Abstract
Background
Cancer-related fatigue (CRF) is the most frequently reported side effect of cancer and its treatment. In previous research, Polarity Therapy (PT), an energy therapy, was shown to reduce CRF in patients receiving radiation. This study reports on a small randomized clinical trial designed to collect preliminary data on the efficacy of PT compared with an active control (massage) and passive control (standard care) for CRF among cancer patients receiving radiation therapy.
Methods
Forty-five women undergoing radiation therapy for breast cancer were randomized to I of 3 weekly treatment conditions. Patients received standard clinical care, 3 modified massages, or 3 PT treatments. CRF and healthrelated quality of life (HRQL) were assessed during baseline and the 3 intervention weeks.
Results
TResults show CRF ratings were reduced after PT. The effect sizes for PT versus modified massage and versus standard care were small when using the primary measure of CRF (Brief Fatigue Inventory) and large when using the secondary measure of CRF (Daily CRF Diaries).The effect size was medium when assessing the benefit of PT on maintaining HRQL compared with standard care with very little difference between the PT and modified massage conditions. Patients’ feedback showed that both the modified massage and PT treatments were deemed useful by radiation patients. Conclusion. The present pilot randomized clinical trial supports previous experimental research showing that PT, a noninvasive and gentle energy therapy, may be effective in controlling CRF. Further confirmatory studies as well as investigations of the possible mechanisms of PT are warranted.
Keywords: cancer-related fatigue, radiation, Polarity Therapy, massage, quality of life, complementary and integrative medicine
Introduction
Cancer-related fatigue (CRF) is the most prevalent and distressing side effect among patients with cancer undergoing radiation therapy, with 70% to 100% of patients complaining of fatigue.1-4 CRF can reduce patients’ quality of life by affecting their abilities to participate in leisure activities, to sustain meaningful relationships and activities with their families, and to work during and after treatment. 5 Recent systematic and meta-analytic reviews of nonpharmalacologic interventions for CRF found effect sizes for exercise to be moderate and for psychosocial interventions to be small to moderate.6,7 Despite these positive findings and the substantial amount of research on CRF that has been conducted to date,8 neither pharmacologic nor nonpharmacologic treatments for CRF are in systematic and widespread use in cancer clinics at this point, and even after these methods are used, CRF often remains a problem.9
Increasingly, patients who are distressed about treatment side effects such as CRF with no clear medical remedy use complementary and alternative medical (CAM) therapies. A recent study reports that 91 % cancer patients use at least one form of CAM during the course of their cancer treatments.10 Included in these CAM therapies is a group of interventions designed to balance the energy fields ofliving organisms and restore a state of well-being through the use of gentle human touch (eg, Healing Touch [HT], Therapeutic Touch [TT], Reiki, and Polarity Therapy [PT]). These interventions, known collectively as energy therapies, focus on restoring energy and a state of energy balance, both of which fatigued patients feel they lack.11 All are premised on 3 assumptions: (a) illness is the result of an impeded energy flow (blockage), ultimately disrupting the natural homeostatic state of the human body, (b) the mind and body have the power to heal themselves, and (c) healing can be facilitated through gentle manipulation of the patient’s immediate energy fields and internal energy flow, known as the “life force.”11,12 Energy therapies are rooted in ancient healing practices of several countries, and cultural influences have resulted in the several variant forms of energy therapy, including the aforementioned HT, TT, Reiki, and PT. 13 We chose to study PT rather than the other, better known energy therapies because of its standardized and comprehensive training system, which requires 155 hours for the initial training of a Polarity therapist and 675 hours to become a registered provider. 14
Based on years of experience, PT is a safe technique that is increasingly used in integrative medical practices. 15 PT has been shown to alter brain wave activity 16 and is reported, anecdotally, to be useful in treating chronic fatigue, fibromyalgia, anxiety, and malaise. 15 PT was also examined in a nonrandomized investigation conducted on 70 hospitalized patients with a wide variety of conditions as well as on 21 hospital medical staff. Both patients and staff reported feelings of peace, rest, or deep relaxation after PT sessions. 17 A recently completed study examined the effectiveness ofPT in reducing stress and depression and improving quality of life for American Indian and Alaskan Native family caregivers of individuals with dementia. A total of 42 family caregivers were randomized to an 8-session trial ofPT or to an enhanced respite control condition (ERC) that included respite care for the person with dementia and a choice of activities for the caregiver. PT participants improved significantly more than ERC participants on stress, depression, bodily pain, vitality, and general health. 18
Several studies demonstrate that energy therapies can alter physiological processes such as skin temperature, blood pressure, brain wave activity, and proliferation of fibroblasts, tenocytes, and osteoblasts in culture. 19-22 Additional studies provide limited evidence that such therapies can reduce pain, fatigue, and anxiety.23-27
Despite these positive preliminary reports, systematic reviews of the studies involving energy therapies have concluded that the scientific evidence substantiating the value of these therapeutic modalities remains inconclusive and that most studies of energy therapies have inconsistent results, suffer from a lack of replication, and generally exhibit a low quality of experimental design.28,29 Benford et al30 also suggests that a lack of consistent evidence of the existence of human energy fields is a cause for much of the resistance from the traditional medical community to accepting and promoting the use of energy therapies such as HT, TT, Reiki, and PT.
