. after conditioning, Experiment 2) it was observed that Paired pups exhibited more sensor presses but also had higher levels of overall motor activity when compared with Yoked pups. This phenomenon may reflect frustration in terms of gaining new access to the previously experienced reinforcers or a decline in memory capabilities related with the target behavior and its contingency with intraoral reinforcement.
Another finding that deserves consideration is the fact that under the present experimental procedures, intake patterns –assessed as percent of body weight gain- did not resemble operant activity patterns. Yoked pups exhibited higher body weight gain than Paired pups. This result is similar to the one observed in previous studies (Bordner et al., 2008; Ponce et al., in press). It is possible that this difference might indicate that increases in behavioral activity (e.g. head and front paw movements) resulting from fluid reinforcement competes with the ability of Paired subjects to regulate liquid consumption. Despite this possibility, palatability of the different reinforcers resulted in differential body weight gain independent of prenatal and conditioning treatments. According to this index of ingestion, pups tended to reject the 6% v/v ethanol solution, a solution which was not found to increase operant performance during acquisition.
When comparing our results to those reported by Bordner et al. (2008), it is important to note some differences between these studies. Sprague Dawley newborns (Bordner et al., 2008) seem more active in terms of physical contacts with the sensor during acquisition and extinction relative to Wistar pups (tested in the present experiments). We cannot discard that strain differences in baseline activity rates may explain these differences. It is possible that higher levels of motor activity during initial phases of training may augment the likelihood of behavioral reinforcement and in doing so, may facilitate the process of operant learning. A second difference between studies is that Sprague-Dawley neonates exhibited optimal conditioning when reinforced with low concentrated ethanol solutions (ranging between 3 and 5% v/v). Our Wistar-derived pups showed operant learning modulated by ethanol reinforcement only if prenatally exposed to the drug. Once again, we cannot discard strain specific differences concerning ethanol affinity or sensitivity to the reinforcing effects of the drug. In terms of consumption, Sprague-Dawley rats (Molina et al., 1986) seem to find ethanol more palatable than Wistar-derived rats (Pepino et al., 2004; Ponce et al., 2004). This comparison only applies in the case of young adults. To our knowledge, strain differences in ethanol affinity have not been explicitly assessed during early stages in ontogeny. Beyond possible strain differences, there are methodological issues that also may play an important role in determining differences between the mentioned studies. For instance, Bordner et al. (2008) infused a volume of 1 µl after each sensor contact, whereas we reinforced Wistar pups with 10 µls. The rationale for increasing the volume was to ensure maximum intraoral sensory stimulation. We cannot discard that this parametric difference may determine differential rates of responding in Sprague-Dawley and Wistar pups. However, this possibility seems unlikely since recent experiments conducted in our laboratory, in which we utilized 1 µl of milk or 3% v/v ethanol as reinforcers, have yielded operant performance similar to that in the present study (Miranda Morales et al., 2008).
Learning that takes place while still in the womb can last for considerable periods of time. In rodents, the effects of intrauterine conditioned taste aversions or familiarization with chemosensory cues are still expressed during adulthood (Gruest et al., 2004; Smotherman, 1982; Stickrod et al., 1982). Fetal learning involving chemosensory stimuli seems to represent a biological adaptation allowing the offspring to develop dietary preferences (Mennella et al., 1995; Schaal et al., 2000). Unfortunately and according to studies conducted during the last two decades, this biological adaptation may apply in the case of ethanol exposure (Abate et al., 2008; Chotro & Arias, 2007; Molina et al., 2007b; Spear & Molina, 2005). Fetal experience with low to moderate ethanol doses has been observed to: (a) increase orienting responses to the odor of ethanol both in rats (Chotro & Spear, 1997; Dominguez et al., 1996; ) and human babies (Faas et al., 2000); (b) increase ethanol’s palatability and consumption of the drug in animal models (Arias & Chotro, 2005a, 2005b; Dominguez et al., 1998); and (c) sensitize the newborn rat to positive reinforcing effects of ethanol (Nizhnikov et al., 2006). The present study demonstrates that prenatal exposure to ethanol predisposes the organism to rapidly vary behavioral patterns in order to gain access to the drug. Considering this result under the light of recent theories that consider addiction as a pathological usurpation of the neural mechanisms of learning and memory (Hyman, 2005), it seems that prenatal ethanol learning changes the organism’s susceptibility to the reinforcing effects of the drug itself or to a sweet-bitter solution.
