Abstract
Background
Studies published prior to 1980 failed to find an association between smoking and colorectal cancer, while subsequent studies reported an association after accounting for a three to four decade initiation period. The aims of this study were to determine the effect of accounting for secondhand smoke (SHS) exposure on the association between smoking and colorectal cancer and to determine the association between SHS and colorectal cancer.
Methods
Approximately 1,200 colorectal cancer cases treated at Roswell Park Cancer Institute between 1982 and 1998 were matched to 2,400 malignancy-free controls. The effect of accounting for SHS exposure was determined by comparing the odds ratios (OR) for each smoking variable in the overall sample and then for those who reported no current SHS exposure.
Results
A small, significant increase in colorectal cancer odds was noted for heavy, long-term smoking males when not accounting for SHS exposure (>45 PY: OR = 1.34; 95% CI 1.04–1.72). OR increased when the analyses were restricted to individuals reporting no current SHS exposure (>45 PY: OR = 2.40; 95% CI 1.36–4.23).
Conclusions
Accounting for SHS exposure resulted in a substantial increase in the odds of colorectal cancer for all smoking variables in this study. Future studies should account for SHS exposure when examining the association between smoking and colorectal cancer.
Keywords: Smoking, Secondhand smoke exposure, Colorectal cancer, Case–control
Introduction
Cigarette smoke contains over 50 known carcinogens, including colorectal carcinogens that reach the digestive tract through direct inhalation, the circulatory system, or direct contact with mucus brought up from the lungs and swallowed [1–5]. Epidemiological studies published before 1980 failed to detect any association between cigarette smoking and colorectal cancer (CRC) [6–11]. Following the increased use of colonoscopy for CRC screening in the 1980s, several studies reported an increase in colorectal adenomas among heavy, long-term cigarette smokers [12–17]. After this point, both case–control [18–23] and cohort studies [13, 14, 24–27] reported significant increases in colorectal cancer risk for individuals who reported having a smoking history of more than 30 years. The biological mechanism remains unclear but may be due to known colorectal carcinogens (tobacco-specific nitrosamines and heterocyclic amines) that are contained in cigarette smoke [28–30]. Despite the evidence demonstrating an association between colorectal cancer risk and a significant smoking history, the Surgeon General concluded in 2004 “the evidence is suggestive but not sufficient to infer a causal relationship” [31].
The discrepancy in results between the early and more recent studies may be due to the failure of the early studies to adequately account for the long time lag between smoking exposure and time of risk [32]. Studies demonstrate that numerous decades must pass before an increase in risk for colorectal cancer from smoking can be observed; an increase in risk is rarely seen in those who smoked for less than 30 years [13, 14, 17]. Additionally, many studies may have suffered from exposure misclassification due to smoking deception or lack of control for secondhand smoke (SHS) exposure [33, 34]. Studies that do not account for SHS exposure may include a number of individuals who were exposed to cigarette smoke through SHS, creating a reference group that is not free of cigarette smoke exposure [33]. This has been previously demonstrated in the case of smoking and breast cancer. Early epidemiological studies examining the association between breast cancer and cigarette smoking found no clear association between the two despite the detection of smoking-specific damage in human breast tissue [35]. Recent evidence shows that when secondhand smoke exposure information was collected and controlled for, a more consistent increase in breast cancer risk from active smoking was observed [36]. Epidemiological studies have also shown that SHS exposure can be associated with organs that are not in direct contact with cigarette smoke, as in the case of SHS exposure and bladder cancer [37–39]. To date, only a few studies have examined the effect of secondhand smoke on colorectal cancer and reported mixed results [40–44]. We decided to investigate the potential association between SHS exposure and colorectal cancer based on the evidence from previous studies involving colorectal cancer and SHS, and additional studies from analogous cancer sites, such as the bladder.
Due to the lack of studies that have controlled for secondhand smoke exposure when examining the association between active smoking and colorectal cancer, we conducted a study investigating these associations. The aims of this study were to determine the effect of accounting for secondhand smoke (SHS) exposure on the association between smoking and colorectal cancer and to determine the association between SHS and colorectal cancer. Detailed information was available on active smoking history, secondhand smoke exposure, and other confounding variables from patients with colorectal cancer who presented for care at the Roswell Park Cancer Institute.
Materials and methods
The study population included men and women who received care at Roswell Park Cancer Institute (RPCI) between 1982 and 1998 and successfully completed an inclusive epidemiological questionnaire after providing informed consent. The case group consisted of 1,203 individuals ranging in age from 40 to 88 diagnosed with primary incident colorectal cancer identified from the RPCI tumor registry. Cases diagnosed prior to the age of 40 were excluded from the analyses due to the likelihood of an underlying genetic predisposition to colorectal cancer. The control group consisted of 2,406 individuals free of any neoplasm (excluding non-melanomatous skin cancer), who were randomly selected from a pool of 7,123 individuals who received care at RPCI during the same period, and ranged in age from 40 to 86. All potential controls were seen at RPCI with the suspicion of neoplastic disease but were not found to have benign or malignant tumors. In addition, potential controls receiving care at the gastrointestinal clinic for conditions associated with an increase in colorectal cancer risk (Crohn’s disease and ulcerative colitis) were excluded. The majority of controls received care at the breast/gynecological clinic, dermatology clinic, genitourinary clinic, head and neck clinic, respiratory clinic, or sarcoma/melanoma clinic. Controls were frequency matched to cases on gender, 5-year age interval, and year of questionnaire completion at a 2:1 ratio.