Polarity Therapy was developed in 1947 by Dr Randolph Stone, an osteopathic and neuropathic physician, after an extensive study of the Ayurvedic medical system in India. 14 Dr Stone began to teach PT in the 1960s, and in 1984 a group of practitioners formed the American Polarity Therapy Association (APTA), a governing body that regulates the profession. A dual level of certification was established with 155 hours of training needed to become an Associate Polarity Practitioner and 675 hours needed to be a Registered Polarity Practitioner. In 2010, approximately 900 practitioners were members of the APTA. 14 A PT session typically lasts 60 to 75 minutes and involves gentle stretching and rocking using different intensities of touch.22 Like other energy therapies, PT is based on the idea that electromagnetic and energy fields exist in the human body and that impedance of proper energy flow in one area of the body negatively affects the entire human being physically, emotionally, mentally, and spiritually.11,15
Polarity Therapy is based on the following assumptions: (a) a primal omnipresent energy exists in the universe, (b) this energy flows through the cranial/sacral core system and out to all parts of the body, (c) this energy flows via positive (top or right) and negative (bottom or left) poles, attracting and repelling in a manner similar to a bar magnet, (d) this energy settles in a harmonic neutral polarization flowing freely and unimpeded within healthy bodies, and (e) the human body possesses a “wireless anatomy” operating at specific frequencies, identified as air, water, fire, and earth. Energy is postulated to step down through this “wireless anatomy” via auras (energy fields in immediate proximity to the body) to chakras (core body energy centers aligned with the spinal column), and from chakras into the physical realm through nerves, muscles, bones, and fascia. The proper flow of energy current is impeded by stress and manifests as fatigue, pain, nausea, illness, and side effects from medical treatments.11,19,31
In our prior study that was designed to treat CRF, 15 women undergoing radiation therapy for breast cancer and experiencing CRF were randomized to receive none, 1, or 2 PT treatments.32 Treatments were given 1 week apart to the patients receiving 2 treatments. There was a statistically significant improvement in both CRF and in health-related quality of life (HRQL) in the 10 patients who received a PT treatment compared with the 5 control patients at the week 1 assessment. Additionally, there was a statistically significant difference among the t3 treatment groups in improvement in CRF at the week 2 assessment with the patients who received 2 PT treatments improving the most. The current randomized clinical trial is a follow-up to that initial feasibility pilot study. The purpose of this study was to examine the efficacy of PT for reducing CRF and improving HRQL in women receiving radiation treatments for breast cancer. We hypothesized that PT, because of its energy balancing features, would be effective in reducing fatigue in patients with breast cancer receiving radiation therapy. We further hypothesized that it would be more effective in doing so than our modified massage control condition that did not have an energy balancing component.
Methods
Patients undergoing radiation therapy for breast cancer were eligible for this study if they had received at least 8 radiation treatments prior to the beginning of the study and were scheduled to receive at least 18 additional treatments. As this experiment was designed as treatment study to reduce fatigue, only patients already experiencing at least a mild level of fatigue during the preceding week were eligible. We determined this at our screening interview and required a response of at least 2 on an 11-point scale, anchored by “0“ = no fatigue and “10“ = worst possible fatigue, that assessed fatigue during the prior week. We note that scores from 4 to 6 are considered moderate on this scale and scores of ≥7 are considered severe.33 Ineligibility criteria included receiving concurrent chemotherapy or interferon treatment, having distant metastases, having a hemoglobin level of <11 g/dL, or taking methylphenidate, modafinil, sedatives, or anxiolytics.
Patients were recruited by a clinical research coordinator with a referral from their treating oncologist and randomly assigned to 1 of 3 trial arms using a computer-generated random table with a block size of 6 to (a) standard care (arm 1), (b) standard clinical care plus 3 modified massages (arm 2), or (c) standard clinical care plus 3 PT treatments (arm 3). Patients in the standard care control arm were offered the option of receiving their choice of 2 Polarity, 2 massage, or 1 of each treatment gratis after completing the entire study in exchange for their participation. Participants were told that this was a study examining the efficacy of Polarity and massage for improving fatigue. Patients were studied for a 4-week period (1 week of baseline plus 3 weeks of intervention) while receiving daily radiation treatments. CRF and HRQL were assessed by questionnaires on Fridays during the 4-week study period. In addition, patients kept a daily fatigue diary during the entire 4-week study period and completed a feedback item at the conclusion of the study. All ancillary treatments for control of symptoms caused by the cancer or its treatment were allowed for all patients and were not standardized. The protocol was approved by the Institutional Review Board of the University of Rochester Medical Center.
Study treatments (modified massage or PT) were given on either Mondays or Tuesdays. All treatments took place in a room dedicated for that purpose in the Department of Radiation Oncology. Polarity treatments were given by 1 of 2 Registered Polarity Practitioners and the modified massages were given by 1 of2 licensed massage therapists with extensive experience in providing massage to cancer patients. All treatments took place on a comfortable massage table with the patient’s shoes removed, but otherwise with patients fully clothed. All treatments began with patients lying on their back and rolling over later in the treatment. Each treatment, whether modified massage or PT, lasted approximately 75 minutes. Both the PT and the massage therapists were instructed to keep conversation with the patient to the minimum necessary to effectively provide the treatment. Specific techniques of the 2 therapies are described in the following subsections.
Polarity Therapy Treatments
The therapist used anatomical hand positions, known as connectors, to examine energy flow, discover trigger points (energy impediments), and restore homeostatic energy flow. Examples of these hand positions include placing both hands over the ears or on the soles of the feet of the participant. These trigger points might have manifested as tenderness, tightness, warmth, coolness, heaviness, density, or any sense of discomfort. They were felt by the therapist and/or communicated by the patient. The hand positions were gentle contact, not manipulative, forceful, or mechanical, and were maintained for a sufficient duration to relieve the trigger point discomfort as discerned by the Polarity Therapist.
Modified Massage Treatments
The modified massage treatments were designed as a control condition for the touch and personal attention received during PT and did not have the energy balancing features of PT. The massage therapists used a modified Swedish massage technique applied over the clothing and without the use of lubricant. Strokes used included compression, light moving touch, and static holds. Areas of the body to be massaged were left to the discretion of the patients and could include back, neck, upper and lower limbs, head, hands, and feet.