ACKNOWLEDGEMENTS
This work was supported by grants from Agencia Nacional dePromoción Científica y Tecnológica (FONCyT, PICT 05-14024) and Fundación Roemmers awarded to J.C.M. and by grants from NIAAA (AA11960, AA013098, AA015992) and NIMH (MH035219) awarded to N.E.S.
References
- Abate P, Pepino MY, Domínguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Pepino MY, Spear NE, Molina JC. Fetal learning with ethanol: correlations between maternal hypothermia during pregnancy and neonatal responsiveness to chemosensory cues of the drug. Alcohol Clin Exp Res. 2004;28:805–815. doi: 10.1097/01.alc.0000125354.15808.24. [DOI] [PubMed] [Google Scholar]
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against "safe" amounts of prenatal exposure. Exp Biol Med (Maywood) 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcohol Clin Exp Res. 2001;25:989–998. [PubMed] [Google Scholar]
- Abate P, Varlinkaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-related prenatal memories: impact on the first suckling response. Alcohol Clin Exp Res. 2002;26:1512–1522. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- Abel EL. Fetal Alcohol Syndrome: behavioral teratology. Psych Bull. 1980;87:29–50. [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O'Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Arch Gen Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Anandam N, Felegi W, Stern JM. In utero alcohol heightens juvenile reactivity. Pharmacol Biochem Behav. 1980;13:531–535. doi: 10.1016/0091-3057(80)90276-2. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005a;29:337–346. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005b;29:337–346. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89:608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Spear NE, Molina A, Molina JC. Rapid acquisition of operant conditioning in 5-day-old rat pups: a new technique articulating suckling-related motor activity and milk reinforcement. Dev Psychobiol. 2007;49:576–588. doi: 10.1002/dev.20236. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the ethiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Blass EM. Suckling: Determinants, changes, mechanisms, and lasting impressions. Dev Psychol. 1990;26:520–533. [Google Scholar]
- Bond NW. Prenatal alcohol exposure and offspring hyperactivity: effects of physostigmine and neostigmine. Neurotoxicol Teratol. 1988;10:59–63. doi: 10.1016/0892-0362(88)90067-0. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, West JR. Early postnatal alcohol exposure acutely and permanently reduces the number of granule cells and mitral cells in the rat olfactory bulb: a stereological study. J Comp Neurol. 1992;324:557–566. doi: 10.1002/cne.903240408. [DOI] [PubMed] [Google Scholar]
- Bordner KA, Molina JC, Spear NE. Analysis of ethanol reinforcement in 1-day-old rats: assessment through a brief and novel operant procedure. Alcohol Clin Exp Res. 2008;32:580–592. doi: 10.1111/j.1530-0277.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Chen WA, Parnell SE, West JR. Early postnatal alcohol exposure produced long-term deficits in brain weight, but not the number of neurons in the locus coeruleus. Brain Res Dev Brain Res. 1999;118:33–38. doi: 10.1016/s0165-3806(99)00128-5. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behav Neurosci. 2000;114:484–495. [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a condiitoned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Kraebel KS, McKinzie DL, Molina JC, Spear NE. Prenatal and postnatal ethanol exposure influences preweanling rat behavioral and autonomic responding to ethanol odor. Alcohol. 1996;13:377–385. doi: 10.1016/0741-8329(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Spear NE. Repeated exposure to moderate doses of alcohol in the rat fetus: evidence of sensitization to toxic chemosensory aspects of alcohol. Alcohol Clin Exp Res. 1997;21:360–367. [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol's motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Delauney-El Allam M, Marlier M, Schaal B. Learning at the breast: preference formation for an artificial scent and its attraction against the odor of maternal milk. Infant Behav Dev. 2006;29:308–321. doi: 10.1016/j.infbeh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- DiLorenzo P, Kiefer S, Rice A, García J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol. 1986;3:55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Domínguez HD, Bocco G, Chotro MG, Spear NE, Molina JC. Operant responding controlled by milk or milk contaminated with alcohol as positive reinforcers in infant rats. Pharmacol Biochem Behav. 1993;44:403–409. doi: 10.1016/0091-3057(93)90482-9. [DOI] [PubMed] [Google Scholar]