Every patient who received care at RPCI between 1982 and 1998 was given the opportunity to complete the 16 page Patient Epidemiology Data System (PEDS) questionnaire. Participation in the survey was voluntary and not related to treatment; all patients were asked to complete the questionnaire and approximately 50% did so. The questionnaire covered information on family history of cancer, alcohol consumption, occupational and environmental exposures, reproductive and medical history, tobacco usage, secondhand tobacco smoke exposure, and diet. Diet was assessed using a food frequency questionnaire (FFQ) of 45 different foods, accounting for more than 90% of the variance of intake of major nutrients. For tobacco history, participants reported on their current smoking status, average amount smoked before their illness, number of years smoked, age at smoking initiation, and number of years since quitting. From these questions, the variable “pack-years” (amount smoked multiplied by years smoked) was calculated. All continuous variables were put into quartiles based on the distribution among controls. For secondhand smoke exposure, participants reported on the number of hours each day they were currently exposed to smoke of others in the (a) home, (b) work, and (c) other locations. The numbers of hours from all three categories were added to create a composite measure of total hours each day exposed to SHS.
Descriptive analyses included Student’s t-test for continuous variables and a χ2 test for categorical variables. Odds ratios (OR) and 95% confidence intervals were calculated from unconditional logistic regression models. Covariates included age (continuous), sex, body mass index (BMI) (continuous), residence, race, education, income, family history, vegetable consumption (continuous), meat consumption (continuous), other tobacco use, alcohol use, and aspirin use. Model selection was performed in a stepwise, forward manner, adding covariates one at a time. In an effort to preserve statistical power, covariates were included only if they changed the observed odds ratio estimates by at least 10% or significantly contributed to explaining the variance in the statistical models. Two separate models were created for each exposure variable: (a) all colorectal cases and matched controls and (b) restricted to those who reported no secondhand smoke exposure. In all logistic regression models, lifetime never-smokers served as the reference group. Statistical significance was defined as p < 0.05.
Results
Table 1 displays the characteristics of the cases and controls in regard to sociodemographic, lifestyle, and dietary variables. Cases and controls were similar in regard to age, gender, education, income, alcohol consumption, fruit consumption, and vegetable consumption. However, cases were significantly more likely to have a higher BMI, live outside Western New York (WNY), be non-Caucasian, have a relative previously diagnosed with CRC, consume less cruciferous vegetables, consume more meat, and take less aspirin.
Table 1.
Characteristics of 1,203 colorectal cancer patients and 2,406 hospital controls, Roswell Park Cancer Institute, 1982–1998
| Characteristic | Cases | Controls | p Value |
|---|---|---|---|
| Age at diagnosis (years) | 63.4 | 63.4 | 0.87 |
| Gender | |||
| % Male | 56.3% | 56.3% | 1.00 |
| Body mass index before illness | 26.5 | 26.1 | 0.02 |
| Race | |||
| % Caucasian | 94.4% | 96.7% | <0.01 |
| Residence | |||
| % Western New York residence | 55.8% | 86.6% | <0.01 |
| % Outside Western New York | 44.2% | 13.4% | |
| Education | |||
| % <High school education | 28.1% | 25.4% | 0.08 |
| % High school graduate or more | 71.9% | 74.6% | |
| Income | |||
| <$16,000/year | 45.8% | 46.0% | 0.90 |
| $16,000–$30,000/year | 30.8% | 31.3% | |
| >$30,000/year | 23.4% | 22.7% | |
| Family history of CRC | |||
| % No 1st degree relative with CRC | 86.9% | 88.9% | 0.08 |
| % With 1st degree relative with CRC | 13.1% | 11.1% | |
| % No relatives with CRC | 81.0% | 85.5% | <0.01 |
| % With any relatives with CRC | 19.0% | 14.5% | |
| Alcohol consumption (drinks/week) | 5.3 | 5.1 | 0.49 |
| Fruit consumption (servings/week) | 8.9 | 8.8 | 0.86 |
| Vegetable consumption (servings/week) | 15.7 | 16.2 | 0.16 |
| Cruciferous vegetable consumption (servings/week) |
3.7 | 4.0 | 0.04 |
| Meat consumption (servings/week) | 23.4 | 20.7 | <0.01 |
| Aspirin (tablets/week) | 2.0 | 2.7 | <0.01 |
| Pack-years | |||
| Never smoker | 40.8% | 43.0% | 0.04 |
| 1-20 Pack-years | 18.2% | 17.8% | |
| 20-40 Pack-years | 18.7% | 20.2% | |
| >41 Pack-years | 22.3% | 19.0% | |
| SHS exposure | |||
| No SHS | 37.2% | 37.1% | 0.09 |
| 0.5–2 h/day | 21.1% | 23.6% | |
| 2–7 h/day | 22.9% | 23.4% | |
| >7 h/day | 18.8% | 15.9% |
In data not shown, the odds ratios and 95% confidence intervals were calculated for the entire study population and those who reported no current exposure to SHS (0 h/day). For the overall population, a borderline significant increase in odds was observed for those with more than 40 total pack-years of smoking (OR = 1.23; 95% CI 1.02–1.48) and more than 15 pack-years of smoking before age 30 (OR = 1.20; 95% CI 1.00–1.44). When the study population was restricted to those who did not report being exposed to SHS, odds ratios increased substantially. Smokers in the highest quartile of amount smoked, years smoked, pack-years, and pack-years prior to age 30 had significantly greater odds of colorectal cancer compared to never-smokers who were not exposed to secondhand smoke. Separate OR for colon cancer and rectal cancer were found to be similar and no interaction was noted between colorectal sub-sites (pinteraction > 0.10).
