Abstract
Context
Pain problems are exceedingly prevalent among psychiatric patients. Moreover, clinical impressions and neurobiological research suggest that physical and psychological aspects of pain are closely related entities. Nonetheless, remarkably few pain-related themes are presently included in psychiatric residency training.
Objective
Our objective is twofold: (1) to provide clinical and scientific rationale for psychiatric training enrichment with basic tenets of pain medicine and (2) to raise the awareness and sensitivity of clinicians, scientists and educators alike to the important yet unmet clinical and public health need.
Results
Three lines of translational research evidence, extracted from the comprehensive literature review, are presented in support of the objective. First, the neuroanatomical and functional overlap between pain and emotion/reward/motivation brain circuits suggests integration and mutual modulation of these systems. Second, psychiatric disorders are commonly associated with alterations in pain processing, whereas chronic pain may impair emotional and neurocognitive functioning. Third, pain may serve as a functional probe for unraveling pathophysiological mechanisms inherent in psychiatric morbidity given its stressful nature for the organism.
Conclusions
Pain training in psychiatry will not only contribute to deeper and more sophisticated insights into pain syndromes but also into psychiatric morbidity at large regardless of patients’ pain status. Furthermore, it will ease artificial boundaries separating psychiatric and medical formulations of brain disorders, thus fostering cross-fertilizing interactions between specialists in various disciplines entrusted with the care of pain patients.
Keywords: Pain, stress, reward, opioid, dopamine, nucleus accumbens, depression, anxiety, post-traumatic, borderline personality, comorbidity, education, interdisciplinary
Introduction
Chronic pain is a public health problem of pandemic proportions that afflicts over 70 million Americans 1, 2 and remains the most common complaint brought to the attention of health care practitioners 3. Its annual cost to the American society is staggering and is estimated to be around $100 billion owing to medical costs and loss of earnings and productivity 4. The current demographic trends 5 and the tremendous shortage of pain experts 6 forecast the need for even greater attention to pain from the medical establishment in consequence of the substantial rise in the overall proportion of geriatric population that is naturally at a heightened risk for the development of pain-related conditions 7. Specifically, 50% of community-dwelling elders and up to 80% of nursing home residents are afflicted with chronic pain 8. The rates of Americans over the age of 65 has increased steadily due to the rise in life expectancy and decline in fertility from 4% at the beginning of the 20th century to 12% nowadays with projected over 20% (70 million) over the next 25 years 9. Pain is exceedingly prevalent in psychiatric samples 10 and since more people will be suffering from pain so will psychiatric patients 11.
One of the most daunting challenges faced by psychiatric practitioners is how to distinguish and approach physical and emotional symptoms arising in the context of pain experienced by their patients. We respond to this question by proposing to integrate fundamental tenets of pain medicine within the core psychiatric residency training curriculum 12. Besides altered pain function 13, 14, psychiatric patients can be noncompliant with pain treatment and additionally develop co-morbid addiction to opiate analgesics 15. On the other hand, chronic pain is frequently associated with anxiety and depressive symptoms 16 as well as with degenerative cortical changes that may further deteriorate affective states and cognition 17, 18.
Sensory areas are recruted by both acute and chronic pain, but the latter is a more complex phenomenon engaging a broader stress-related neural network with emotional, motivational and cognitive components 19 that converge on circuits such as the mesocorticolimbic dopaminergic and endogenous opioid, involved in salience/reward/motivational mechanisms in addition to responses to pain, more generally stressors and their regulation 20. With this premise of neurobiological overlap between the processing of pain and of other stressful and emotionally salient signals, the present paper considers the aspects of pain that are related to stress, reward and motivational regulation and are pertinent to psychiatric morbidity. First, we compare data on the neural mechanisms that underlie physical and emotional types of “pain”, and this comparison is taken as the basis for the assumption of overlap between these frequently related entities. Second, we summarize the mechanisms involved in the mediation of pain and analgesia, as they relate to normal processing of emotions, reward and motivation as well as their alterations encountered in psychiatric patients. The same section also covers specific background demonstrating that pain-related stimuli provide a reliable functional probe of the brain reward/salience/motivation circuitry and may thus have heuristic value for the inquiry into neurobiology of psychiatric illnesses. Next we provide specific clinical context on the association between psychiatric disorders and pain conditions. Finally, the two last sections cover psychiatrists’ training in pain medicine and the importance of this endeavor.
