Abstract
The recently published Rome III criteria reflect current understanding of functional gastrointestinal disorders. These criteria include definitions of these conditions and their pathophysiologic subtypes and offer guidelines for their management. At the 2006 Annual Scientific Meeting of the American College of Gastroenterology, a panel of experts discussed these criteria as they pertain to irritable bowel syndrome, functional dyspepsia, and chronic constipation. This article reviews the panel’s findings, highlights the differences between the Rome II and III criteria, and summarizes best treatment options currently available to practitioners and their patients.
Functional gastrointestinal (GI) disorders, such as irritable bowel syndrome (IBS), functional dyspepsia, and chronic constipation, pose an extensive healthcare burden and negatively affect quality of life. The total cost of caring for an IBS patient may reach thousands of dollars; in fact, symptoms of IBS are second only to the common cold for causing work absenteeism. 1 Similarly, patients and their insurers spend considerable amounts of money for the diagnosis and treatment of chronic constipation, an uncomfortable and distressing condition for which few satisfactory therapies are available.2,3
Over the past 15 years, the evolving definition of functional bowel disorders has been driven by advanced understanding of symptom patterns. Currently, functional GI disorders are classified into six major domains according to anatomical distribution, from the esophagus to anorectum. These disorders are believed to be caused by disturbed motor and sensory function, altered immune function and inflammation, and dysregulation of the central and enteric nervous systems. Consensus-based definitions spearheaded by the Rome committee for various functional GI disorders were launched in May of this year during Digestive Disease Week 2006.
During the annual scientific meeting of the American College of Gastroenterology, held in Las Vegas, Nevada, October 20–25, 2006, a panel of experts reviewed the Rome III criteria and their recommended therapeutic interventions. Participants included Nicholas J. Talley, MD, PhD, FACG, Professor of Medicine at the Mayo Clinic College of Medicine, Rochester; Minnesota; Charlene M. Prather, MD, MPH, Associate Professor of Internal Medicine at St. Louis University, Missouri; and Satish S.C. Rao, MD, PhD, FACG, Professor of Internal Medicine and Director of Neurogastroenterology and GI Motility at the University of Iowa Hospital and Clinic, Iowa City.
Irritable Bowel Syndrome
Irritable bowel syndrome affects about 10%–20% of adolescents and adults; it predominantly occurs in females. 4,5 As with other functional GI disorders, treating the symptoms of IBS is expensive in terms of dollars and patient discomfort and anguish.6,7
Rome III Criteria for IBS
Irritable bowel syndrome is characterized by abdominal pain or discomfort associated with disturbed defecation or a change in bowel habit (Table 1).8 Based upon bowel patterns at a particular point in time, the disorder may be categorized further into four groups (Table 2).8 An individual with symptoms that do not correlate with either diarrhea- or constipation-predominant IBS is categorized as having either mixed IBS (meeting criteria for both diarrhea- and constipation-predominant IBS) or unsubtyped IBS (insufficient abnormality of stool consistency to meet any of the three types). Alarming symptoms may suggest involvement of structural or biochemical abnormalities, yet such findings are not required as exclusionary criteria for the condition’s diagnosis.8
Table 1.
Rome III Diagnostic Criteria* for Irritable Bowel Syndrome
Recurrent abdominal pain or discomfort† more than 3 days per month over the previous 3 months associated with two or more of the following:
|
Symptom onset greater than 6 months prior to the diagnosis, with the above criteria fulfilled for the past 3 months
Discomfort means an uncomfortable sensation not described as pain
Adapted from Longstreth et al8
Table 2.
Irritable Bowel Syndrome (IBS) Subtypes by Predominant Stool Pattern
|
Bristol Stool Form Scale 1–2
Bristol Stool Form Scale 6–7
In the absence of antidiarrheals or laxatives
Adapted from Longstreth et al8
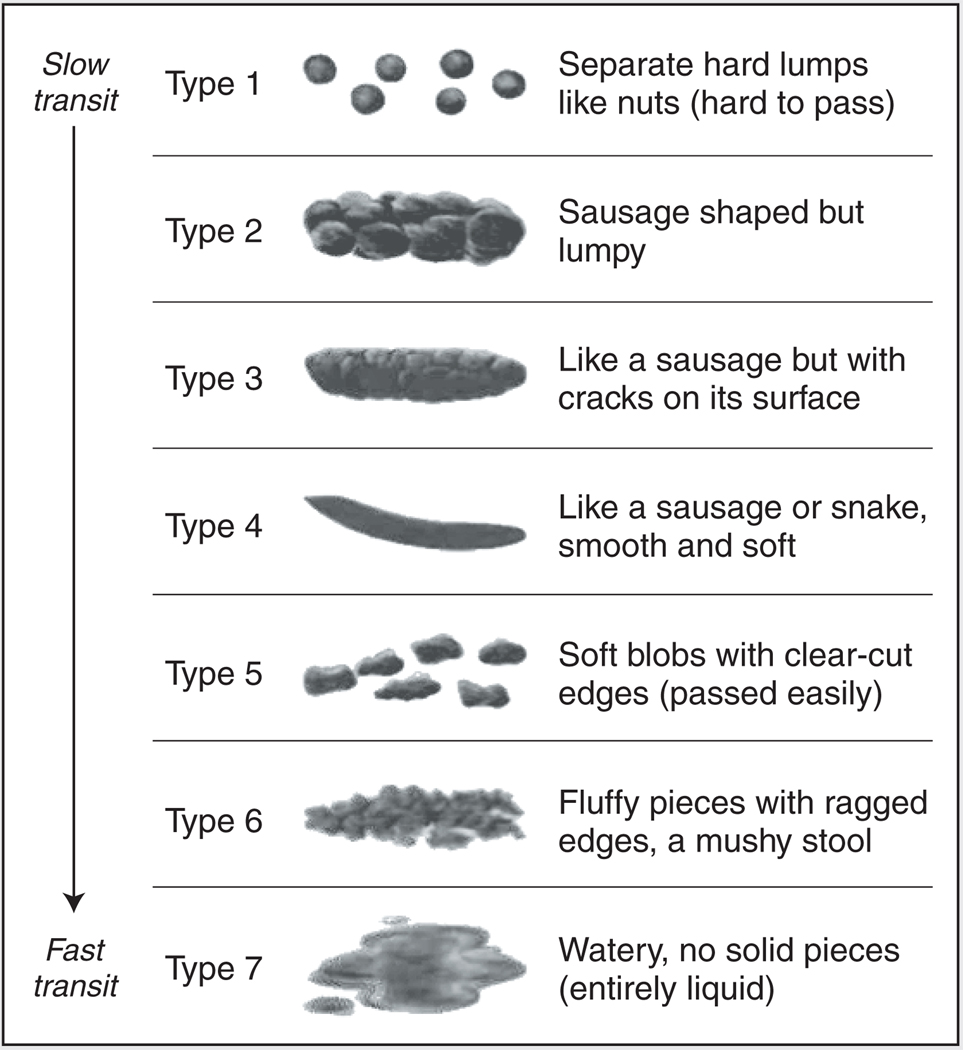