Measures
Fatigue
The revised Brief Fatigue Inventory (BFI) was the primary outcome measure for this study. The BFI is a self-report 9-item questionnaire that has adequate reliability and validity.33 The first 3 items on the BFI ask patients to rate their levels of fatigue “now,” “at its “worst,” and “usual,” respectively, for the last 24 hours. Patients respond on the 11-point scales that are bounded by 0 = no fatigue and 10 = fatigue as bad as you can imagine. The remaining questions asked patients to rate their fatigue interference with several HRQL domains, including general activity, walking, mood, work, and relations with others. These items were bounded by 0 = does not interfere and 10 = interferes completely.
Daily Fatigue Diaries completed prior to going to bed were used to assess fatigue at its worst during the day on an 11-point scale, anchored by 0 = not present and 10 = as bad as you can imagine. This measure of fatigue was taken from a symptom inventory created at The University of Texas M. D. Anderson Cancer Center, Houston.34,35 The single-item assessments of fatigue done on rating scales over multiple time points are often used in research on treatment-related side effects,36-40 and fatigue assessed in this manner is less confounded with depression than are multi-item fatigue measures.37 The daily diary fatigue scores and BFI scores were directly correlated in the current study (r = .73).
Health-related quality of life
HRQL was assessed using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale. The FACIT-F is a 28-item HRQL scale that is part of a system of measurement developed specifically for use in cancer clinical trials. This particular version of the instrument includes the 4 original subscales, commonly known as the FACT-G, which assess different domains of HRQL (emotional, physical, social, and functional). The FACIT-F also includes 13 additional questions directly related to the impact of fatigue on quality of life. In this study, the total score from all 41 items was used to measure health-related quality of life, because this targeted instrument uses questions that focus more on the specific cancer and fatigue issues surrounding quality of life and, as a result, tends to be more responsive to clinically important changes compared with generic instruments. This measure was developed through extensive interviews with cancer patients and their oncology professionals, and it has been validated in a series of studies in 542 cancer patients. The measure has demonstrated very good test-retest reliability as well as validity and has a scale range from 0 to 164.41-45
Feedback
At the end of the study, patients in the 2 intervention groups were asked the following question: “Based upon your experience with the study treatment you received, would you recommend it or similar therapies to other patients receiving radiation therapy?” Patients provided responses on a 5-point scale anchored by 1 = strongly do not recommend and 5 = highly recommend.
Statistical Analyses
The primary outcome variable for this study was change in fatigue from baseline as assessed by the BFI total at the end of the third week of the intervention. Per the study protocol, the primary objective for conducting this pilot study was to generate effect size estimates for the development of a planned RO1 submission. Although statistical power was limited by the small sample size (N = 45), the efficacy of the intervention for reducing fatigue (as assessed with the BFI and the daily diary) and in improving HRQL using the FACIT-F was examined.
Analysis of covariance (ANCOVA) models were used to determine whether the intervention had an impact on fatigue and quality of life. No significant deviation from linearity was found (P > .05). One-way ANCOVA was used where the response for a given week was modeled with the treatment arm as the independent variable and baseline score as the covariate. Cohen’s d effect sizes (calculated as the difference between estimated marginal means divided by the standard deviation) were used to determine the magnitudes of the interventions. Despite the small sample size and the primary aim of this study to provide effect size estimates, a repeated measures analysis was performed to evaluate the effects of the three treatment arms across the three weeks. The outcome change score was the response. Baseline outcome, Week, Treatment, and Week × Treatment interaction were the fixed effects. Patient-specific residual variation was modeled as an unstructured covariance pattern. Maximum likelihood (ML) estimation was used for parameter estimates, and the F tests were performed using the Kenward-Roger degrees of freedom adjustment.46 Post hoc tests of the differences between the treatments were adjusted with the Tukey-Kramer multiple comparisons method.47 Because of the small sample size and large error variances, the effectiveness of these computations were checked by a Bayesian analysis with non-informative Jeffrey’s priors and Markov chain Monte Carlo sampling to make inferences about the posterior distribution of the parameter estimates. We found the results of the 2 analyses very similar, adding credence to the validity of the analysis.
Results
Patient Sample
A total of 45 women with CRF receiving radiation treatments for breast cancer took part in this 3-arm, randomized pilot clinical trial. Two of the patients, both randomized to the PT treatment condition, provided only partial baseline data because they changed their minds about participating and withdrew before fully commencing the study. These patients withdrew before completing baseline data because they felt they were too busy to continue to be a part of the study. Data from the remaining 43 (96%) patients are included in the analyses. Three of these 43 patients were only partially evaluable because of missing data (no week 3 or 4 data for one patient, no diaries for one patient, and partial diaries for one other patient).
Of the 43 patients included in the analyses, 38 were Caucasian, 4 were African American, and 1 was Hispanic. The patients ranged in age from 34 to 84 years, with a mean of 52.9 years. Sixty-one percent of the patients were married. Sixty-three percent had graduated from or attended college, and an additional 28% were high school graduates. See Table 1 for additional demographic and clinical information. Fifteen patients were randomized to arm 1 (standard care) with an equal number randomized to arm 2 (modified massage). Arm 3 (PT) had 13 patients. All study subjects received their radiation and study-related treatments as outpatients. An examination of between-group differences at baseline revealed no significant demographic differences; however, the 3 study arms did differ significantly on baseline CRF as assessed by the BFI: mean Arm1 = 1.8, mean Arm2 = 3.0, mean Arm3 = 3.7 (P = .03). Similar differences existed for the baseline Daily Diary CRF at its worst (P = .003). In light of this important clinical difference at baseline among groups, ANCOVAs controlling for baseline scores were used in all subsequent analyses comparing groups. No differences between groups was found for FACIT-F (P = .18).