Physical and Emotional Pain may Represent Two sides of the Same Coin
In addition to the epidemiological association between pain and psychiatric disorders 10, there are clinical, even diagnostic features of pain syndromes that point to neuropsychiatric origin. Pain’s definition by the International Association for the Study of Pain (IASP) as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage" 21, recognizes its multifaceted bio-psycho-social nature with causes that extend well beyond direct physical harm. This is expressed in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) as the Axis I diagnostic category of the Pain Disorder, which is encoded in three of the five diagnostic criteria, viz., A. Pain…is of sufficient severity to warrant clinical attention; B. Pain causes clinically significant distress or impairment in social, occupational, or other important areas of functioning; C. Psychological factors are judged to have an important role in the onset, severity, exacerbation or maintenance of pain 22. Pain arising from physical sources is classified among the DSM-IV-TR Axis III disorders, solely reserved for medical conditions 22. In the reality the distinction between Axis I and III Pain Disorders is not that obvious and both pain categories share clinical characteristics, symptom severity and functional impairment 23. Realization of such blurring of diagnostic boundaries is rooted in the lay language where the “pain” term is used interchangeably in both physical and emotional contexts 24.
Pain and the Brain: Implications for Emotional and Motivational Processing
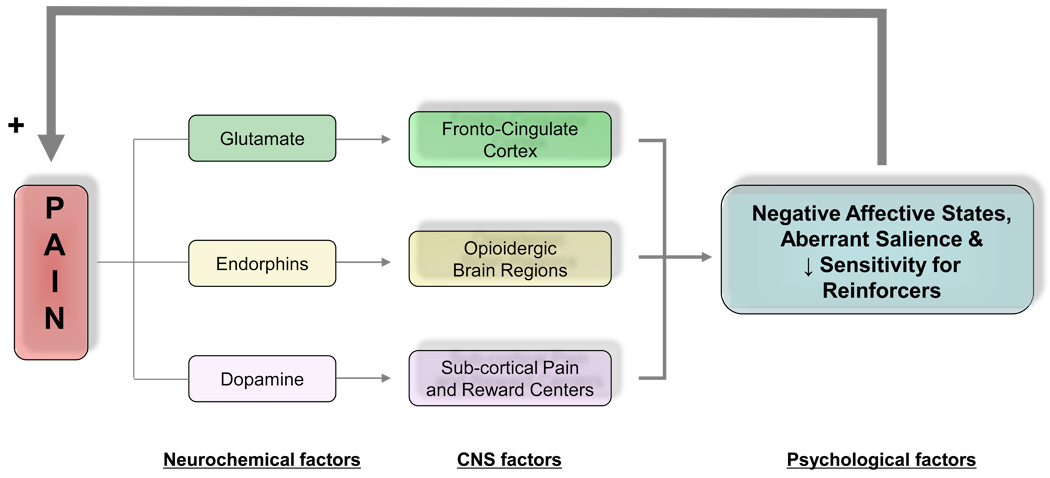
Acute pain signals real or perceived tissue damage from environmental threats 25. Contextually, albeit not clinically similar, cancer pain is also etiologically linked to direct tissue damages due to malignancy or to surgical, chemotherapy or radiotherapy treatments 26, 27. Chronic non-malignant pain is not a unitary sensation evoked by a local injury, inflammation or other tissue pathology but is a complex state modulated by genetic, environmental, cognitive and emotional factors 28, 29. Majority of non-malignant chronic pain cases are neuropathic viz., caused by alterations in the central nervous system 19. Such centralization is conceptualized as an independent neuropsychopathological disease state, occurring at the level of the spinal cord pathways and emotional/motivational circuits, respectively manifested in exaggerated response to painful (hyperalgesia) and non-painful (allodynia) stimuli in combination with negative affective states and persistent drive to eliminate pain via behavioral or pharmacological measures 19, 30, 31. The apparent intricate link between pain and emotions/motivations underscores the importance of dynamic interactions between these and other (e.g., neurochemical and neuroanatomical) factors for understanding of health and psychopathology (Figure 1).