Historically, variations in patient and physician perception have made the diagnosis of “constipation” and “diarrhea” difficult. For example, straining to defecate may occur with soft or watery stools; on the other hand, the stool may be solid, yet defecation is frequent.9 Stool form reflects intestinal transit time, and the Rome committee recommends use of the Bristol Stool Form Scale to classify patients into the four IBS subtypes (Figure 1).8–10 This scale considers constipation to be related to IBS types 1 and 2 and diarrhea to be linked with IBS types 6 and 7.8,10
Figure 1.
Bristol Stool Form Scale. Adapted from Lewis and Heaton.10
Pathophysiology
Mechanisms that explain the presence of visceral hypersensitivity and GI motor disturbances in IBS are emerging. Some recent studies point to postinfectious changes, inflammation, bacterial overgrowth, and neurotransmitter alteration as potential mechanisms of IBS.
Postinfectious and Inflammatory IBS
IBS may follow a bout of bacterial gastritis. For example, Marshall and others11 reported a higher incidence of IBS among individuals exposed to municipal water contamination than among controls (27.5% vs 10.1%, respectively; P < 0.01). Furthermore, the incidence of IBS was even higher among individuals with clinically documented gastroenteritis than among controls (36.2% vs 10.1%; P < 0.01). Mearin et al12 found that the relative risk of developing IBS after Salmonella gastroenteritis increased by eightfold over the subsequent year, thereby supporting the role of postinfectious gastroenteritis in the development of IBS.
Some IBS patients also have subtle gut mucosal inflammation and immune activation. For example, an increase in mast cells, neutrophils, natural killer cells, eosinophils, and intraepithelial lymphocytes has been recorded among individuals with documented postinfectious and non-postinfectious IBS when compared with controls.13,14 Such increased immune activation also is manifested as a shift toward inflammatory cytokine profiles with an abnormal interleukin (IL)-10/IL-12 ratio and an increased level of IL-1β.15,16
Bacterial Overgrowth
The GI motor disturbances seen in IBS may lead to qualitative or quantitative changes in bacterial flora that promote bacterial overgrowth and increase bacterial fermentation/gas. This production of excess gas induces such symptoms as abdominal discomfort and bloating.17
The association between bacterial overgrowth and IBS is supported by studies showing improved IBS symptoms after antibiotic treatment. For example, a recent double-blind, randomized, placebo-controlled study by Pimentel et al18 assigned participants meeting the Rome I IBS criteria to receive either 400 mg of rifaximin three times daily for 10 days or placebo. The rifaximin-treated patients achieved a significant global IBS symptom improvement (36%) when compared with the placebo group (21%).
Promising evidence supports the role of bacterial overgrowth in IBS and eradication of such colonies for management of the disorder. However, the expert panel warned physicians to accept this approach cautiously, noting that more work to confirm these observations is needed.
Neurotransmitter Alteration and IBS
Alterations in neurotransmitters may lead to visceral hypersensitivity and GI motor disturbances. In particular, serotonin, or 5-hydroxytryptamine (5-HT), and its effects on the GI system are the subject of active research. Biologically, alterations in the levels or sensing mechanism of serotonin may lead to IBS, since this neurotransmitter is a major regulator of the peristaltic reflex and sensory relays in the gut.19
Two studies support this claim. Whereas Dunlop’s team20 reported that the release of serotonin fell among patients with constipation-predominant IBS and rose in individuals with the diarrhea-predominant form of the disorder, Coates et al21 showed that IBS patients exhibited lower levels of gut mucosal serotonin and serotonin reuptake transporter than did controls.
Updates in IBS Treatment
Initial IBS management includes education, reassurance, and investigation of psychosocial issues.21
Helping Patients Help Themselves
In a randomized study, Robinson’s team22 found that introducing a self-help guidebook to IBS patients resulted in a 60% reduction in their primary-care consultations (P < 0.001) and a drop in their perceived symptom severity (P < 0.001) when compared with controls.