Table 1.
Clinical Characteristics and Demographics
| n | |
|---|---|
| Educational level | |
| High school diploma | 28 |
| Attended college | 13 |
| Married | 27 |
| Stage of the disease | |
| Stage 0 | 4 |
| Stage I | 13 |
| Stage II | 16 |
| Stage III | 9 |
| Stage IV | 0 |
| Unknown | 3 |
| Treatment details | |
| Current radiation | 45 |
| Current chemotherapy | 0 |
| Prior chemotherapy | 28 |
| Prior surgery | 35 |
| Current hormone therapy | 12 |
| Age in years; mean (range) | 52.9 (24-84) |
Primary Analysis of Cancer-Related Fatigue
All means and standard deviations for the BFI are provided in Table 2. Mean changes were in a positive direction in regards to PT, but these changes did not reach statistical significance. Repeated measures analysis with the BFI score as the dependent variable showed no significant overall differences among the 3 treatment arms (P = .72), or week-dependent treatment differences (P = .20). Despite the fact that the repeated measures analysis revealed no significant differences, we proceeded with individual ANOVAs because of the primary exploratory pilot nature of the study.
Table 2.
Fatigue and Quality of Life at Baseline and Weeks 1 to 3a
| Fatigue: BFI |
Fatigue: Daily Diary |
HRQL: FACIT-F |
||||
|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | |
| Baseline | ||||||
| Massage | 3.0 ± 1.7 | 2.0-3.9 | 3.8 ± 1.5 | 2.9-4.7 | 117.5 ± 23.3 | 104.6-130.3 |
| Polarity | 3.7 ± 2.3 | 2.5-5.0 | 5.3 ± 2.6 | 3.9-6.8 | 110.4 ± 28.8 | 94.5-126.4 |
| Control | 1.8 ± 1.4 | 1.1-2.6 | 2.3 ± 1.9 | 1.2-3.5 | 127.1 ±7.0 | 117.7-136.6 |
| Week 1 | ||||||
| Massage | 2.8 ± 1.9 | 1.7-3.8 | 4.0 ± 1.7 | 3.0-5.1 | 116.6 ± 24.7 | 103.0-130.3 |
| Polarity | 3.7 ± 2.3 | 2.3-5.1 | 4.8 ± 2.8 | 3.2-6.4 | 109.8 ±29.3 | 92.1-127.6 |
| Control | 2.2 ± 1.5 | 1.4-3.1 | 3.2 ± 2.0 | 2.1-4.4 | 121.5 ± 20.2 | 110.3-132.7 |
| Week 2 | ||||||
| Massage | 3.1 ± 2.0 | 1.9-4.2 | 4.6 ± 2.2 | 3.3-5.9 | 117.3 ± 24.8 | 103.6-131.1 |
| Polarity | 2.9 ± 2.6 | 1.2-4.5 | 4.0 ± 2.5 | 2.3-5.6 | 114.3±31.1 | 94.6-134.1 |
| Control | 2.2 ± 1.4 | 1.4-3.0 | 2.8 ± 1.6 | 2.0-3.7 | 120.9 ± 18.0 | 111.0-130.9 |
| Week 3 | ||||||
| Massage | 3.0 ± 2.2 | 1.8-4.3 | 4.5 ± 2.1 | 3.3-5.8 | 113.4 ± 27.9 | 98.0-128.9 |
| Polarity | 3.6 ± 2.8 | 1.8-5.4 | 4.5 ± 2.8 | 2.7-6.2 | 105.6 ± 35.6 | 82.9-128.2 |
| Control | 2.5 ± 1.5 | 1.7-3.3 | 3.2 ± 1.8 | 2.2-4.2 | 116.5 ± 20.4 | 105.2-127.8 |
Note: BFI = Brief Fatigue Inventory; HRQL = health-related quality of life; FAClT-F = Functional Assessment of Chronic Illness Therapy-Fatigue scale; SD = standard deviation; 95% CI = 95% confidence interval.
Means are raw, unadjusted values.
Three separate ANCOVAs controlling for baseline on the BFI scores for weeks 1, 2, and 3, respectively, revealed no significant differences (P = .77, P = .14, and P = .84, respectively). The adjusted mean change scores were as follows: week 1, standard care = −0.10, modified massage = 0.18, PT = −0.19; week 2, standard care = −0.20, modified massage = −0.11, PT = 0.82; week 3, standard care = −0.43, modified massage = −0.11, PT = −0.05 (see Figure 1; note that a positive score in this figure denotes a decrease in CRF).
Figure 1.
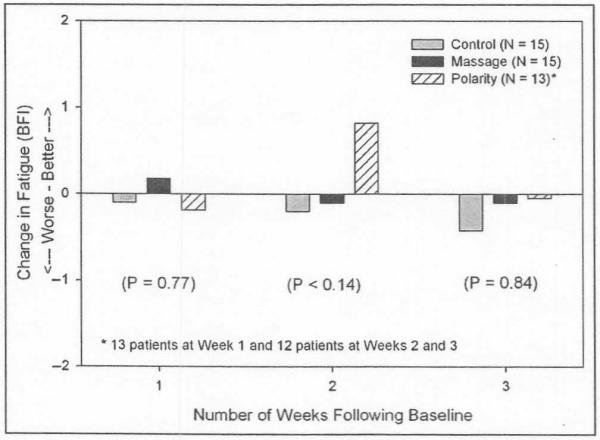
Change in fatigue (assessed by the Brief Fatigue Inventory [BFI]) at 1, 2, and 3 weeks following baseline by treatment group
An ANCOVA with the BFI score averaged across the 3 weeks as the dependent variable and controlling for baseline was also conducted. An ANCOVA with the BFI score averaged across the 3 weeks as the dependent variable and controlling for baseline revealed no significant difference between the mean changes of PT, modified massage or standard care (P = .64). Overall, the 15 patients randomized to the standard care group had an estimated average increase in CRF of 0.25 points (13%) during weeks 1 to 3 compared with baseline. In contrast, the 12 patients receiving PT had an average decrease in CRF of 0.24 points (6%) at the follow-up assessments compared with baseline. The patients receiving modified massage had a very small increase in CRF of 0.01 points (<1%). Using the common standard deviation for the entire sample of 1.23, the Cohen’s d effect size on these change scores for PT compared with standard care was 0.40 (small) and for PT compared with modified massage was 0.21 (small).