Figure 1. Schematic Overview of the Interface between Neurobiological and Psychological Factors Involved in Chronic Pain Experience.
Several lines of evidence link pain to emotional, motivational and reward processing. At the fronto-cingulate cortical level chronic pain conditions may cause brain reorganization via glutamatergic mechanisms 179 including cortical thinning resulting in mild to moderate impairments in emotional and cognitive function with ensuing negative affective states and compromised decision making capacity 12,17. Dysphoric emotional states render pain stimuli more aversive leading to additional psychological impairments (i.e., feedforward phenomenon). With regard to subcortical systems, acute pain is associated with increased dopaminergic trafficking within mesolimbic dopaminergic pathways 33,124. Chronic pain exerts an opposite action by decreasing dopaminergic transmission in the same neural structures 180 and is accompanied by decreased motivation towards normally pleasurable stimuli 87, clinically manifested as anhedonia. Central opioidergic systems that are dysregulated in chronic pain conditions further contribute to affective disturbances.
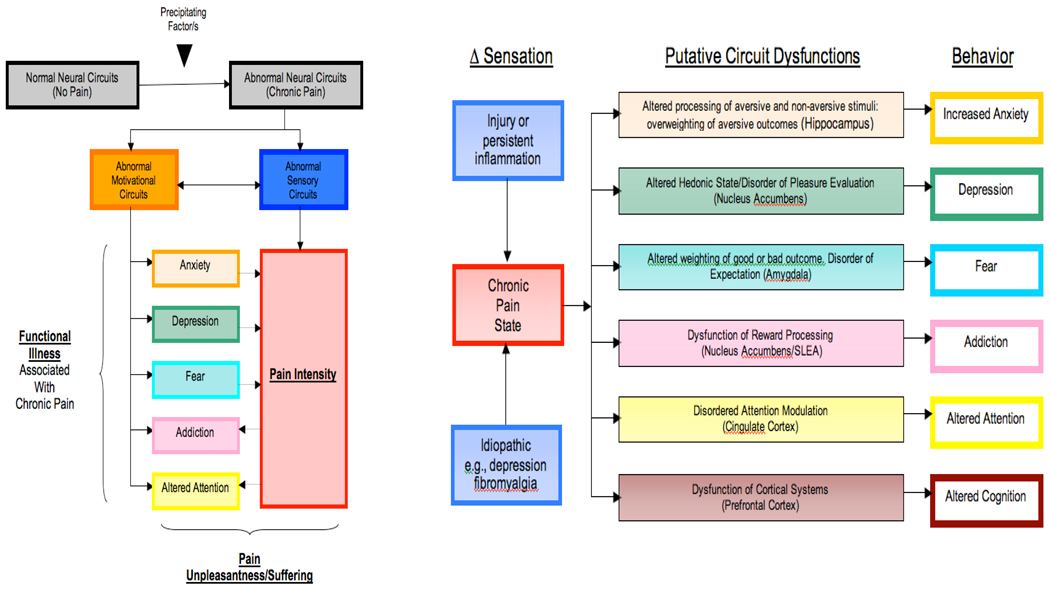
Figure 2 schematically displays a somewhat representative compilation of the interactions between sensory and emotional systems with ensuing affective states and symptomatology. Evidence of such interaction is provided by functional magnetic resonance imaging (fMRI) work showing that grief-related emotional pain activates classical physical pain circuitry (e.g., periaqueductal gray, insula and the anterior cingulate cortex) 32 while physical pain in humans activates reward/motivational circuits (e.g., nucleus accumbens [NAc] and ventral tegmentum [VT]) 33–35. Furthermore, acute emotional or physical pain in patients with the respective chronic conditions (i.e., complicated grief and back pain) via exposure to reminders of the deceased32 or thermal pain 36 robustly engages the key reward and reinforcement structure, namely the NAc. These finding suggest that emotional and physical pain may become a reinforced stimulus in some conditions (e.g., complicated grief and chronic back pain) thus providing partial explanations for their unremitting course. In a broader sense, critical for the survival mechanisms pain system, is embedded within extensive emotion/reward/motivation circuitry, representing a neural network responsible for continued existence of individuals and species via pursuit of food, water, and sex as well as via learning, decision making and adaptation to stress. Considering survival significance of social affiliations, their pleasurable acquisitions and painful losses may have evolutionarily tapped the same reward systems 37; an idea consistent with the reward-pain continuum hypothesis promulgated by Baruch Spinoza. A practical offshoot of the reward-aversion continuum is that pain serves as a functional probe of the brain reward and motivational systems 33. This is an important contribution, given the paucity of reliable probes and the pivotal role played by reward and motivation in the course of psychiatric illnesses.