Table 2 displays the odds ratios and corresponding confidence intervals for the entire female study population and for females who reported no current exposure to SHS (0 h/day). For the overall female population, no increase in colorectal cancer odds was noted for females with the highest exposure categories. Women who smoked 37 or more years (OR = 1.02; 95% CI 0.75–1.38) and 37 or more pack-years (OR = 1.05; 95% CI 0.78–1.41) had no increase in odds when compared to never smokers. When the analyses were restricted to women who reported no secondhand smoke exposure, odds ratios rose for all smoking exposures, including women who smoked 37 or more years (OR = 1.46; 95% CI 0.80–2.66) and 37 or more pack-years (OR = 1.63; 95% CI 0.92–2.88). However, the odds ratios did not achieve statistical significance despite increasing in magnitude. In results not shown, no interaction was detected for colorectal sub-site analyses (pinteraction > 0.05).
Table 2.
Odds ratios and 95% confidence intervals for colorectal cancer and various active smoking exposures, females only, Roswell Park Cancer Institute, 1982–1998
| Cases (%) | Control (%) | Overall female population (n = 1,578) |
Restricted to females with no current SHS exposure (n = 480) |
|||
|---|---|---|---|---|---|---|
| Odds ratioa | 95% Confidence interval |
Odds ratioa | 95% Confidence interval |
|||
| Smoking status | ||||||
| Never smoker | 284 (54.0) | 563 (53.5) | 1.00 | Referent | 1.00 | Referent |
| Ever smoker | 242 (46.0) | 489 (46.5) | 0.98 | 0.80–1.21 | 1.37 | 0.91–2.05 |
| Amount smoked | ||||||
| Never smoker | 284 (54.0) | 563 (53.6) | 1.00 | Referent | 1.00 | Referent |
| <1 Pack/day | 85 (16.2) | 166 (15.8) | 1.02 | 0.76–1.37 | 1.24 | 0.72–2.12 |
| 1 Pack/day | 102 (19.4) | 202 (19.2) | 1.00 | 0.76–1.33 | 1.11 | 0.66–1.89 |
| >1 Pack/day | 55 (10.5) | 119 (11.3) | 0.92 | 0.65–1.30 | 1.35 | 0.68–2.65 |
| Years smoked | ||||||
| Never smoker | 284 (54.1) | 563 (53.5) | 1.00 | Referent | 1.00 | Referent |
| 1–23 Years | 89 (17.0) | 163 (15.5) | 1.08 | 0.80–1.45 | 1.08 | 0.63–1.84 |
| 24–36 Years | 69 (13.1) | 165 (15.7) | 0.83 | 0.60–1.13 | 1.04 | 0.58–1.87 |
| 37 or More years | 83 (15.8) | 161 (15.3) | 1.02 | 0.75–1.38 | 1.46 | 0.80–2.66 |
| Pack-years (PY) | ||||||
| Never smokers | 284 (54.1) | 563 (53.6) | 1.00 | Referent | 1.00 | Referent |
| 1–17 PY | 89 (17.0) | 161 (15.3) | 1.10 | 0.82–1.47 | 1.33 | 0.80–2.22 |
| 18–36 PY | 65 (12.4) | 162 (15.4) | 0.80 | 0.58–1.10 | 0.59 | 0.29–1.22 |
| 37 or More PY | 87 (16.6) | 164 (15.6) | 1.05 | 0.78–1.41 | 1.63 | 0.92–2.88 |
| PY before age 30 | ||||||
| No PY before 30 | 312 (59.4) | 608 (57.8) | 1.00 | Referent | 1.00 | Referent |
| 1–6 PY | 75 (14.3) | 155 (14.7) | 0.94 | 0.69–1.28 | 0.99 | 0.57–1.75 |
| 7–14 PY | 73 (13.9) | 141 (13.4) | 1.01 | 0.74–1.38 | 1.34 | 0.74–2.41 |
| 15 or More PY | 65 (12.4) | 148 (14.1) | 0.86 | 0.62–1.18 | 1.03 | 0.55–1.94 |
Adjusted for various factors changing risk>10% [(a) age, (b) sex, (c) BMI, (d) WNY residence, (e) race, (f) education, (g) income, (h) family history, (i) vegetable intake, (j) meat intake, (k) other tobacco use, (l) alcohol use, or (m) aspirin use]
Table 3 displays the odds ratios and corresponding confidence intervals for the entire male study population and for males who reported no current exposure to SHS (0 h/day). For the overall male population, a significant increase in colorectal cancer odds was observed for males who smoked more than 1 pack a day (OR = 1.48; 95% CI 1.15–1.90), smoked 46 or more pack-years (OR = 1.34; 95% CI 1.04–1.72), and smoked 20 or more pack-years before the age of 30 (OR = 1.37; 95% CI 1.07–1.75). Once again, odds ratios rose substantially when the analyses were restricted to males who reported no current exposure to secondhand smoke. Colorectal cancer odds for males who smoked more than 1 pack a day (OR = 2.02; 95% CI 1.22–3.36), smoked 46 or more pack-years (OR = 2.40; 95% CI 1.36–4.23), and smoked 20 or more pack-years (OR = 2.55; 95% CI 1.43–4.52) were more than double compared to never-smokers with no secondhand smoke exposure. In results not displayed, no interaction was noted between the colon and rectal sub-sites (pinteraction > 0.05).