Secondary Analysis of Cancer-Related Fatigue
All means and standard deviations for the daily diary fatigue scores are provided in Table 2. The analyses of CRF as assessed by the daily diary were also in a positive direction in regard to the effectiveness of PT for reducing CRF. Repeated measures analysis with the daily diary score as the dependent variable showed significant overall differences between the 3 treatment arms (P = .05), with no dependence on the week (P = .33). Tukey-Kramer post hoc tests revealed a significant difference between massage and Polarity (P = .04) with Polarity demonstrating a larger reduction in daily diary CRF scores across all 3 weeks.
Three separate ANCOVAs controlling for baseline on the daily diary scores for weeks 1, 2, and 3, respectively, revealed no significant differences at week 1 (P = .51), but there were statistical trends at weeks 2 and 3 (P = .07 and P = .09, respectively). The adjusted mean change scores were as follows: week 1, standard care = −0.57, modified massage = −0.23, PT = 0.20; week 2, standard care = −0.01, modified massage = −0.80, PT = 0.89; week 3, standard care = −0.59, modified massage = −0.74, PT = 0.65. At each of the three intervention weeks, CRF levels were lower than at baseline for the patients receiving PT, but CRF levels were greater than at baseline for patients randomized to standard care or modified massage treatment conditions (see Figure 2).
Figure 2.
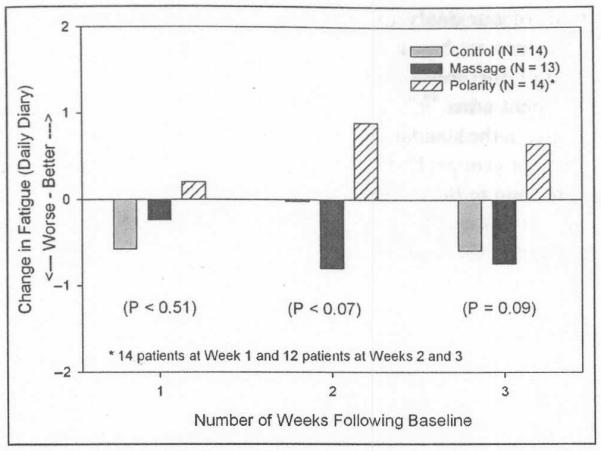
Change in fatigue (assessed daily on a 0 to 10 scale) at 1, 2, and 3 weeks following baseline by treatment group
An ANCOVA with the daily diary score averaged across the 3 weeks as the dependent variable and controlling for baseline revealed a statistical trend for differences between the study arms (P = .08). Patients receiving PT had an average decrease in CRF of 0.59 points (11 %) across all 3 weeks. In contrast, patients randomized to the standard care group had an average increase in CRF of 0.39 points (17%) across all 3 weeks, and patients receiving modified massage had an average increase of 0.59 points (16%) across all 3 weeks. Using the common standard deviation for the entire sample of 1.23, the Cohen’s d effect sizes on these change scores for PT compared with standard care was large at 0.80 and even larger for PT compared to modified massage at 0.96.
Analysis of Health-Related Quality of Life
All means and standard deviations for the FACIT-F are provided in Table 2. Repeated measures analysis with the FACIT-F score as the dependent variable showed no significant overall differences between the 3 treatment arms (P = .21), and no dependence of treatment effects on week (P = .49). Despite the fact that the repeated measures analysis revealed no significant differences, we proceeded with individual ANOVAs because of the primary exploratory pilot nature of the study.
Three separate ANCOVAs controlling for baseline on the FACIT-F scores for weeks 1, 2, and 3, respectively, demonstrated positive results. Both massage and PT patients reported less deterioration in HRQL than standard care patients, but these results did not reach statistical significance. P-values were .31, .09, and .64 for the 3 study weeks, respectively. The adjusted mean change scores were as follows: week 1, standard care = −5.32, modified massage = −0.86, PT = −0.51; week 2, standard care = − 5.46, modified massage = −0.21, PT = 4.52; week 3, standard care = −9.80, modified massage = −4.13, PT = −4.31 (note: a positive score in these analyses denotes an increase in HRQL).
An ANCOVA with the FACIT-F score averaged across the 3 weeks as the dependent variable and controlling for baseline showed no significant differences between the study arms (P = .24). Participants in all conditions showed a decrease in average HRQL scores across all 3 weeks. Patients randomized to the Polarity group had the smallest decrease in HRQL of 0.21 points (0.6%) across all 3 weeks. Patients randomized to the massage group showed a decrease of 1.73 points (<1%) in HRQL across all 3 weeks. Patients randomized to the standard care group demonstrated the largest decrease of 6.88 points (5.4%) in HRQL across all 3 weeks. Using the common standard deviation for the entire sample of 10.8, the Cohen’s d effect sizes for these change scores comparing PT and standard care was moderate at 0.66 and comparing PT to modified massage was small at 0.18.