Figure 2. Pain and Emotions (Adapted from 33).
A: Pain produces changes in emotional states with ensuing psychiatric symptomatology and these effects are bidirectional, that is to say, negative affective states can augment the perceived intensity of pain.
B: Altered Sensation and Functional Changes in Brain Regions that subserve emotional states and cognition.
Co-morbidity of Pain and Psychiatric Disorders
Provided that the emotional brain circuitry abnormalities are arising in psychiatrically healthy people exposed chronic pain 18, a compelling a fortiori argument is that propensity for pain condition is worsened by neuropsychopathology affecting the same neural structures. A model partially suited to explain this link is the diathesis-stress theory postulating preexisting sometimes subclinical traits that manifest in the form of overt psychopathology in the context of prolong pain-induced stress 16, 38,39.
The list of psychiatric ailments associated with heightened pain prevalence in at least one extant epidemiological survey would stretch the entire DSM-IV-TR diagnostic range from the Disorders Usually First Diagnosed in Infancy, Childhood or Adolescence to Other Conditions that may be a Focus of Clinical Attention. For the purpose of the present discussion we address four common and representative syndromes, namely, the major depressive disorder (MDD), borderline personality disorder (BPD), addiction and post-traumatic stress disorder (PTSD).
Pain and MDD
Elicitation of depressed vs. happy affective states in laboratory settings respectively worsen and improves pain experience in healthy individuals and in chronic pain patients 40, 41, 42. Accordingly, a substantial proportion of MDD patients suffer from chronic pain 43, the intensity of which increases with the severity of depressive symptomatology 44. On the other hand, sustained pain leads to increased negative affective states 45, 46 and eventually to high MDD prevalence in chronic pain patients 47 so that, in comparison to pain alone or MDD with other medical conditions, comorbid MDD-pain patients present greater severity of depressive symptoms and substantially poorer treatment outcomes 48.
In order to link MDD neuropathology and pain, painful heat stimuli were applied to the dorsal forearm of depressed patients during fMRI 13. Among other functional alterations, depressed patients had proportionally (to depressive symptomatology) heightened right amygdala activity when anticipated and actually experienced painful stimuli 13. Other lines of evidence supporting recursive, partly shared neural systems by MDD and pain processing include (a) serotonergic and noradrenergic pathways’ involvement in mood regulation and in the control over ascending pain stimuli, explaining therapeutic pain successes of dual action serotonergic/noradrenergic reuptake inhibitors and tricyclic antidepressants 49; (b) other shared treatment modalities (e.g., transcranial magnetic or vagus nerve stimulation) and (c) abnormalities of the analgesic opioidergic system in MDD patients and the involvement of this neurotransmitter in emotion regulation 50. Taken together, these findings support the idea that MDD and pain can trigger and perpetuate each other owing to overlapping neural and emotional alterations. Therefore, assessment of pain function may provide important diagnostic and therapeutic leads in MDD, which is projected to become the second most common cause of disability after the heart disease 51.