Table 3.
Odds ratios and 95% confidence intervals for colorectal cancer and various active smoking exposures, males only, Roswell Park Cancer Institute, 1982–1998
| Cases (%) | Control (%) | Overall male population (n = 2,031) |
Restricted to males with no current SHS exposure (n = 448) |
|||
|---|---|---|---|---|---|---|
| Odds ratioa | 95% Confidence interval |
Odds ratioa | 95% Confidence interval |
|||
| Smoking status | ||||||
| Never smoker | 205 (30.3) | 468 (34.6) | 1.00 | Referent | 1.00 | Referent |
| Ever smoker | 472 (69.7) | 886 (65.4) | 1.22 | 1.00–1.48 | 1.99 | 1.31–3.04 |
| Amount smoked | ||||||
| Never smoker | 205 (30.3) | 468 (34.6) | 1.00 | Referent | 1.00 | Referent |
| <1 Pack/day | 81 (12.0) | 178 (13.2) | 1.06 | 0.76–1.47 | 1.63 | 0.87–3.05 |
| 1 Pack/day | 174 (25.7) | 335 (24.8) | 1.22 | 0.94–1.59 | 1.66 | 1.00–2.76 |
| >1 Pack/day | 217 (32.1) | 372 (27.5) | 1.48 | 1.15–1.90 | 2.02 | 1.22–3.36 |
| ptrend = 0.01 | ptrend = 0.01 | |||||
| Years smoked | ||||||
| Never smoker | 205 (30.3) | 468 (34.6) | 1.00 | Referent | 1.00 | Referent |
| 1–21 Years | 145 (21.4) | 291 (21.5) | 1.13 | 0.88–1.47 | 1.39 | 0.82–2.37 |
| 22–37 Years | 181 (26.8) | 298 (22.0) | 1.38 | 1.08–1.77 | 1.71 | 1.01–2.88 |
| 38 or More years | 145 (21.4) | 296 (21.9) | 1.12 | 0.86–1.44 | 2.29 | 1.32–3.98 |
| ptrend > 0.10 | ptrend = 0.01 | |||||
| Pack-years (PY) | ||||||
| Never smokers | 206 (30.4) | 469 (34.6) | 1.00 | Referent | 1.00 | Referent |
| 1–22 PY | 146 (21.6) | 296 (21.9) | 1.12 | 0.87–1.45 | 1.29 | 0.76–2.19 |
| 23–45 PY | 156 (23.0) | 301 (22.2) | 1.18 | 0.92–1.52 | 1.81 | 1.08–3.02 |
| 46 or More PY | 169 (25.0) | 288 (21.3) | 1.34 | 1.04–1.72 | 2.40 | 1.36–4.23 |
| ptrend = 0.06 | ptrend = 0.01 | |||||
| PY before age 30 | ||||||
| No PY before 30 | 214 (31.6) | 488 (36.1) | 1.00 | Referent | 1.00 | Referent |
| 1–10 PY | 137 (20.2) | 279 (20.6) | 1.12 | 0.86–1.45 | 1.82 | 1.08–3.08 |
| 11–19 PY | 154 (22.7) | 299 (22.1) | 1.17 | 0.91–1.51 | 1.47 | 0.88–2.47 |
| 20 or More PY | 172 (25.4) | 287 (21.2) | 1.37 | 1.07–1.75 | 2.55 | 1.43–4.52 |
| ptrend = 0.02 | ptrend = 0.01 | |||||
Adjusted for various factors changing risk>10% [(a) age, (b) sex, (c) BMI, (d) WNY residence, (e) race, (f) education, (g) income, (h) family history, (i) vegetable intake, (j) meat intake, (k) other tobacco use, (l) alcohol use, or (m) aspirin use]
Figure 1 presents the colorectal cancer odds ratios for exposure to secondhand smoke among lifetime never smokers. No increase in odds was noted for the overall population (OR = 1.18; 95% CI 0.84–1.67) and for women (OR = 0.97; 95% CI 0.61–1.53) who were exposed to more than 7 h of secondhand smoke a day. Although a statistically significant trend was detected for men, the increase in odds for those who were exposed to more than 7 h of SHS a day was not significant (OR = 1.58; 95% CI 0.93–2.69).
Fig. 1.