Feedback Analysis
Responses to our feedback question indicated that both the PT and massage interventions were well-received and that the modified massage intervention served as a credible control group. At week 1, 46.7% and 42.7% of the patients in massage and Polarity, respectively, gave a rating of 5 out of 5 in favor of using and recommending these CAM therapies. Favorability of the CAM therapies increased over the 3 weeks for both groups. At week 3, patients receiving massage gave an average rating of 4.71, with 10 of the 14 patients (71.4%) responding with a rating of 5. The numbers were comparable but slightly lower in the PT group with an average rating of 4.33 with 8 of the 12 patients (67.7%) responding with a rating of 5.
We examined the possibility that higher baseline fatigue in the PT group, compared with the other groups, could mean that the benefits for PT might be an artifact caused by regression to the mean rather than a true treatment effect. The mean daily diary measure of fatigue at baseline for the 41 evaluable patients was 3.81 on the 0 to 10 scale, and 17 of the 41 patients had a baseline fatigue above this level. Of these 17 patients, 3 were randomized to standard care and 5 to modified massage. For these 8 patients, average fatigue increased from a mean of 5.4 at baseline to a mean of 5.5 at the other 3 assessments averaged together. Six of these 8 patients had an overall increase in fatigue between baseline and the later assessments, showing there was no regression to the mean in the standard care and modified massage treatment groups for patients starting with high baseline fatigue.
Discussion
Overall, this study provides further support for studying Polarity Therapy, a CAM approach, for reducing CRF and increasing QOL in cancer patients undergoing radiation. Although the effect sizes of PT compared with standard care and the modified massage were small when assessed by the BFI, we found large effect sizes for PT compared with both standard care and the modified massage conditions when we assessed CRF by the daily diary. We also found medium effect sizes for both PT and modified massage compared to standard care on maintaining HRQL, suggesting that both PT and massage might be effective in improving quality of life in patients with cancer undergoing radiation.
Why did we find a large difference in effect size between our 2 fatigue measures? The single-item daily measure of fatigue we used is actually the third question from the BFI, verbatim, and that question correlated at .73 with the weekly BFI total score in our study. Despite its high correlation with the BFI, we think the daily diary measure showed a larger effect size because of its greater ability to detect daily fluctuations in fatigue that are common in patients undergoing cancer treatments. Jacobsen et al48 speak to this point in their systematic review and meta-analysis of psychological and activity-based interventions for CRF. They note that CRF can fluctuate considerably over short periods of time in patients undergoing chemotherapy or radiotherapy and suggest that daily or shorter assessments may be more appropriate for capturing variability in CRF. Frequent assessment of other psychological (eg, depression) orphysiological constructs (eg, temperature) is known to increase the reliability of the measured variable.
Examination of the direction and magnitude of change in HRQL showed an overall average decrease over time in all three groups, with no statistically significant differences among the groups. Although the differences were not statistically significant, an examination of effect sizes showed the both the massage and PT groups showed a medium effect size benefit in comparison to the control group.
An anomaly in our data shows up in our BFI and HRQL measures in the patients receiving PT. That is, there is a relatively large increase in HRQL and decrease in fatigue, as assessed by the BFI, at week 2 compared with week 3. No such anomaly is seen in the other treatment conditions. We have no explanation for this finding other than speculation that it may be because of the small sample size and the fact that both measures were given weekly. It is possible that even 2 or 3 patients having a good day, or a bad day, when the measures were completed could account for the anomaly.
In addition to its relatively small sample size, this study has some recognized limitations. First, results may be specific to radiation treatment regimens and/or to female patients with breast cancer. Further confirmatory research is necessary to determine if the intervention would be efficacious for patients with other diagnoses and for those who are receiving other treatments such as surgery or chemotherapy. A second limitation is the possibility that our positive findings are merely an artifact of report bias on the patients’ part or some other nonspecific factor that was not adequately controlled for because the study was not blinded. Our use of a credible control group limits this concern but does not eliminate it. A third limitation is that we did not control for other measures patients may have taken to control their fatigue. This limitation is mitigated by our use of a randomized study design and a pre-post assessment that allowed patients to act as their own controls. It is also possible that our findings are a statistical artifact, that is, regression to the mean, related to the fact that despite randomization, patients receiving the PT intervention had a significantly higher level of fatigue than the control group patients during the baseline period. Although possible, we think this is unlikely. Our analyses showing there was no regression to the mean in the standard care and modified massage treatment groups for patients starting with high baseline fatigue argues against a regression to the mean phenomenon having occurred in the PT group because it did not occur in the other 2 treatment groups even for patients who had average fatigue well above the mean. In addition, we used ANCOVA in all of our analyses as a method of controlling for this difference in baseline fatigue. ANCOVA is an accepted method for controlling for baseline differences between treatment arms.49,50 It is also possible that the low level of fatigue in the standard care group at baseline, compared with the other groups, had an effect on the analyses not related to regression to the mean that compromises their validity. We suggest that our finding be interpreted cautiously.
Despite the positive findings from our initial study32 and that of Korn et al,18 described in the Introduction, and the findings of small to larger effect sizes, depending on the measure, for PT in reducing CRF in the present study, we do not believe the accumulated evidence is strong enough to recommend its use to treat CRF other than in a research setting. Larger studies showing statistical significance are needed before such a recommendation can be made. Larger studies are also needed to determine which aspects of fatigue (ie, somatic, cognitive, or functional) are best alleviated by PT. We suggest that the research design we used, discussed in detail below, is an appropriate model for such investigations.