Pain and BPD
Emotion dysregulation, affective lability and impulsive behavior in BPD patients are reported to be associated with increased pain thresholds 52 that predict dissociative symptoms and negative affect 53. In comparison to healthy subjects, BPD patients demonstrated enhanced opioid neurotransmission during molecular imaging with positron emission tomography 54 in conjunction with greater pain-induced fMRI signal changes in the prefrontal cortex but lower activity in the amygdala and anterior cingulate. These are regions that interface emotion, pain and stress regulation and where the endogenous opioid system plays key modulatory roles55. Impulsivity, a trait characterizing not only BPD patients but also those at risk for addictive disorders, was also associated with greater endogenous opioid function in similar brain regions of healthy people 56.
Pain and Addiction
Numerous epidemiological surveys documented that drug addiction (including prescription opioids) is particularly prevalent among pain patients 16,57,58. The mechanisms of this link are likely to be multifactorial and to involve both psychosocial and neurobiological causes 59. Among neurobiological factors, the brain reward and motivational system probably plays an important role. The mesoaccumbens dopamine pathway, extending from the VT of the midbrain to the forebrain regions such as the NAc is the crucial component of this system 60–63. Physiological significance of the increases in the NAc’ dopamine, a common element of the rewarding effects of natural rewards as well as drugs of abuse 64, 65, is a subject of complementary hypotheses 20, 66, 67 including but not limited to the subjective pleasure or “high” (reward) sought by drug users as well as motivation, appraisal of stimuli salience, stress, learning and decision making 68–72.
Both acute pain 35, 73, 74 and euphorogenic drugs 64, 65 activate dopamine transmission in the brain reward circuitry including the NAc, whereas prolong periods of pain 75, 76 or of drug consumption 77–79 produce the opposite effect, accompanied by reduced motivation towards normally pleasurable stimuli 80–83. These findings can be interpreted in the context of the incentive motivation theory 34, viewing the brain reward function as comprised from core motivational and emotional processes, respectively termed 'wanting' and 'liking'. Pain- or drug-induced changes in the mesolimbic dopaminergic circuitry underlying the wanting but not liking purposes may be responsible for transforming regular wanting responses into heightened incentive salience assigned to pain or drug-related cues. Such incentive sensitization, construed as an animal homolog of human craving 34, may be responsible for drug seeking driven by the desire to ameliorate an inadequately treated pain (i.e., pseudo-addiction; 84) or to avoid a feared opioid withdrawal (i.e., therapeutic dependence; 85).
A closely related concept, derived from primate work, is the aberrant learning theory suggesting that learning of new rewarding or aversive experiences is encoded via interactions between tonic (baseline) and phasic spikes in dopaminergic neurons; phasic firing predicts unanticipated stimuli 86. Hence, neural adaptations to excessive dopaminergic bombardment in response to pain may lead to low signal-to-noise detection capability for natural rewards 87 along with profound overlearning of the motivational significance of cues that either predict painful episodes or are associated with painful experience 86.
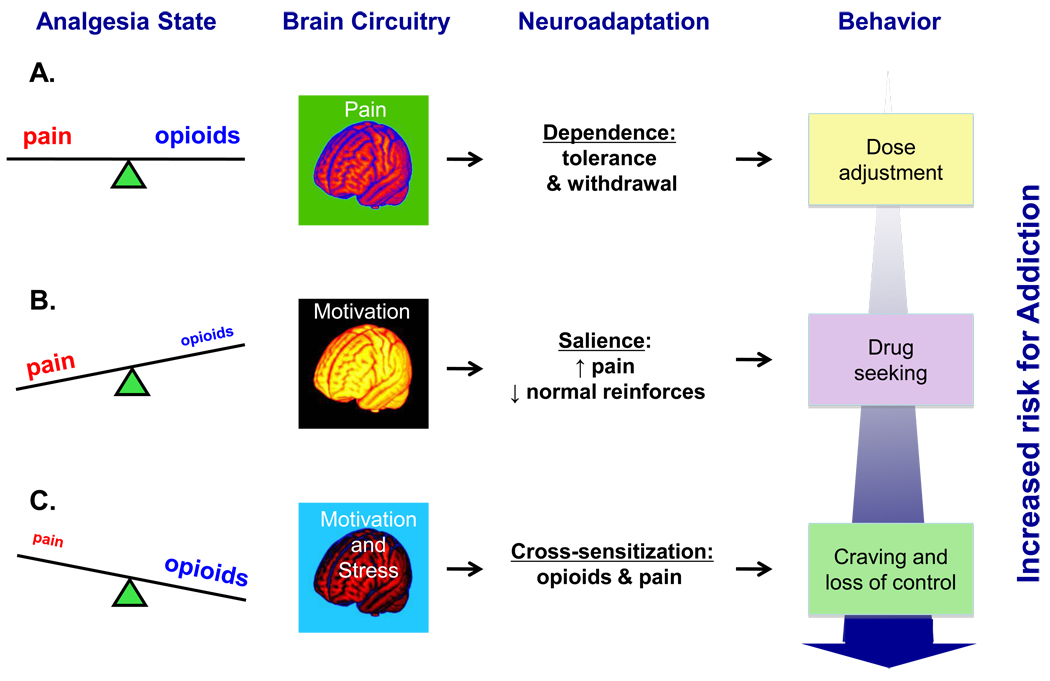