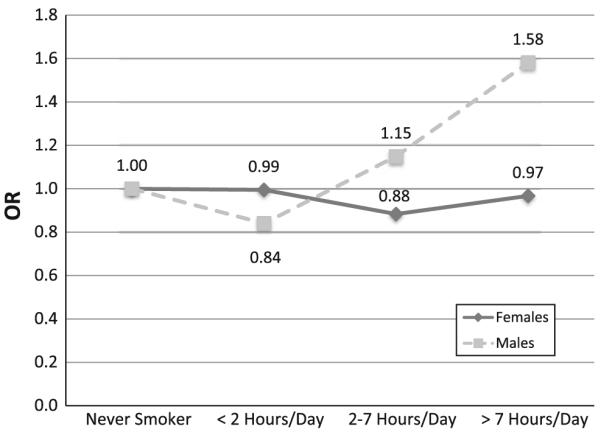
Odds ratios for colorectal cancer and daily hours of secondhand smoke (SHS), lifetime never-smokers only, Roswell Park Cancer Institute, 1982–1998
Discussion
This study found that heavy, long-term cigarette smoking was associated with slightly increased odds of colorectal cancer for males but not females. Of particular importance, this study found the odds of colorectal cancer for smokers significantly increased when SHS exposure was accounted for in the exposure assessment. This is one of the first studies to demonstrate these findings. The results of this study are in agreement with several other studies that reported an increase in risk among individuals with decades of smoking exposure and which were conducted during a similar time period [18, 20–23], although not all studies conducted during this time period supported an association between smoking and colorectal cancer [45–49].
Our goal of restricting analyses to those who reported no current exposure to secondhand smoke was to create a reference group of lifetime never-smokers who were not exposed to the smoke of others. We believe this allowed for a more accurate assessment of cigarette smoke exposure among the reference group compared to studies that did not account for SHS, where there may be a higher degree of exposure misclassification. When this restriction was applied, odds ratios among every exposure category rose, similar to the observed association between cigarette smoking and breast cancer [36]. The increase in odds may be due to a reduction in exposure misclassification, in which the reference group was not exposed to any cigarette smoke, either through their own or others’ behavior. We also tested the possibility of confounding, but SHS exposure was not associated with both the outcome and exposure. Because this is one of the first studies to control for secondhand smoke exposure, future studies should attempt to collect this information and control for it in statistical analyses.
Although our results found no increase in the odds of colorectal cancer of never smoking females exposed to 7 or more hours a day of SHS, we observed a 58% non-significant increase in odds for never smoking males exposed to 7 or more hours of SHS each day. The lack of statistical significance may be due to a lack of statistical power; fewer than 40 never smoking male colorectal cancer cases reported being exposed to 7 or more hours a day of SHS. Additionally, the results from the small number of studies that examined the association between SHS and colorectal cancer risk were also mixed [40–44]. Our results were similar to several other studies [40, 41, 43], but different from another study that found SHS exposure to be protective against colorectal cancer [42] and from another study that found SHS exposure to increase colorectal cancer risk in women only [44]. Based on the results of this study and others, further research is needed to determine the association between secondhand smoke exposure and colorectal cancer risk.
The increase in colorectal cancer odds was seen only in males in the highest exposure categories, which supports the hypothesis of a 30- to 40-year induction period to observe an increase in risk [32]. The biological mechanism underlying these observations remains unclear. Colorectal carcinogens specific to cigarette smoke and other smoke byproducts can reach the colon through direct inhalation, the circulatory system, or through direct exposure to tobacco carcinogens brought up from the lungs by the ciliated epithelial cells, swallowed and passed through the intestines [1–5]. Recent research demonstrates that chemicals in cigarette smoke can promote cellular proliferation in colorectal adenocarcinomas, accelerate tumor growth, and decrease apoptosis in the colon [50, 51]. However, not every study that accounted for the 30–40 year induction period reported an association between cigarette smoking and colorectal cancer [45–48], indicating other factors may play a role in the association of CRC and smoking. Many of the studies that were published subsequent to 1980 and reported null associations were conducted among European populations. In Europe, never-smokers are exposed to greater amounts of SHS than in the United States [52–55], perhaps creating a larger amount of exposure misclassification. This explanation is supported by a biological rationale, in which SHS contains a number of potent carcinogens [56]. SHS is a mixture of smoke exhaled by the smoker and smoke curling of the end of a cigarette [57], and has a carcinogenic profile similar to that of smoke directly inhaled by active smokers [58]. However, it is logical to expect the risk for active smoking to be greater than the risk from SHS exposure due to the higher concentration of smoke exposure for an active smoking compared to passive smoking [57]. It is possible that a lack of SHS exposure assessment in these studies played a role in creating misclassification bias, contributing to the reported null associations.
It remains unclear why a significant association was observed for males but not females. The difference in results may be due to the lack of a large number of women with a heavy smoking history; approximately 20% of males had more than 40 pack-years of smoking compared to just 10% of women. The difference in odds of SHS exposure and colorectal cancer between men and women may be due to the fact that our SHS exposure assessment did not capture the critical time period of exposure or perhaps due to chance based on a small sub-sample.
Several methodological issues should be considered when the interpreting the results of this hospital-based case–control study. As with the large majority of case–control studies, bias may have played a role in the results. Approximately 50% of eligible patients seen at Roswell Park Cancer Institute completed the PEDS questionnaire. Unfortunately, there is no way to tell whether those individuals who refused to complete the instrument differed from participants with respect to smoking exposure. Others authors who have published manuscripts using the PEDS database and faced the same methodological issues have consistently replicated established epidemiological associations [59–61]. The use of hospital controls might introduce bias, due to the possibility that controls would have a higher smoking rate than the general population [62, 63]. If controls did have a higher rate of smoking, the odds ratios were most likely attenuated and the true odds may indeed be higher. Misclassification bias may be present, as participants were asked to recall their entire smoking history. However, there is no evidence the bias was differential in nature, and a non-differential bias would again attenuate the odds ratios. Lastly, SHS exposure was only assessed for the current time (when the questionnaire was completed) and not throughout adulthood. It is possible the assessment of current SHS exposure does not accurately represent SHS exposure throughout adulthood and most likely represents an underestimation of SHS exposure, as clean indoor air laws were enacted and the proportion of quitters increased over time.