The expert panel recommended the following resources for IBS patients:
Pharmacologic Therapy
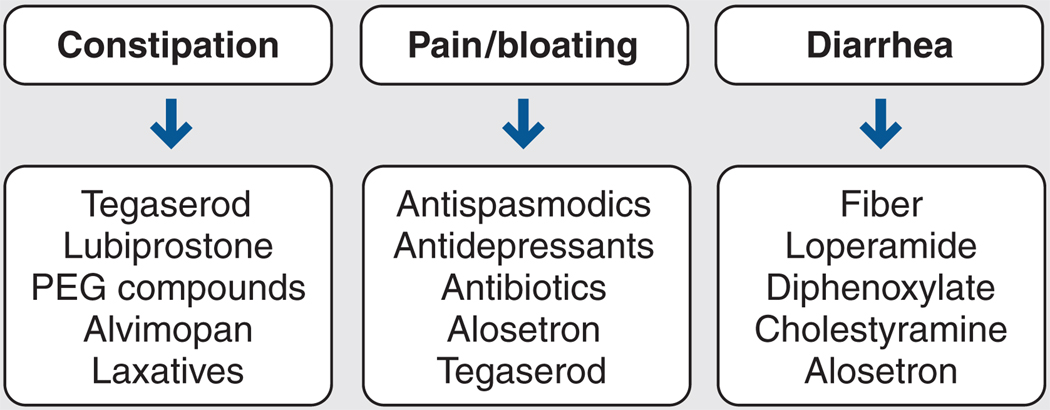
Drug therapy mainly targets IBS symptoms. The types of drugs available are divided into three main categories (Figure 2).23
Figure 2.
Pharmacologic therapy for irritable bowel syndrome. PEG = polyethylene glycol. Adapted from Prather.23
Fiber supplements
According to anecdotal reports, some patients with diarrhea experience a firming of stools after using fiber supplements; however, some studies showed no clear benefit with use of these products.24–26 When prescribing a fiber supplement to patients with diarrhea, start at a low dose and progressively titrate the dose up as tolerated to minimize the bloating that patients frequently experience.
Loperamide
Loperamide is a µ-opioid-receptor agonist that reduces intestinal secretion, slows colonic transit, and increases resting anal sphincter tone.27 When compared with placebo, loperamide reduces diarrhea in IBS patients with this predominant symptom.25,26
Opioid-receptor activation reduces visceral pain via peripheral (spinal afferents) and central mechanisms; however, loperamide has not been shown to alter abdominal pain or other IBS symptoms when compared with placebo.25,26
Alosetron
5-HT plays an important role in visceral sensitivity, gut absorption/secretion, and motility.19 The 5-HT type 3 (5-HT3) receptor antagonists may be useful in reducing colonic transit time and in treating diarrhea.
There is convincing evidence that a drug in this class, alosetron, may delay colonic transit time.28,29 A meta-analysis of randomized trials showed that alosetron is effective against diarrhea-predominant IBS.30 However, because of its suspected side effects (eg, ischemic colitis, colonic ischemia), this drug is only approved for restricted use.31,32 Recently, a meta-analysis showed that the incidence of ischemic colitis related to use of the drug is low (1.1 per 1,000 patient-years of alosetron use) and is rarely associated with long-term sequelae or serious morbidity.33
Functional Dyspepsia
Functional dyspepsia is a clinical syndrome characterized by chronic or recurrent upper abdominal pain or discomfort having no identifiable cause.34 This disorder is not a monosymptomatic entity; several predominant symptoms may be present, including epigastric pain (22%), abdominal fullness (24%), bloating (15%), vomiting (3%), belching (8%), early satiety (12%), nausea (10%), and heartburn (6%).35 These predominant symptoms change over time, making functional dyspepsia difficult to define and classify.34
Rome III Criteria for Functional Dyspepsia
After reviewing available evidence, the Rome committee proposed updated diagnostic criteria for functional dyspepsia. As shown in Table 3,34 the Rome III criteria definition of functional dyspepsia includes at least one of the following: bothersome postprandial fullness, early satiation, epigastric pain, and epigastric burning; patients also must have no evidence of structural disease that would likely explain their symptoms, including upper endoscopic findings. The patient must meet the criteria for 3 months and must begin experiencing symptoms for at least 6 months before diagnosis.34
Table 3.
Rome III Diagnostic Criteria for Functional Dyspepsia
Symptom onset greater than 6 months prior to the diagnosis, with the following criteria fulfilled for the past 3 months:
|
| Postprandial Distress Syndrome |
At least one of the following:
|
| Epigastric Pain Syndrome |
Must include all of the following:
|
Adapted from Tack et al34
Functional dyspepsia may be divided into postprandial distress syndrome (characterized by postprandial fullness and/or early satiation) and epigastric pain syndrome (characterized by epigastric pain). Dyspepsia is considered to be a continuum of various gastric complaints; thus, a diagnosis of functional dyspepsia does not preclude the inclusion of gastroesophageal reflux disease (GERD) and/or IBS in its symptom complex.34
Updates in Treatment of Functional Dyspepsia
Initial management of functional dyspepsia includes reassurance, education, smoking cessation, consumption of several small and low-fat meals each day, and avoidance of coffee, alcohol, and nonsteroidal anti-inflammatory agents; however, no evidence exists that these interventions are effective.36 Other treatment modalities include psychotherapy, cognitive-behavioral therapy, and hypnotherapy; although a few studies have shown benefit of psychological therapy in functional dyspepsia,37 additional studies must be done to establish its efficacy.