The above ideas provide rich opportunities for neuropsychiatric clinicians and researches alike to decipher seemingly elusive interactions between pain and analgesia with respect to the addiction on opioid analgesics 88–91. Three potential classes of interaction may (co)exist between pain and opioids, including competition, independence (i.e., additive effects) and synergism. Competition (Figure 3A) may be the most notable interaction as opioid analgesics, when administered at the adequate dose, restore the homeostatic equilibrium 67, 92 by eliminating pain while pain offsets opioids’ reinforcing properties 89, 93. Dependence, including tolerance and withdrawal, may nonetheless sometimes occur 88, 94 during opioid pharmacotherapy, but it qualitatively differs from addiction by lacking the compulsive nature and loss of control 67, 95–97.
Figure 3. Schematic diagram of potential mechanisms involved in drug-related motivational changes during adequate-, under- or over-treatment of pain with opioid analgesics.
(A) Pain relief owing to adequate analgesia restores homeostatic equilibrium and seldom produces addiction 67, 92 which nonetheless may arise 15, 88 in the context of intricate relationships among various genetic, clinical, pharmacokinetic and psychosocial factors. Dependence on opioid analgesics, including tolerance (i.e., diminution of drug’s effectiveness) and resultant withdrawal 88, 94 is a more likely outcome and it calls for a gradual and judicial dose escalation 181. (B) Inadequately treated or untreated pain activates dopaminergic ventral striatal neurotransmission involved in motivational processing (15) leading to heightened incentive salience attribution to pain and to pain-related stimuli. Opioid analgesics is one of such stimuli that becomes a sensitized motivational target capturing greater attentional resources and leading to expenditure of greater behavioral effort relative to normal reinforcers 93, 104, 105, 182. Even though this state is viewed as pseudo- rather than genuine addiction 84, the latter’s features may predominate with time, given the potential opioid overuse in the form of ill-fated attempts to ‘self-medicate’ perceivably intolerable pain and pain-related anxiety 67. (C) Similar to pain effects discussed in the preceding caption, changes in the mesolimbic dopaminergic circuitry induced by opioids, taken at the doses exceeding the homeostatic need for pain alleviation, are responsible for transforming regular motivational drives into heightened incentive salience assigned to opioids or to opioid-related cues, that is to say drug craving 34. Additional critical aspect of opioid overuse in the context of an ongoing pain condition is the amplification of the physical and emotional aspects of pain. The latter emotional component, recently termed “hyperkatifeia” 67, is purportedly mediated via norepinephrine and consequent corticotropin-releasing factor hypersecretion within the extended amygdala structures underlying anxiety and fear 183, 184 namely the central nucleus of the amygdala 185, 186 and the bed nucleus of the stria terminalis 187–189. Notably, such a cross-sensitization phenomenon is typical of addictive substances and entails a situation when prior exposure to one stimulus (e.g., drug) increases subsequent response to itself 111–113, 190 and to a different stimulus (e.g., stress; 114, 116, 189). Hence, a common outcome in pain patients could be generation of the ‘spiraling distress cycle’ 79, 191 whereby opioid overuse provoked by enhanced pain salience (see Caption B) produces additional deterioration in the pain 117–120 and emotional 67 problems, leading to further opioid consumption that may eventually produce a transition from excessive opioid use to bona fide addiction
To our knowledge, no studies to date directly investigated whether under- or over treated pain has competitive, independent, or synergistic effects on the development of addiction. Opioid analgesics and pain share the ability to affect extracellular dopamine concentrations in the NAc. The latter robustly activate dopaminergic neurotransmission both via inhibition of the GABA neurons 98 and by direct action at the mesocorticolimbic dopamine system and at the related structures e.g., the VT 99, the NAc 100 and the hypothalamus 101. Pain likewise produces DA releases in the NAc with the involvement of the