Strengths of this study include a large number of male colorectal cancer cases with a significant smoking history. As previous publications have shown, an increase in risk is rarely observed for individuals who smoked for less than 30 years. Even though a large number of male cases had a significant smoking history, the number of females who smoked for more than 30 years was much lower. This number was further reduced when the analysis was restricted to those without secondhand smoke exposure. The low number of cases resulted in reduced statistical power, perhaps accounting for the lack of statistical significance for the increase in colorectal cancer odds among long-term, heavy smoking women not exposed to secondhand smoke. This study was able to control for a large number of potential confounding variables including alcohol consumption, BMI, vegetable consumption, meat consumption, aspirin use, family history, and other sociodemographic variables. The addition of covariates to logistic regression models had little effect on odds ratios. Lastly, another strength of this study was a detailed exposure assessment of active and passive smoking, which allowed for the calculation of numerous exposure measures in which to examine the association between smoking and colorectal cancer.
Conclusion
In summary, this hospital-based case–control study found a significant increase in colorectal cancer odds for long-term, heavy smoking males but not for females. When the sample was restricted to individuals not exposed to secondhand smoke, all odds ratios substantially increased. This is one of the first studies to account for secondhand smoke exposure in an effort to reduce potential misclassification bias. Future studies should collect information pertaining to secondhand smoke exposure and subsequently control for its effect. No effect was observed for colorectal cancer odds and secondhand smoke exposure among lifetime never smokers, although a statistical trend for increasing SHS exposure among males with CRC was found. Further research is needed to determine the effect of secondhand smoke exposure on colorectal cancer risk.
Acknowledgments
Source of Support This work was supported by National Cancer Institute R03-CA097780 and R25-CA102618.
Footnotes
Rights and permissions All authors have participated in the drafting of the manuscript and support its submission for review.
Contributor Information
Luke J. Peppone, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave., Box 704, Rochester, NY 14642, USA
Mary E. Reid, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY, USA
Kirsten B. Moysich, Department of Epidemiology, Roswell Park Cancer Institute, Buffalo, NY, USA
Gary R. Morrow, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave., Box 704, Rochester, NY 14642, USA
Pascal Jean-Pierre, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave., Box 704, Rochester, NY 14642, USA.
Supriya G. Mohile, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave., Box 704, Rochester, NY 14642, USA
Tom V. Darling, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave., Box 704, Rochester, NY 14642, USA
Andrew Hyland, Department of Health Behavior, Roswell Park Cancer Institute, Buffalo, NY, USA.
References
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. World Health Organization . Tobacco smoke and involuntary smoking. IARC Press; Lyon, France, Geneva: 2004. International Agency for Research on Cancer. distributed by IARC Press and the World Health Organization Distribution and Sales. [Google Scholar]
- 2.Kune GA, Kune S, Vitetta L, et al. Smoking and colorectal cancer risk: data from the Melbourne Colorectal Cancer Study and brief review of literature. Int J Cancer. 1992;50:369–372. doi: 10.1002/ijc.2910500307. [DOI] [PubMed] [Google Scholar]
- 3.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 4.Yamasaki E, Ames BN. Concentration of mutagens from urine by absorption with the nonpolar resin XAD-2: cigarette smokers have mutagenic urine. Proc Natl Acad Sci USA. 1977;74:3555–3559. doi: 10.1073/pnas.74.8.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppone LJ, Mahoney MC, Cummings KM, et al. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol. 2008;134:743–751. doi: 10.1007/s00432-007-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R, Gray R, Hafner B, et al. Mortality in relation to smoking: 22 years’ observations on female British doctors. Br Med J. 1980;280:967–971. doi: 10.1136/bmj.280.6219.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doll R, Peto R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J. 1976;2:1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham S, Dayal H, Swanson M, et al. Diet in the epidemiology of cancer of the colon and rectum. J Natl Cancer Inst. 1978;61:709–714. [PubMed] [Google Scholar]
- 9.Hammond EC, Horn D. Smoking and death rates; report on forty-four months of follow-up of 187,783 men. II. Death rates by cause. J Am Med Assoc. 1958;166:1294–1308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 10.Kahn HA. The Dorn study of smoking and mortality among U.S. veterans: report on eight and one-half years of observation. Natl Cancer Inst Monogr. 1966;19:1–125. [PubMed] [Google Scholar]