- Domínguez HD, López MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol´s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65:103–112. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- Domínguez HD, López MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Domínguez HD, López MF, Molina JC. Interactions between perinatal and neonatal associative learning defined by contiguous olfactory and tactile stimulation. Neurobiol Learn Mem. 1999;71:272–288. doi: 10.1006/nlme.1998.3882. [DOI] [PubMed] [Google Scholar]
- Faas AE, Spontón ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22:7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- Flory GS, Langley CM, Pfister JF, Alberts JR. Operant learning for a thermal reinforcer in 1-day-old rats. Dev Psychobiol. 1997;30:41–47. doi: 10.1002/(sici)1098-2302(199701)30:1<41::aid-dev4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–242. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity taste.I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Gruest N, Richer P, Bernard H. Emergence of long-term memory for conditioned aversion in the rat fetus. Dev Psychobiol. 2004;44:189–198. doi: 10.1002/dev.20004. [DOI] [PubMed] [Google Scholar]
- Hall WG. Feeding and behavioral activation in infant rats. Science. 1979;205:206–209. doi: 10.1126/science.451591. [DOI] [PubMed] [Google Scholar]
- Hoffmann H, Molina JC, Kucharski D, Spear NE. Further examination of ontogenetic limitations on conditioned taste aversion. Dev Psychobiol. 1987;20:455–463. doi: 10.1002/dev.420200409. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyson RL, Rudy JW. Ontogenesis of learning: II. Variation in the rat’s reflexive and learned responses to acoustic stimulation. Dev Psychobiol. 1984;17:263–283. doi: 10.1002/dev.420170307. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Kiefer SW. Alcohol, palatability, and taste reactivity. Neurosci Biobehav Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Morrow NS, Metzler CW. Alcohol aversion generalization in rats: specific disruption of taste and odor cues with gustatory neocortex or olfactory bulb ablations. Behav Neurosci. 1988;102:733–739. doi: 10.1037//0735-7044.102.5.733. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol. 1990;7:115–120. doi: 10.1016/0741-8329(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Kozlov AP, Varlinskaya EI, Spear NE. Ethanol, saccharine, and quinine: early ontogeny of taste responsiveness and intake. Alcohol Clin Exp Res. 2008;32:294–305. doi: 10.1111/j.1530-0277.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Crawford J, Spear NE. Characteristics and consequences of free-feeding ethanol ingestion during the first two postnatal weeks of the rat. Alcohol Clin Exp Res. 1998;22:1615–1622. [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Gatto GJ, Levy AD, Yoshimoto K, Lumeng L, Li TK. CNS mechanisms of alcohol self-administration. Alcohol Alcohol Suppl. 1993;2:463–467. [PubMed] [Google Scholar]
- Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics. 2001;107:E88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20:207–209. doi: 10.1093/chemse/20.2.207. [DOI] [PubMed] [Google Scholar]
- Miranda Morales RS, Molina JC, abate P. Interactions between pre- and postnatal ethanol experiences: participation of the endogenous opioid system upon newborn and infantile responsiveness to ethanol. 14th biennial scientific meeting of the International Society for Comparative Psychology (ISCP), Bs As; 2008. p. 57. [Google Scholar]
- Molina JC, Pautassi RM, Truxel E, Spear NE. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007a;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxel E, Spear NE. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Serwatka J, Spear NE. Alcohol drinking patterns of young adult rats as a function of infantile aversive experiences with alcohol odor. Behav Neural Biol. 1986;46:257–271. doi: 10.1016/s0163-1047(86)90191-3. [DOI] [PubMed] [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. The International society for developmental psychobiology 39th annual meeting symposium: Alcohol and development: beyond fetal alcohol syndrome. Dev Psychobiol. 2007b;49:227–242. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Health, and Institute of Laboratory Animal Resources, Commission on Life Sciences. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol exposure increases ethanol reinforcement in neonatal rats. Alcohol Clin Exp Res. 2006a;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Petrov ES, Varlisnkaya EI, Spear NE. Newborsn rats' first suckling experience: taste differentiation and suckling plasticity. Physiol Behav. 