The gastrointestinal (GI) tract plays a key role in both the clinical manifestations and pathogenesis of HIV infection. As a mucosal surface, the GI tract serves as an important barrier between pathogens in the external environment and the body's sterile internal environment [1]. The tight epithelial junctions, as well as the local immune system of the GI tract, protect against pathogenic organisms. However, in the face of HIV infection, normal defenses are disrupted, leading to a wide range of clinical and pathogenic consequences.
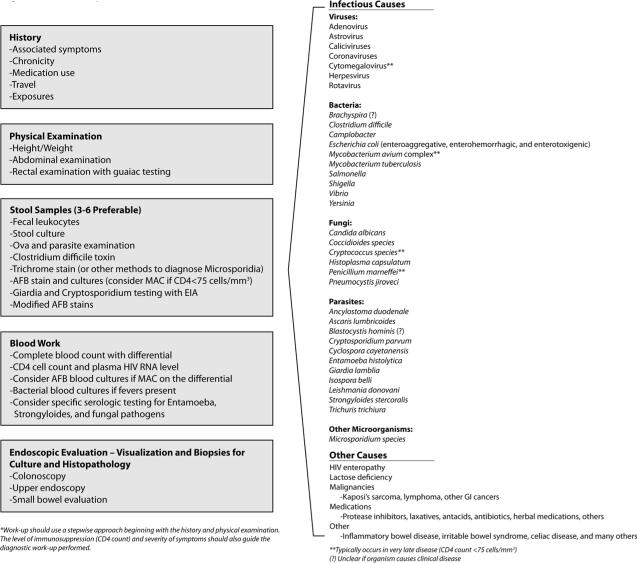
GI symptoms are reported by 50–70% of HIV-infected persons, with even higher percentages among those residing in the developing world [2, 3]. Diarrhea, the most common GI complaint, can occur during both acute HIV infection and advanced disease. Within days of HIV infection, an intense infiltration of virus-laden lymphocytes is present within the bowel wall and may manifest as diarrhea during seroconverting illness [4, 5]. Over time, chronic changes ensue with diminution of the protective mucosal barrier. Opportunistic infections may occur as the CD4 T cell count falls below 100–200 cells/mm3 including a myriad of viral, bacterial, fungal, and parasitic pathogens (Figure 1) [2, 5, 6].
Figure 1.
Work-up of Diarrhea in the HIV Patient*
Beyond the risk for opportunistic pathogens, HIV itself alters the structure and function of the GI tract. In 1984, Kotler et al. noted histological changes in the GI tracts of HIV patients in the absence of other defined infectious or malignant etiologies and termed the condition “HIV enteropathy” [7]. The diagnosis of HIV enteropathy is one of exclusion, requiring a thorough work-up of all other potential causes (Figure 1).
The pathogenesis of HIV enteropathy is the result of both direct and indirect effects of the virus - gp120 negatively affects tubulin depolymerization, and induction of local cytokines (e.g., interleukin (IL)-6, IL-10, tumor necrosis factor) causes altered epithelial ionic balances and enterocyte apoptosis [1, 8, 9]. These changes result in both structural and immunological abnormalities. On histological sampling of the small intestine and colon, villous atrophy, crypt hyperplasia, epithelial hypoproliferation, and CD4+ (CD4+CD45RA−CD69+CCR5+) depletion within the lamina propria are seen [5, 10, 11]. The associated inflammation, increased permeability, and malabsorption (of bile acids and vitamin B12) all contribute to the diarrhea that can occur with HIV enteropathy.
With the advent of highly active antitretroviral therapy (HAART), opportunistic etiologies, along with HIV enteropathy, dramatically decreased [12, 13]. HAART not only improves the systemic immune system, but also the local cellular immunity of the GI tract. Hence, HAART became the cornerstone for both the prevention and treatment of opportunistic GI infections and HIV enteropathy [2]. The case described by Ebama et al highlights this important point [14]; despite therapeutic trials with antimicrobials and nutritional support, diarrhea persisted until HAART was begun. Studies have demonstrated that HIV enteropathy frequently improves within a week of HAART initiation [12].
Why then are we still discussing diarrhea among HIV-infected persons in the HAART era? Although HAART has clearly reduced the impact of some GI conditions, diarrhea remains an important cause of morbidity and mortality among HIV-infected persons. Reasons for this are severalfold. First, in locations where HAART is not universally available, diarrhea due to opportunistic infections and HIV enteropathy remains a leading cause of death. Further, in the U.S. since 45% of patients are diagnosed late in the disease course [15], wasting and diarrhea, unfortunately, remain common diagnoses – this point is highlighted by the case reported by Ebama et al [14]. Moreover, in areas where the diagnosis and management of HIV occurs early, other etiologies of diarrhea have appeared including diarrhea associated with the antiretroviral medications themselves (e.g., protease inhibitors), which can impact both the patients' quality of life as well as medication adherence [6]. Finally, even with HAART, HIV patients may have persistent HIV-related pathogenesis occurring within the GI tract.
Over the past several years, the importance of the GI system as a preferential site of HIV replication and continued CD4 T cell destruction has been realized [16]. Studies have shown that within days of infection there is massive depletion of CD4 T cells within the gut-associated lymphoid tissue (GALT) [17]. Since the reconstitution of CD4 T cells is slower and less complete in the GI tract compared to the peripheral blood [18], the GI tract remains an important site of HIV pathogenesis, even during the HAART era [19].