- 11.Rogot E, Murray JL. Smoking and causes of death among U.S. veterans: 16 years of observation. Public Health Rep. 1980;95:213–222. [PMC free article] [PubMed] [Google Scholar]
- 12.Boutron MC, Faivre J, Dop MC, et al. Tobacco, alcohol, and colorectal tumors: a multistep process. Am J Epidemiol. 1995;141:1038–1046. [PubMed] [Google Scholar]
- 13.Giovannucci E, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. J Natl Cancer Inst. 1994;86:192–199. doi: 10.1093/jnci/86.3.192. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Rimm EB, Stampfer MJ, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994;86:183–191. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson JS, Neugut AI, Murray T, et al. Cigarette smoking and other behavioral risk factors for recurrence of colorectal adenomatous polyps (New York City, NY, USA) Cancer Causes Control. 1994;5:215–220. doi: 10.1007/BF01830239. [DOI] [PubMed] [Google Scholar]
- 16.Potter JD, Bigler J, Fosdick L, et al. Colorectal adenomatous and hyperplastic polyps: smoking and N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers Prev. 1999;8:69–75. [PubMed] [Google Scholar]
- 17.Terry MB, Neugut AI. Cigarette smoking and the colorectal adenoma-carcinoma sequence: a hypothesis to explain the paradox. Am J Epidemiol. 1998;147:903–910. doi: 10.1093/oxfordjournals.aje.a009379. [DOI] [PubMed] [Google Scholar]
- 18.Le Marchand L, Wilkens LR, Kolonel LN, et al. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787–4794. [PubMed] [Google Scholar]
- 19.Luchtenborg M, White KK, Wilkens L, et al. Smoking and colorectal cancer: different effects by type of cigarettes? Cancer Epidemiol Biomarkers Prev. 2007;16:1341–1347. doi: 10.1158/1055-9965.EPI-06-0519. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb PA, Storer BE, Marcus PM. Cigarette smoking in relation to risk of large bowel cancer in women. Cancer Res. 1995;55:4906–4909. [PubMed] [Google Scholar]
- 21.Slattery ML, Potter JD, Friedman GD, et al. Tobacco use and colon cancer. Int J Cancer. 1997;70:259–264. doi: 10.1002/(sici)1097-0215(19970127)70:3<259::aid-ijc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Slattery ML, Samowtiz W, Ma K, et al. CYP1A1, cigarette smoking, and colon and rectal cancer. Am J Epidemiol. 2004;160:842–852. doi: 10.1093/aje/kwh298. [DOI] [PubMed] [Google Scholar]
- 23.Slattery ML, West DW, Robison LM, et al. Tobacco, alcohol, coffee, and caffeine as risk factors for colon cancer in a low-risk population. Epidemiology. 1990;1:141–145. doi: 10.1097/00001648-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Chao A, Thun MJ, Jacobs EJ, et al. Cigarette smoking and colorectal cancer mortality in the cancer prevention study II. J Natl Cancer Inst. 2000;92:1888–1896. doi: 10.1093/jnci/92.23.1888. [DOI] [PubMed] [Google Scholar]
- 25.Heineman EF, Zahm SH, McLaughlin JK, et al. Increased risk of colorectal cancer among smokers: results of a 26-year follow-up of US veterans and a review. Int J Cancer. 1994;59:728–738. doi: 10.1002/ijc.2910590603. [DOI] [PubMed] [Google Scholar]
- 26.Hsing AW, McLaughlin JK, Chow WH, et al. Risk factors for colorectal cancer in a prospective study among U.S. white men. Int J Cancer. 1998;77:549–553. doi: 10.1002/(sici)1097-0215(19980812)77:4<549::aid-ijc13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Knekt P, Hakama M, Jarvinen R, et al. Smoking and risk of colorectal cancer. Br J Cancer. 1998;78:136–139. doi: 10.1038/bjc.1998.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 29.Ito N, Hasegawa R, Sano M, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1991;12:1503–1506. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 30.Wu WK, Wong HP, Luo SW, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone from cigarette smoke stimulates colon cancer growth via beta-adrenoceptors. Cancer Res. 2005;65:5272–5277. doi: 10.1158/0008-5472.CAN-05-0205. [DOI] [PubMed] [Google Scholar]
- 31.Alexandrov K, Rojas M, Kadlubar FF, et al. Evidence of anti-benzo[a]pyrene diolepoxide-DNA adduct formation in human colon mucosa. Carcinogenesis. 1996;17:2081–2083. doi: 10.1093/carcin/17.9.2081. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:725–731. [PubMed] [Google Scholar]
- 33.Corbo GM, Agabiti N, Pistelli R, et al. Parental smoking and lung function: misclassification due to background exposure to passive smoking. Respir Med. 2007;101:768–773. doi: 10.1016/j.rmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Pell JP, Haw SJ, Cobbe SM, et al. Validity of self-reported smoking status: comparison of patients admitted to hospital with acute coronary syndrome and the general population. Nicotine Tob Res. 2008;10:861–866. doi: 10.1080/14622200802023858. [DOI] [PubMed] [Google Scholar]
- 35.Terry PD, Rohan TE. Cigarette smoking and the risk of breast cancer in women: a review of the literature. Cancer Epidemiol Biomarkers Prev. 2002;11:953–971. [PubMed] [Google Scholar]
- 36.Johnson KC. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer. 2005;117:619–628. doi: 10.1002/ijc.21150. [DOI] [PubMed] [Google Scholar]
- 37.Alberg AJ, Kouzis A, Genkinger JM, et al. A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am J Epidemiol. 2007;165:660–666. doi: 10.1093/aje/kwk047. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Yuan JM, Skipper PL, et al. Environmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles County. Cancer Res. 2007;67:7540–7545. doi: 10.1158/0008-5472.CAN-07-0048. [DOI] [PubMed] [Google Scholar]