2002;76:181–198. doi: 10.1016/s0031-9384(01)00672-2. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006b;120:267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Spear NE. Reinforcing effects of central ethanol injections in newborn rats. Alcohol Clin Exp Res. 2006;30:2089–2096. doi: 10.1111/j.1530-0277.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Molina JC, Spear NE. Central reinforcing effects of ethanol are blocked by catalase inhibition. Alcohol. 2007;41:525–534. doi: 10.1016/j.alcohol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Molina JC, Spear NE. Infant rats exhibit aversive learning mediated by ethanol's orosensory effects but are positively reinforced by ethanol’s post-ingestive effects. Pharmacol Biochem Behav. 2008;88 doi: 10.1016/j.pbb.2007.09.012. 393-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41:421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear N, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats after nursing experiences with an ethanol-intoxicated dam. Alcohol Clin Exp Res. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- Pepino MY, López MF, Spear NE, Molina JC. Infant rats respond differently to alcohol after nursing from an alcohol-intoxicated dam. Alcohol. 1999;18:189–201. doi: 10.1016/s0741-8329(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: effects on suckling plasticity. Behav Neurosci. 2001;115:1318–1331. [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Pueta M, Abate P, Haymal OB, Spear NE, Molina JC. Ethanol exposure during late gestation and nursing in the rat: effects upon maternal care, ethanol metabolism and infantile milk intake. Pharmacol Biochem Behav. 2008;91:21–31. doi: 10.1016/j.pbb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueta M, Abate P, Spear NE, Molina JC. Interactions between ethanol experiences during late gestation and nursing: effects upon infantile and maternal responsiveness to ethanol. Inter J Comp Psychology. 2005;18:207–224. [Google Scholar]
- Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. J Neurobiol. 1992a;23:1574–1600. doi: 10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Organization of the stretch response to milk in the rat fetus. Dev Psychobiol. 1992b;25:33–49. doi: 10.1002/dev.420250104. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Examining the role of endogenous opioids in learned odor-stroke associations in infant rats. Dev Psychobiol. 2006;48:71–78. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water- sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–153. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier B, Soussignan R. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behav Neurosci. 1998;112:1438–1449. doi: 10.1037//0735-7044.112.6.1438. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Human foetuses learn odours form their pregnant mother's diet. Chem Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Odor aversion learning by the rat fetus. Phsysiol Behav. 1982;29 doi: 10.1016/0031-9384(82)90322-5. 769-61. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Petrov ES, Varlinskaya EI. Experimental study of the first suckling episode: rat pups ingest fluids through a surrogate nipple. Behav Neurosci. 1997;111:1383–1394. [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to alcohol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;25:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Spear NE, Riccio DC. Memory: Phenomena and Principles. Allyn & Bacon: Needham Heights, MA; 1994. [Google Scholar]
- Spear NE, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22:401–412. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiol Behav. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Developing a sense of safety: the neurobiology of neonatal attachment. Ann N Y Acad Sci. 2003;1008:122–131. doi: 10.1196/annals.1301.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcers in infancy: classical conditioning using stroking or intra-oral infusions of milk as UCs. Dev Psychobiol. 1988;21:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- Truxel E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28:1200–1211. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]
- Young C, Olney WJ. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol Dis. 2006;22:548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effects of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav Neurosci. 2007;121:1306–1315. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]