An important consideration in choosing the design for this study, described in the Methods section, was our desire to control for nonspecific treatment factors that might inflate the actual effectiveness of the PT intervention. We considered using a nontreatment control group in which participants simply answered the study questionnaires. We felt that this strategy, however, would not adequately control for benefits that patients might derive merely from having the opportunity to relax in a soothing environment and from the element of simple human touch and interaction. These benefits, although not strictly related to the Polarity treatment itself, are nevertheless possible. We therefore decided to use a modified massage control condition which served as a control for time, attention, touch, expectancy, and simply lying down for 75 minutes. In addition, a conventional nontreatment control group was added to provide for important comparisons between the intervention and standard care. We note that even though the modified massage treatment was perceived as equally credible as the PT, it was less effective, as hypothesized, in alleviating fatigue. Credible control conditions such as this one have been recommended for CAM studies that cannot be adequately blinded.51,52
The choice of patients with breast cancer receiving radiation treatments as the study population provided important logistical advantages that enhanced the study design. Because these patients were treated daily, we were able to standardize the treatment protocol in important ways. For example, all study treatments (ie, Polarity or modified massage) were given early in the week, on a Monday or Tuesday, and all self-reported assessments, other than the daily diary, took place at the end of the week on Friday evening. Having the primary assessment period a couple of days after the treatment, as opposed to the same or next day, provided a meaningful time frame in which to assess outcomes that was not likely clouded by transient benefits of the intervention. In addition, the intraweek treatment and assessment schedule (ie, intervention completed by Tuesday and evaluation on Friday) allowed us to avoid potential error variance in our measurements by excluding nontreatment weekend days from our assessments.
Limiting the study to female patients with breast cancer with no distant metastasis at initial diagnosis increased our ability to detect significant intervention effects by eliminating potential variance from differences relating to cancer type, extent, location, gender, and/or treatments. Studying only this group provided a sample limited in heterogeneity, thereby allowing for greater ease in data interpretation.
Conclusion
The present study supports previous experimental research showing that PT, a noninvasive and gentle energy therapy, may be effective in controlling CRF. PT is an integrative medicine energy therapy based on the Ayurvedic medical system used for thousands of years in India and evidence of its efficacy could lead to further, potentially fruitful, explorations of this ancient medical practice and/or of other energy therapies such as HT, TT, and Reiki. Further confirmatory studies as well as studies investigating the possible mechanisms of PT are warranted.
Acknowledgments
The authors would like to thank the study therapists for their time and contributions: Judy Moss, Sharon Bailey, Jill Scheltz, and Karen Cole.
Funding The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by NCCAM Grant 5R21AT2531 and NCI Grants 1K07CA120025 and K07CA 132916. Clinical Trials.gov identifier: NCT00288795.
Footnotes
Declaration of Conflicting Interests The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Danjoux C, Gardner S, Fitch M. Prospective evaluation of fatigue during a course of curative radiotherapy for localised prostate cancer. Support Care Cancer. 2007;15:1169–1176. doi: 10.1007/s00520-007-0229-8. [DOI] [PubMed] [Google Scholar]
- 2.Lavdaniti M, Patiraki E, Dafni U, Katapodi M, Papathanasoglou E, Sotiropoulou A. Prospective assessment of fatigue and health status in Greek patients with breast cancer undergoing adjuvant radiotherapy. Oncol Nurs Forum. 2006;33:603–610. doi: 10.1188/06.ONF.603-610. [DOI] [PubMed] [Google Scholar]
- 3.Turriziani A, Mattiucci GC, Montoro C, et al. Radiotherapyrelated fatigue: Incidence and predictive factors. Rays. 2005;30:197–203. [PubMed] [Google Scholar]
- 4.Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustian KM, Palesh O, Heckler CE, et al. Cancer-related fatigue interferes with activities of daily living among 753 patients receiving chemotherapy: A URCC CCOP study. J Clin Oncol. 2008 May 20;26(Supp1) abstr 9500. [Google Scholar]
- 6.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of nonpharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 7.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD006953.pub2. CD006953. doi: 10.1002/14651858.CD006953.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean-Pirre P, Mustian KM, Kohli S, Roscoe JA, Hickok JT, Morrow GR. Community-based clinical oncology research trials for cancer-related fatigue. J Support Oncol. 2006;4:511–516. [PubMed] [Google Scholar]
- 9.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. In: Marty M, Pecorelli S, editors. Fatigue and Cancer. Elsevier; Amsterdam, Netherlands: 2001. pp. 3–16. [Google Scholar]
- 10.Yates JS, Mustian KM, Morrow GR, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806–811. doi: 10.1007/s00520-004-0770-7. [DOI] [PubMed] [Google Scholar]
- 11.Sills F. The Polarity Process: Energy as a Healing Art. North Atlantic Books; Berkeley, CA: 2002. [Google Scholar]
- 12.Weil A. Spontaneous Healing. Alfred A. Knopf; New York, NY: 1995. [Google Scholar]
- 13.Markides EJ. Complementary energetic practices: An exploration into the world of Maine women healers. Dissertation Abstracts International A. 1996;57:1509. [Google Scholar]
- 14.Anonymous [Accessed October 11, 2010]; American Polarity Therapy Association Website. http://www.polaritytherapy.org.