- 39.Samanic C, Kogevinas M, Dosemeci M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006;15:1348–1354. doi: 10.1158/1055-9965.EPI-06-0021. [DOI] [PubMed] [Google Scholar]
- 40.de Verdier M Gerhardsson, Plato N, Steineck G, et al. Occupational exposures and cancer of the colon and rectum. Am J Ind Med. 1992;22:291–303. doi: 10.1002/ajim.4700220303. [DOI] [PubMed] [Google Scholar]
- 41.Nishino Y, Tsubono Y, Tsuji I, et al. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control. 2001;12:797–802. doi: 10.1023/a:1012273806199. [DOI] [PubMed] [Google Scholar]
- 42.Sandler RS, Sandler DP, Comstock GW, et al. Cigarette smoking and the risk of colorectal cancer in women. J Natl Cancer Inst. 1988;80:1329–1333. doi: 10.1093/jnci/80.16.1329. [DOI] [PubMed] [Google Scholar]
- 43.Slattery ML, Edwards S, Curtin K, et al. Associations between smoking, passive smoking, GSTM-1, NAT2, and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:882–889. [PubMed] [Google Scholar]
- 44.Verla-Tebit E, Lilla C, Hoffmeister M, et al. Exposure to environmental tobacco smoke and the risk of colorectal cancer in a case–control study from Germany. Eur J Cancer Prev. 2009;18:9–12. doi: 10.1097/CEJ.0b013e3282f0c06c. [DOI] [PubMed] [Google Scholar]
- 45.D’Avanzo B, La Vecchia C, Franceschi S, et al. Cigarette smoking and colorectal cancer: a study of 1,584 cases and 2,879 controls. Prev Med. 1995;24:571–579. doi: 10.1006/pmed.1995.1091. [DOI] [PubMed] [Google Scholar]
- 46.Nyren O, Bergstrom R, Nystrom L, et al. Smoking and colorectal cancer: a 20-year follow-up study of Swedish construction workers. J Natl Cancer Inst. 1996;88:1302–1307. doi: 10.1093/jnci/88.18.1302. [DOI] [PubMed] [Google Scholar]
- 47.Olsen J, Kronborg O. Coffee, tobacco and alcohol as risk factors for cancer and adenoma of the large intestine. Int J Epidemiol. 1993;22:398–402. doi: 10.1093/ije/22.3.398. [DOI] [PubMed] [Google Scholar]
- 48.Tavani A, Gallus S, Negri E, et al. Cigarette smoking and risk of cancers of the colon and rectum: a case–control study from Italy. Eur J Epidemiol. 1998;14:675–681. doi: 10.1023/a:1007545607059. [DOI] [PubMed] [Google Scholar]
- 49.Baron JA, de Verdier M Gerhardsson, Ekbom A. Coffee, tea, tobacco, and cancer of the large bowel. Cancer Epidemiol Biomarkers Prev. 1994;3:565–570. [PubMed] [Google Scholar]
- 50.Wong HP, Yu L, Lam EK, et al. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Wong HP, Yu L, Lam EK, et al. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci. 2007;97:279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- 52.Haw SJ, Gruer L. Changes in exposure of adult nonsmokers to secondhand smoke after implementation of smoke-free legislation in Scotland: national cross sectional survey. BMJ. (Clinical research ed) 2007;335:549. doi: 10.1136/bmj.39315.670208.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jefferis BJ, Thomson AG, Lennon LT, et al. Changes in environmental tobacco smoke (ETS) exposure over a 20-year period: cross-sectional and longitudinal analyses. Addiction (Abingdon, England) 2009;104:496–503. doi: 10.1111/j.1360-0443.2008.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Sanchez JM, Fernandez E, Fu M, et al. Assessment of exposure to secondhand smoke by questionnaire and salivary cotinine in the general population of Barcelona, Spain (2004–2005) Prev Med. 2009;48:218–223. doi: 10.1016/j.ypmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Pirkle JL, Bernert JT, Caudill SP, et al. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schick S, Glantz SA. Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tob Control. 2006;15:424–429. doi: 10.1136/tc.2006.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Witschi H, Espiritu I, Peake JL, et al. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997;18:575–586. doi: 10.1093/carcin/18.3.575. [DOI] [PubMed] [Google Scholar]
- 58.Jinot J, Bayard SP, United States, Environmental Protection Agency, Office of Health and Environmental Assessment et al. Respiratory health effects of passive smoking: lung cancer and other disorders. Office of Health and Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency; Washington, DC: 1992. [Google Scholar]
- 59.Menezes RJ, Swede H, Niles R, et al. Regular use of aspirin and prostate cancer risk (United States) Cancer Causes Control. 2006;17:251–256. doi: 10.1007/s10552-005-0450-z. [DOI] [PubMed] [Google Scholar]
- 60.Mettlin C, Croghan I, Natarajan N, et al. The association of age and familial risk in a case–control study of breast cancer. Am J Epidemiol. 1990;131:973–983. doi: 10.1093/oxfordjournals.aje.a115617. [DOI] [PubMed] [Google Scholar]
- 61.Moysich KB, Mettlin C, Piver MS, et al. Regular use of analgesic drugs and ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:903–906. [PubMed] [Google Scholar]
- 62.Morabia A, Stellman SD, Wynder EL. Smoking prevalence in neighborhood and hospital controls: implications for hospital-based case–control studies. J Clin Epidemiol. 1996;49:885–889. doi: 10.1016/0895-4356(96)00026-1. [DOI] [PubMed] [Google Scholar]
- 63.Wynder EL, Stellman SD. The “over-exposed” control group. Am J Epidemiol. 1992;135:459–461. doi: 10.1093/oxfordjournals.aje.a116312. [DOI] [PubMed] [Google Scholar]