Medical treatment options for functional dyspepsia remain limited, and available studies do not address the newly defined subgroups of epigastric pain syndrome and postprandial distress syndrome. In addition, up to 60% of patients respond to placebo, which further limits tests of pharmaceutical effectiveness in this patient population.38
Gastric Acid Suppression and Helicobacter pylori Eradication
Several lines of compelling evidence point to gastric acid suppression as the first-line drug therapy for functional dyspepsia. A Cochrane systematic review39 showed histamine-2 (H2)-receptor antagonists and proton-pump inhibitors to be superior to placebo in managing the disorder. Another meta-analysis of controlled, randomized trials40 both confirmed this finding and showed acid suppression to be cost-effective in treating functional dyspepsia. The benefit of acid suppression against functional dyspepsia may be related to unrecognized GERD or nonerosive reflux disease.40–42
Eradication of H pylori also appears to help treat functional dyspepsia. Another Cochrane systematic review43 concluded that H pylori eradication therapy has a statistically significant effect in treating H pylori-positive, nonulcer dyspepsia, and an economic model suggested that H pylori eradication also may be cost-effective. Thus, H pylori eradication is recommended for functional dyspepsia.
Antidepressants
Few studies have investigated the effectiveness of antidepressants against functional dyspepsia. A small, randomized, crossover trial of seven patients showed that 50 mg of amitriptyline increased tolerance to aversive visceral sensations when compared with placebo administration.44
Despite the limited data, treatment with tricyclic antidepressants (eg,10–25 mg of imipramine or desipramine at night) or a selective serotonin reuptake inhibitor (eg, 10 mg of escitalopram or 20 mg of sertraline in the morning) is recommended after standard therapy with a proton-pump inhibitor or H pylori eradication fails.45
Prokinetic Agents
Prokinetic agents, such as metoclopramide, domperidone, and cisapride, reduce symptoms of functional dyspepsia. These drugs have proven more effective than has placebo against the disorder, especially in patients presenting with predominant symptoms of fullness, bloating, or nausea.39,46
However, patients must be monitored closely when using these agents. For example, metoclopramide must be used cautiously in the elderly because of undesired side effects (eg, tardive dyskinesia).47 Domperidone is not available in the United States because of its risk of cardiotoxicity48; cisapride was withdrawn from the US market because it caused QT-interval prolongation and rare cases of fatal arrhythmia.49
5-HT Agonists/Antagonists
The 5-HT pathway is important to visceral sensitivity, so its alteration may treat functional dyspepsia effectively.27 One randomized, controlled trial showed that alosetron caused significantly greater reductions in the severity and frequency of functional dyspepsia symptoms than did placebo (P < 0.001).50
Because a link between alosetron and ischemic colitis was suggested, the drug is not used for functional dyspepsia; however, no causal relationship between the two has been established.51 However, a recent meta-analysis concluded that alosetron use is rarely associated with serious morbidity and is associated with a low incidence of ischemic colitis (1.1 cases per 1,000 patient-years of alosetron use).33
Chronic Constipation and Functional Anorectal Disorders
There have been advances in the understanding of symptom patterns of chronic constipation and functional anorectal disorders, and updated criteria have been based on new scientific evidence. These criteria are designed to enhance clinical recognition and develop better scientific understanding of these disorders and to standardize their management.
Rome III Criteria for Functional Constipation
Functional constipation presents as persistently difficult, infrequent, and seemingly incomplete defecation that does not fulfill the IBS criteria. The subjective and objective criteria for functional constipation are summarized in Table 4.8
Table 4.
Rome III Diagnostic Criteria for Functional Constipation
Symptom onset more than 6 months prior to the diagnosis, with the following criteria fulfilled for the past 3 months:
|
Adapted from Longstreth et al8
A comparison of the Rome II criteria and the Rome III criteria shows two main changes. First, the more recent criteria are consistent with those for other functional bowel disorders, as the frequency of bowel movements now is > 25% instead of ≥ 25%. Second, the newer criteria permit laxative-induced loose stools when treating functional constipation, because studies using Rome II criteria yielded a lower prevalence of the disorder than did those using Rome I.52
Functional Anorectal Disorders
Functional anorectal disorders include fecal incontinence, anorectal pain, and disorders of defecation; they are defined by specific symptoms, as shown in Table 5.53
Table 5.
Functional Anorectal Disorders
| Functional fecal incontinence |
| Functional anorectal pain |
|
| Functional defecation disorders |
|
Adapted from Bharucha et al53
Rome III Criteria for Functional Fecal Incontinence
Functional fecal incontinence is defined as uncontrolled leakage of fecal material for at least 3 months in an individual over 4 years of age (Table 6).53 Because it is unclear when the passage of flatus is abnormal, leakage of flatus alone should not be considered a symptom of fecal incontinence.
Table 6.