The reasons for the incomplete immune reconstitution in the GI tract may be attributed to 1) persistent viral replication (due to low local concentrations of antiretrovirals or overexpression of multidrug transporters); 2) differential loss of certain CD4 T cell subtypes (e.g., producing IL-17); and 3) immune activation and inflammation in the gut microenvironment [18, 20, 21]. Of particular importance is that disturbances in mucosal integrity may result in microbial translocation. Several groups have detected elevated levels of lipopolysachharides (LPS) and other bacterial products within the systemic circulation (despite the receipt of HAART), which may contribute to immune activation leading to local and systemic CD4 T cell activation and cell death, and ultimately HIV progression [4, 22]. Of note, the degree of immune activation has been shown to predict HIV progression better than the plasma HIV viral load [23].
Although HAART has dramatically changed the course of HIV, it has yet to fully restore normal life expectancy among those infected [24]. Part of the reason may be residual involvement of the GI tract; ongoing immune activation and inflammation may play a role in the development of conditions contributing to excess mortality in this population. Further studies of HIV-induced pathogenic processes within the gut are needed [25].
In summary, the GI tract was recognized early in the epidemic as an important site of HIV-related complications including opportunistic infections and HIV enteropathy. Remarkable progress in understanding the role of the GI system during both early and late phases of HIV infection has been made over the past 30 years. However, the GI system remains at the center of HIV pathogenesis, and novel therapeutic strategies addressing ongoing local HIV replication and immune activation are needed. Such steps may be the missing link for HIV-infected persons to achieve complete virologic control, and to one day achieve the normal life expectancy that all desire.
Acknowledgments
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
Footnotes
Conflict of Interest: None. The author has no financial interest in this work.
The content of this publication is the sole responsibility of the author and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This work is original and has not been published elsewhere.
References
- 1.Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19:107–117. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cello JP, Day LW. Idiopathic AIDS enteropathy and treatment of gastrointestinal opportunistic pathogens. Gastroenterology. 2009;136:1952–1965. doi: 10.1053/j.gastro.2008.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johanson JF. Diagnosis and management of AIDS-related diarrhea. Can J Gastroenterol. 1996;10:461–468. doi: 10.1155/1996/739845. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sestak K. Chronic diarrhea and AIDS: insights into studies with non-human primates. Curr HIV Res. 2005;3:199–205. doi: 10.2174/1570162054368084. [DOI] [PubMed] [Google Scholar]
- 6.Wallace MR, Brann OS. Gastrointestinal manifestations of HIV infection. Curr Gastroenterol Rep. 2000;2:283–293. doi: 10.1007/s11894-000-0020-1. [DOI] [PubMed] [Google Scholar]
- 7.Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 8.Maresca M, Mahfoud R, Garmy N, et al. The virotoxin model of HIV-1 enteropathy: involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J Biomed Sci. 2003;10:156–166. doi: 10.1007/BF02256007. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz H, Rokos K, Florian P, et al. Supernatants of HIV-infected immune cells affect the barrier function of human HT-29/B6 intestinal epithelial cells. AIDS. 2002;16:983–991. doi: 10.1097/00002030-200205030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Zeitz M, Ullrich R, Schneider T, et al. HIV/SIV enteropathy. Ann N Y Acad Sci. 1998;859:139–148. doi: 10.1111/j.1749-6632.1998.tb11118.x. [DOI] [PubMed] [Google Scholar]
- 11.Clayton F, Snow G, Reka S, et al. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. 1997;107:288–292. doi: 10.1111/j.1365-2249.1997.236-ce1111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotler DP, Shimada T, Snow G, et al. Effect of combination antiretroviral therapy upon rectal mucosal HIV RNA burden and mononuclear cell apoptosis. AIDS. 1998;12:597–604. doi: 10.1097/00002030-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Mönkemüller KE, Call SA, Lazenby AJ, et al. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am J Gastroenterol. 2000;95:457–462. doi: 10.1111/j.1572-0241.2000.01768.x. [DOI] [PubMed] [Google Scholar]
- 14.Ebama NH, Wehbeh W, Rubin D. HIV enteropathy: case report and review of advances in pathogenesis, diagnosis, and treatments. Infect Dis Clin Pract. 2010 [Google Scholar]
- 15.Shouse RL, Kajese T, Hal HI, et al. Late HIV Testing – 34 states, 1996–2005. MMWR. 2009;58:661–665. [PubMed] [Google Scholar]
- 16.Nannini EC, Okhuysen PC. HIV1 and the gut in the era of highly active antiretroviral therapy. Curr Gastroenterol Rep. 2002;4:392–398. doi: 10.1007/s11894-002-0009-z. [DOI] [PubMed] [Google Scholar]
- 17.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 18.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knox TA, Spiegelman D, Skinner SC, et al. Diarrhea and abnormalities of gastrointestinal function in a cohort of men and women with HIV infection. Am J Gastroenterol. 2000;95:3482–3489. doi: 10.1111/j.1572-0241.2000.03365.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones K, Bray PG, Khoo SH, et al. P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS. 2001;15:1353–1358. doi: 10.1097/00002030-200107270-00004. [DOI] [PubMed] [Google Scholar]
- 21.Cecchinato V, Trindade CJ, Laurence A, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 23.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 24.The Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]