- 15.Korn L. Polarity therapy. In: Novey DW, editor. Clinician’s Complete Reference to Complementary and Alternative Medicine. Mosby; Philadelphia, PA: 2000. pp. 423–434. [Google Scholar]
- 16.Benford MS, Talnagi J, Doss DB, Boosey S, Arnold LE. Gamma radiation fluctuations during alternative healing therapy. Altern Ther Health Med. 1999;5:51–56. [PubMed] [Google Scholar]
- 17.Clifford D. Hospital study shows benefits of polarity therapy. Energy: Newslett Am Polarity Ther Assoc. 1997;12:1. [Google Scholar]
- 18.Korn LE, Logsdon RG, Polissar NL, Gomez-Beloz A, Waters T, Ryser R. A randomized trial of a CAM therapy for stress reduction in American Indian and Alaskan Native family caregivers. Gerontologist. 2009;49:368–377. doi: 10.1093/geront/gnp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone R. Health Building: The Conscious Art of Living Well. CRCS Publications; Sebastopol, CA: 1985. [Google Scholar]
- 20.Jhaveri A, Walsh SJ, Wang Y, McCarthy M, Gronowicz G. Therapeutic touch affects DNA synthesis and mineralization of human osteoblasts in culture. J Orthop Res. 2008;26:1541–1546. doi: 10.1002/jor.20688. [DOI] [PubMed] [Google Scholar]
- 21.Gronowicz GA, Jhaveri A, Clarke LW, Aronow MS, Smith TH. Therapeutic touch stimulates the proliferation of human cells in culture. J Altern Complement Med. 2008;14:233–239. doi: 10.1089/acm.2007.7163. [DOI] [PubMed] [Google Scholar]
- 22.Engebretson J, Wardell D. Experience of a Reiki session. Altern Ther Health Med. 2002;8:48–53. [PubMed] [Google Scholar]
- 23.Olson K, Hanson J. Using Reiki to manage pain: a preliminary report. Cancer Prev Control. 1997;1:108–113. [PubMed] [Google Scholar]
- 24.Aghabati N, Mohammadi E, Esmaiel Z Pour. The effect of therapeutic touch on pain and fatigue of cancer patients undergoing chemotherapy. Evid Based Complement Altern Med. 2010;7:375–381. doi: 10.1093/ecam/nen006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrero MS. The effects of therapeutic touch on state-train anxiety level of oncology patients. Masters Abstracts International. 1985;42:3. [Google Scholar]
- 26.Turner JG, Clark AJ, Gauthier DK, Williams M. The effect of therapeutic touch on pain and anxiety in burn patients. J Adv Nurs. 1998;28:10–20. doi: 10.1046/j.1365-2648.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 27.Wardell DW, Engebretson J. Biological correlates of Reiki touch healing. J Adv Nurs. 2001;33:439–445. doi: 10.1046/j.1365-2648.2001.01691.x. [DOI] [PubMed] [Google Scholar]
- 28.Winstead-Fry P, Kijek J. An integrative review and meta-analysis of therapeutic touch research. Altern Ther Health Med. 1999;5:58–67. [PubMed] [Google Scholar]
- 29.Lee MS, Pittler MH, Ernst E. Effects ofReiki in clinical practice: A systematic review of randomised clinical trials. Int J Clin Pract. 2008;62:947–954. doi: 10.1111/j.1742-1241.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 30.Benford MS, Schwartz GER, Russek LGS, Boosey S. Exploring the concept of energy in touch-based healing. In: Novey DW, editor. Clinician ’s Complete Reference to Complementary and Alternative Medicine. Mosby; Philadelphia, PA: 2000. pp. 483–493. [Google Scholar]
- 31.Stone R. Polarity Therapy. CRCS Publications; Reno, NV: 1986. [Google Scholar]
- 32.Roscoe JA, Matteson SE, Mustian KM, Padmanaban D, Morrow GR. Treatment of radiotherapy-induced fatigue through a nonpharmacological approach. Integ Cancer Ther. 2005;4:8–13. doi: 10.1177/1534735404273726. [DOI] [PubMed] [Google Scholar]
- 33.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Cleeland CS, Anderson KO. Expert Opinions on Methodology: Development of Cancer CAM Symptom Research. National Cancer Institute; Bethesda, MD: 2003. Assessment of cancer-related symptoms: relevance for CAM research methodology; pp. 67–90. The NCI’s Office of Cancer Complementary and Alternative Medicine (NCI Publication Number 03-5427) [Google Scholar]
- 36.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist. 2007;12(Suppl 1):11–21. doi: 10.1634/theoncologist.12-S1-11. [DOI] [PubMed] [Google Scholar]
- 37.Sloan JA, Berk L, Roscoe JA, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007;25:5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 38.Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 39.Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 40.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005;30:433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Cella D. The Functional Assessment of Cancer Therapy–Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34:13–19. [PubMed] [Google Scholar]
- 42.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 43.Cella DF. Quality-of-life—concepts and definition. J Pain Symptom Manage. 1994;9:186–192. doi: 10.1016/0885-3924(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 44.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy–Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 45.Winstead-Fry P, Schultz A. Psychometric analysis of the Functional Assessment of Cancer Therapy–General (FACT-G) scale in a rural sample. Cancer. 1997;79:2446–2452. [PubMed] [Google Scholar]
- 46.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 47.Kramer CY. Extension of multiple range tests to group means with unequal numbers of replication. Biometrics. 1956;12:309–310. [Google Scholar]
- 48.Jacobsen PB, Donovan KA, Vadaparampil ST, et al. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26:660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belin TR, Normand ST. The role of ANCOVA in analyzing experimental data. Psychiatr Ann. 2009;39:753–760. [Google Scholar]
- 50.Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrow GR, Hickok JT, Roscoe JA, Matteson SE. Expert Opinions on Methodology: Development of Cancer CAM Symptom Research. National Cancer Institute; Bethesda, MD: 2003. Theory, hypothesis development, and randomized clinical trials in complementary and alternative medicine: a beginning at separating science from political science; pp. 91–104. (Publication Number 03-5427) [Google Scholar]
- 52.Miller FG, Emanuel EJ, Rosenstein DL, Straus SE. Ethical issues concerning research in complementary and alternative medicine. JAMA. 2004;291:599–604. doi: 10.1001/jama.291.5.599. [DOI] [PubMed] [Google Scholar]