Rome III Diagnostic Criteria for Functional Fecal Incontinence
Recurrent uncontrolled passage of fecal material in a patient at least 4 years of age and more than one of the following:
|
| Symptoms must persist for over 3 months |
Exclusion of all of the following:
|
Adapted from Bharucha et al53
In general, the spectrum of functional incontinence is broader than that of fecal incontinence. According to the Rome III criteria, functional fecal incontinence may be associated with such organic disorders as dementia, multiple sclerosis, and Crohn’s disease, because the relationship between structural disturbances and the disorder is unclear and asymptomatic patients may have small anal sphincter defects. However, if anal sphincter electromyography demonstrates abnormal innervation (eg, pudendal neuropathy), organic diseases that may lead to denervation/ reinnervation changes (eg, dementia, multiple sclerosis, and diabetes) are excluded as the cause of incontinence.53
Chronic Proctalgia
Chronic proctalgia and proctalgia fugax are the two functional anorectal pain disorders. Chronic proctalgia may be broken down into levator ani syndrome (tenderness during posterior traction on the puborectalis muscle) or unspecified anorectal pain, depending upon the presence or absence of puborectalis tenderness during digital rectal examination (Table 7).53 The distinction between these two types is emphasized by modified nomenclature featured in the Rome III criteria.
Table 7.
Rome III Diagnostic Criteria for Chronic Proctalgia
Symptom onset more than 6 months prior to the diagnosis, with all of the following criteria fulfilled for the past 3 months:
|
| Levator Ani Syndrome |
| Fulfilled criteria for chronic proctalgia and tenderness during posterior traction on the puborectalis |
| Unspecified Functional Anorectal Pain |
| Fulfilled criteria for chronic proctalgia but no tenderness during posterior traction on the puborectalis |
Adapted from Bharucha et al53
Proctalgia Fugax
Proctalgia fugax is distinguished from chronic proctalgia by the duration, frequency, and character of the pain. This disorder is manifested as a sudden, severe pain in the anal area that lasts several seconds to minutes and then disappears completely (Table 8).8
Table 8.
Rome III Diagnostic Criteria for Proctalgia Fugax
Must include all of the following criteria:
|
| For research purposes: criteria must be fulfilled for 3 months |
| Clinical practice: diagnosis and evaluation may be made before 3 months |
Adapted from Longstreth et al8
For research purposes, the diagnostic criteria for proctalgia fugax must be fulfilled for 3 months or more. However, in clinical practice, its diagnosis, evaluation, and treatment may take place before 3 months pass.53
Functional Defecation Disorders
Functional defecation disorders are defined as paradoxical contraction or inadequate relaxation of the pelvic floor muscles along with fulfillment of the Rome III criteria for functional constipation (Table 9).53 This anorectal disorder may be broken down further as either dyssynergic defecation (inappropriate pelvic floor contraction or sphincter relaxation with adequate propulsive forces during attempted defecation) or inadequate defecatory propulsion (inadequate propulsive forces during attempted defecation with or without dyssynergic defecation).
Table 9.
Rome III Diagnostic Criteria for Functional Defecation Disorders
Symptom onset more than 6 months prior to the diagnosis, with all of the following criteria fulfilled for the past 3 months:
|
| Dyssynergic Defecation |
| Inappropriate contraction of the pelvic floor or less than 20% relaxation of basal resting sphincter pressure with adequate propulsive forces during attempted defecation |
| Inadequate Defecatory Propulsion |
| Inadequate propulsive forces or less than 20% relaxation of the anal sphincter during attempted defecation. |
Adapted from Bharucha et al53
According to both the Rome II and Rome III criteria, the diagnosis for functional defecation disorders requires both abnormal diagnostic test results and the presence of defecation symptoms53; a list of symptoms alone does not distinguish patients with functional defecatory disorders from those with functional constipation. Further, the diagnostic criteria for dyssynergia also are the same for both maladies. However, studies using balloon expulsion and rectal barium evacuation showed that inadequate rectal propulsive force causes impaired evacuation54,55; thus, the revised criteria highlight the possibility that functional defecation disorders may be caused by inadequate propulsive forces.
Updates in Treatment of Chronic Constipation and Functional Anorectal Disorders
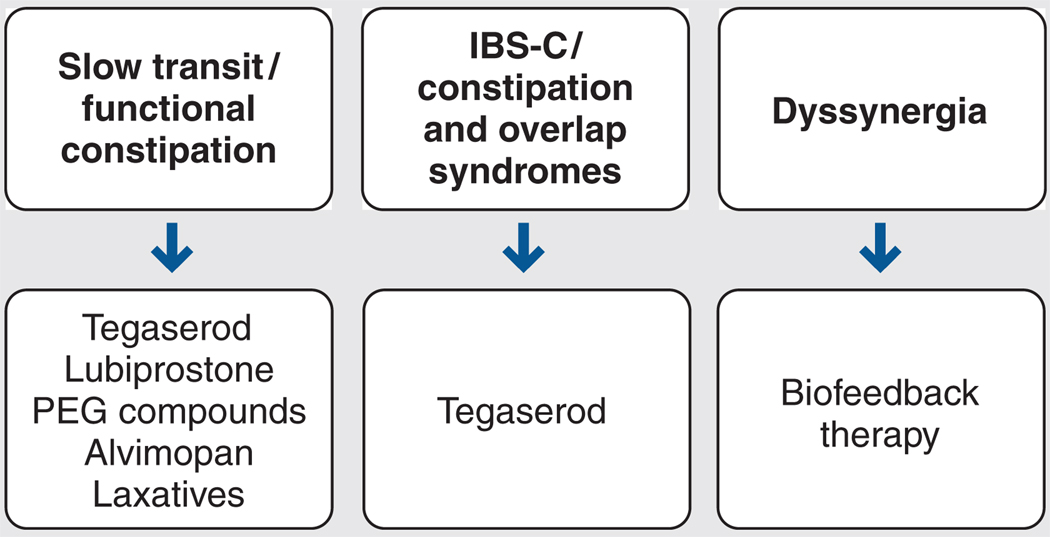
As illustrated in Figure 3,56 a pathophysiologic approach to managing chronic constipation is recommended. Classes of medications marketed to manage chronic constipation include bulking agents, osmotic and stimulatory laxatives, stool softeners, lubricants, and newer receptor-based therapies (eg, tegaserod, lubiprostone, and alvimopan). However, patients with dyssynergic defecation may benefit more from biophysical therapies than from pharmacotherapy.
Figure 3.
Pathophysiology-based treatment for chronic constipation. IBS-C = constipation-predominant irritable bowel syndrome; PEG = polyethylene glycol. Adapted from Rao.56
The literature shows no clear benefit when chronically constipated patients used bulking agents (eg, psyllium, calcium polycarbophil, methylcellulose, bran, aloe vera),24 stimulant laxatives (eg, bisacodyl, senna),57 or stool softeners (eg, docusate sodium, docusate calcium).57,58 However, several high-quality, placebo-controlled, randomized trials have shown osmotic laxatives (eg, polyethylene glycol solutions) and lactulose to be effective in this population.57,59,60
Serotonin-Receptor Agonists/Antagonists
Approximately 90% of serotonin is found within the enterochromaffin cells of the GI tract.61 When 5-HT interacts with 5-HT3 and 5-HT type 4 (5-HT4) receptors, it promotes peristalsis and modulates fluid content of the stool and visceral sensation via 5-HT4 receptors.62 Such findings resulted in the development of 5-HT4 receptor agonists.
Tegaserod, a partial 5-HT4 receptor agonist, was approved by the US Food and Drug Administration (FDA) for long-term treatment of chronic idiopathic constipation in men and women younger than 65 years of age. Kamm and others63 performed a placebo-controlled, randomized clinical trial that compared placebo with 2 mg and 6 mg of tegaserod given twice daily to patients with chronic constipation (defined as fewer than three complete spontaneous bowel movements per week). At 4 weeks, a significantly greater number of spontaneous bowel movements were noted among patients receiving 2 mg (41.4%) or 6 mg (43.2%) of tegaserod twice daily than among the placebo group (25.1%; P < 0.0001). These results were confirmed by a similarly designed, international, multi-center study.63 The high-quality designs of such trials and reproducibility of the findings led to tegaserod receiving a “grade A” recommendation for treatment of chronic constipation from two systematic reviews.57,58
Chloride-Channel Activators
Chloride channels are located on the apical surfaces of the gut mucosal epithelial cells. As negatively charged chloride ions enter the intestinal lumen through these channels, so do sodium ions and water passively to maintain isoelectric neutrality.64
Lubiprostone, a bicyclic fatty acid, is the first chloride-channel activator to be approved by the FDA. In a randomized clinical trial that compared placebo with 24 µg of lubiprostone given twice daily to patients with chronic constipation for up to 4 weeks, Johanson’s team65 found the drug to effectively increase the number of spontaneous bowel movements, decrease straining, and improve stool consistency when compared with placebo (P < 0.002 for all outcome measurements). Johanson et al66 also showed that lubiprostone improved abdominal bloating, discomfort, and severity of constipation when compared with baseline examination for up to 24 weeks (P < 0.001).
Adverse effects associated with lubiprostone therapy included nausea, diarrhea, headache, and abdominal pain.
Peripheral µ-Opioid Antagonists
Another class of medications being tested against chronic constipation, peripheral µ-opioid antagonists may be particularly useful in managing opioid-induced constipation. In initial physiologic studies, a drug in this class, alvimopan, accelerated whole gut transit time and reversed the constipating and gut-slowing effects of codeine phosphate when compared with placebo at 24 and 48 hours (P < 0.05).67
Biofeedback Therapies
Functional defecation disorders also may be managed by pelvic floor training or biofeedback therapies.68–71 Chiarioni and others72 recently reported that biofeedback sessions were more effective than were continuous polyethylene glycol for treating pelvic floor dyssynergia (80% vs 22% improvement, respectively; P < 0.001), noting that the benefits of biofeedback persisted for at least 2 years. Furthermore, this method also produced greater reductions in straining, sensations of incomplete evacuation and anorectal blockage, use of enemas and suppositories, and abdominal pain (P < 0.01 for all parameters). The superiority of biofeedback therapy to alternative treatments for patients with pelvic floor dyssynergia-type constipation also was confirmed by two other randomized controlled trials.73,74
Conclusion
The symptoms of functional bowel disorders vary and may include both GI and extraintestinal complaints. Alarm symptoms suggest the possibility of structural disease, but they do not exclude a diagnosis of functional bowel disorder. Thus, the patient’s diagnosis should be based on a symptom complex that fulfills the accepted Rome criteria.
Over the past 15 years, the definition of functional bowel disorder has evolved with advances in the understanding of symptom patterns; currently, this knowledge is reflected in the Rome III criteria. Historically, management of functional bowel disorders has been frustrating; today, however, it is possible to design effective therapies using a pathophysiologic approach.
The cure of functional bowel disorders ultimately requires scrutiny of basic research results to better understand the underlying pathogenesis. With time, evidence-based therapy will help us to specifically target these physical conditions—and not just their symptoms.
Acknowledgments
Dr. Shih is supported by a gastroenterology training grant (T32 DK07180-30) from the National Institutes of Health and by the Specialty Training and Advanced Research (STAR) Program at the University of California, Los Angeles.
Biographies
Dr. Shih is a Gastroenterology Fellow at Cedars-Sinai Inflammatory Bowel Disease Center and the University of California, Los Angeles.

Dr. Kwan is a Resident in Internal Medicine at Cedars-Sinai Medical Center, Los Angeles, California.

References
- 1.Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci. 1989;34:606–611. doi: 10.1007/BF01536339. [DOI] [PubMed] [Google Scholar]
- 3.Rantis PC, Jr, Vernava AM, 3rd, Daniel GL, Longo WE. Chronic constipation—is the work-up worth the cost? Dis Colon Rectum. 1997;40:280–286. doi: 10.1007/BF02050416. [DOI] [PubMed] [Google Scholar]
- 4.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 5.Longstreth GF. Definition and classification of irritable bowel syndrome: current consensus and controversies. Gastroenterol Clin North Am. 2005;34:173–187. doi: 10.1016/j.gtc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, Longstreth GF, Knight K, et al. Quality of life in managed care patients with irritable bowel syndrome. Manag Care Interface. 2004;17:24–28. 34. [PubMed] [Google Scholar]
- 7.Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98:600–607. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300:439–440. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery Walkerton Health Study Investigators. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Mearin F, Perez-Oliveras M, Perello A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Quigley EM. Changing face of irritable bowel syndrome. World J Gastroenterol. 2006;12:1–5. doi: 10.3748/wjg.v12.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 16.Shih DQ, Targan SR. The immunological basis of inflammatory bowel disease. World J Gastroenterol. 2006 In press. [Google Scholar]
- 17.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 19.Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358:2061–2068. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- 20.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 21.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Robinson A, Lee V, Kennedy A, et al. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of irritable bowel syndrome. Gut. 2006;55:643–648. doi: 10.1136/gut.2004.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prather CM. Irritable bowel syndrome; Presented at the 2006 American College of Gastroenterology Annual Scientific Meeting; Las Vegas, Nevada. 2006. Oct 25, [Google Scholar]
- 24.Jones MP, Talley NJ, Nuyts G, Dubois D. Lack of objective evidence of efficacy of laxatives in chronic constipation. Dig Dis Sci. 2002;47:2222–2230. doi: 10.1023/a:1020131126397. [DOI] [PubMed] [Google Scholar]
- 25.Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003460.pub2. CD003460. [DOI] [PubMed] [Google Scholar]
- 26.Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum AL. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:1253–1269. doi: 10.1111/j.1365-2036.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Bueno L, de Ponti F, Fioramonti J, Lydiard RB, Tack J. Pharmacological and pharmacokinetic aspects of functional gastrointestinal disorders. Gastroenterology. 2006;130:1421–1434. doi: 10.1053/j.gastro.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 28.Talley NJ, Phillips SF, Haddad A, et al. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci. 1990;35:477–480. doi: 10.1007/BF01536922. [DOI] [PubMed] [Google Scholar]
- 29.Gore S, Gilmore IT, Haigh CG, Brownless SM, Stockdale H, Morris AI. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther. 1990;4:139–144. doi: 10.1111/j.1365-2036.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 30.Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15:79–86. doi: 10.1046/j.1365-2982.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 31.Beck IT. Possible mechanisms for ischemic colitis during alosetron therapy. Gastroenterology. 2001;121:231–232. doi: 10.1053/gast.2001.26046. [DOI] [PubMed] [Google Scholar]
- 32.Callahan MJ. Irritable bowel syndrome neuropharmacology: a review of approved and investigational compounds. J Clin Gastroenterol. 2002;35:S58–S67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]
- 33.Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069–1079. doi: 10.1111/j.1572-0241.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 34.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 35.Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 2006;130:296–303. doi: 10.1053/j.gastro.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Talley NJ, Weaver AL, Zinsmeister AR. Smoking, alcohol, and nonsteroidal anti-inflammatory drugs in outpatients with functional dyspepsia and among dyspepsia subgroups. Am J Gastroenterol. 1994;89:524–528. [PubMed] [Google Scholar]
- 37.Calvert EL, Houghton LA, Cooper P, Morris J, Whorwell PJ. Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology. 2002;123:1778–1785. doi: 10.1053/gast.2002.37071. [DOI] [PubMed] [Google Scholar]
- 38.Veldhuyzen van Zanten SJ, Cleary C, Talley NJ, et al. Drug treatment of functional dyspepsia: a systematic analysis of trial methodology with recommendations for design of future trials. Am J Gastroenterol. 1996;91:660–673. [PubMed] [Google Scholar]
- 39.Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, Forman D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD001960. CD001960. [DOI] [PubMed] [Google Scholar]
- 40.Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329–1337. doi: 10.1053/j.gastro.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Tack J, Caenepeel P, Arts J, Lee KJ, Sifrim D, Janssens J. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut. 2005;54:1370–1376. doi: 10.1136/gut.2004.053355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talley NJ, Meineche-Schmidt V, Pare P, et al. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies) Aliment Pharmacol Ther. 1998;12:1055–1065. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 43.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD002096.pub2. CD002096. [DOI] [PubMed] [Google Scholar]
- 44.Mertz H, Fass R, Kodner A, Yan-Go F, Fullerton S, Mayer EA. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol. 1998;93:160–165. doi: 10.1111/j.1572-0241.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 45.Talley NJ. Dyspepsia. Gastroenterology. 2003;125:1219–1226. doi: 10.1016/s0016-5085(03)01245-9. [DOI] [PubMed] [Google Scholar]
- 46.Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689–696. doi: 10.1111/j.1572-0241.2001.03521.x. [DOI] [PubMed] [Google Scholar]
- 47.Pasricha PJ, Pehlivanov N, Sugumar A, Jankovic J. Drug insight: from disturbed motility to disordered movement—a review of the clinical benefits and medicolegal risks of metoclopramide. Nat Clin Pract Gastroenterol Hepatol. 2006;3:138–148. doi: 10.1038/ncpgasthep0442. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad N, Keith-Ferris J, Gooden E, Abell T. Making a case for domperidone in the treatment of gastrointestinal motility disorders. Curr Opin Pharmacol. 2006 doi: 10.1016/j.coph.2006.07.004. In press. [DOI] [PubMed] [Google Scholar]
- 49.Craig JS. Cisapride and intestinal dysmotility: the final word? Biol Neonate. 2005;88:276–277. doi: 10.1159/000087624. [DOI] [PubMed] [Google Scholar]
- 50.Talley NJ, Van Zanten SV, Saez LR, et al. A dose-ranging, placebo-controlled, randomized trial of alosetron in patients with functional dyspepsia. Aliment Pharmacol Ther. 2001;15:525–537. doi: 10.1046/j.1365-2036.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 51.Gallo-Torres H, Brinker A, Avigan M. Alosetron: ischemic colitis and serious complications of constipation. Am J Gastroenterol. 2006;101:1080–1083. doi: 10.1111/j.1572-0241.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- 52.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235. doi: 10.1023/a:1013208713670. [DOI] [PubMed] [Google Scholar]
- 53.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 54.Halligan S, Thomas J, Bartram C. Intrarectal pressures and balloon expulsion related to evacuation proctography. Gut. 1995;37:100–104. doi: 10.1136/gut.37.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 56.Rao S. Constipation and pelvic floor disorders; Presented at the 2006 American College of Gastroenterology Annual Scientific Meeting; Las Vegas, Nevada. 2006. Oct 25, [Google Scholar]
- 57.Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100 suppl 1:S5–S21. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- 58.Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 59.Freedman MD, Schwartz HJ, Roby R, Fleisher S. Tolerance and efficacy of polyethylene glycol 3350/electrolyte solution versus lactulose in relieving opiate induced constipation: a double-blinded placebo-controlled trial. J Clin Pharmacol. 1997;37:904–907. doi: 10.1002/j.1552-4604.1997.tb04264.x. [DOI] [PubMed] [Google Scholar]
- 60.Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999;44:226–230. doi: 10.1136/gut.44.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn J, Ehrenpreis ED. Emerging treatments for irritable bowel syndrome. Expert Opin Pharmacother. 2002;3:9–21. doi: 10.1517/14656566.3.1.9. [DOI] [PubMed] [Google Scholar]
- 62.Cash BD, Chey WD. Review article: the role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment Pharmacol Ther. 2005;22:1047–1060. doi: 10.1111/j.1365-2036.2005.02696.x. [DOI] [PubMed] [Google Scholar]
- 63.Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. doi: 10.1111/j.1572-0241.2005.40749.x. [DOI] [PubMed] [Google Scholar]
- 64.Basavappa S, Vulapalli SR, Zhang H, Yule D, Coon S, Sundaram U. Chloride channels in the small intestinal cell line IEC-18. J Cell Physiol. 2005;202:21–31. doi: 10.1002/jcp.20085. [DOI] [PubMed] [Google Scholar]
- 65.Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Phase III efficacy and safety of RU-0211, a novel chloride channel activator, for the treatment of constipation. Gastroenterology. 2003;124:A-48. [Google Scholar]
- 66.Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Multicenter open-label study of oral lubiprostone for the treatment of chronic constipation: phase III patient assessments of the effects of lubiprostone, chloride channel-2 (CIC-2) activator, for the treatment of constipation. Am J Gastroenterol. 2005;100:S331. Abstract 903. [Google Scholar]
- 67.Gonenne J, Camilleri M, Ferber I, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:784–791. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- 68.Kawimbe BM, Papachrysostomou M, Binnie NR, Clare N, Smith AN. Outlet obstruction constipation (anismus) managed by biofeedback. Gut. 1991;32:1175–1179. doi: 10.1136/gut.32.10.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bleijenberg G, Kuijpers HC. Biofeedback treatment of constipation: a comparison of two methods. Am J Gastroenterol. 1994;89:1021–1026. [PubMed] [Google Scholar]
- 70.Cox DJ, Sutphen J, Borowitz S, Dickens MN, Singles J, Whitehead WE. Simple electromyographic biofeedback treatment for chronic pediatric constipation/encopresis: preliminary report. Biofeedback Self Regul. 1994;19:41–50. doi: 10.1007/BF01720669. [DOI] [PubMed] [Google Scholar]
- 71.Rao SS, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Dig Dis Sci. 1997;42:2197–2205. doi: 10.1023/a:1018846113210. [DOI] [PubMed] [Google Scholar]
- 72.Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Rao SS, Kincade KJ, Schulze KS, et al. Biofeedback therapy for dyssynergic constipation: randomized controlled trial. Gastroenterology. 2005;128:A268. [Google Scholar]
- 74.Heymen S, Scarlett Y, Jones K, Drossman D, Ringel Y, Whitehead WE. Randomized controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Am J Gastroenterol. 2005;100:S335. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]