GABA-ergic mechanisms 102, 103. Chronic pain perpetuation and addiction development may result from the dysregulation of these systems. For instance, activation of mesolimbic dopaminergic pathways involved in motivational processing 35 by inadequately treated or untreated pain (Figure 3B) leads to heightened incentive salience attribution to pain and to pain-related stimuli 86. Opioid analgesics providing quick pain relief (i.e., negative reinforcement) can thus become a sensitized motivational target capturing greater attentional resources and resulting in drug craving clinically manifested in expenditure of greater behavioral effort relative to normal reinforcers to seek and to obtain opioids 93, 104, 105. Even though this state is viewed as pseudo- rather than genuine addiction 84, the latter features may predominate with time, giving raise to opioids’ overuse in the form of ill-fated attempts to ‘self-medicate’ perceivably intolerable pain and pain-related anxiety 67. Such an outcome is relatively uncommon and its likelihood is increased with prior history of drug abuse, non-compliance with treatment recommendations and disability litigation 106–108.
Changes in the mesolimbic dopaminergic circuitry induced by opioids, taken at the doses exceeding the homeostatic need for pain alleviation (Figure 3C), may be responsible for transforming regular motivational drives into heightened incentive salience assigned to opioids or to opioid-related cues, that is to say drug craving 34. Additional critical aspect of opioid overuse in the context of an ongoing pain condition is the amplification of “hyperkatifeia” or negative affective states and of physical pain itself 67, 109, 110.
This may be a variant of cross-sensitization when exposure to one stimulus (e.g., addictive drug) increases subsequent response to itself 111–113 and to a different stimulus (e.g., stress; 114–116). Hence, a common outcome in pain patients could be generation of the ‘spiraling distress cycle’ 79, whereby opioid overuse provoked by the heightened pain salience produces additional deterioration in the pain 117–20 and emotional 67 problems, leading to further opioid consumption that may eventually produce a transition from an excessive opioid use to the bona fide addiction. Given such an autonomous, self-sustaining feedforward loop, it is plausible that pain combined with excessive opioids brings about synergistic processes favoring addictive phenomena. In sum, safe and effective care of pain patient requires thorough understanding of the complex interplay among pain-, analgesia- and addiction-related factors.
Pain and PTSD
Anxiety syndromes are also commonly comorbid with chronic pain 121. A conditioned fear and anxiety syndrome, PTSD, could be yet another example of reward/motivational circuitry involvement in the association of chronic pain and psychopathology. Several lines of evidence implicate pain in the course of PTSD. Neuroanatomically, in addition to their involvement in the pain processing 33, 35,122–125, dopamine terminal fields including the NAc, amygdala and medial prefrontal cortex also play key roles in stress, aversive responses and in PTSD 70, 126–129. From the pathophysiological perspective, peritraumatic pain is recognized among PTSD’s independent risk factors 130 while morphine analgesia reduces the severity and even prevents the appearance of PTSD symptomatology 131–134.
A number of mechanisms may contribute to the PTSD-pain comorbidity that carries a substantially poorer prognosis than each condition taken in isolation 135–137. Pain, paired with emotional trauma and its recollections, can become a conditioned stimulus that evokes fear and anxiety responses that in turn augment subjective pain perception and its neural correlates 138, 139 leading to additional deterioration and avoidance of pain- and trauma-related situations 140–143. Such a “mutual maintenance” 144 cycle may be evident in PTSD symptoms emergence caused by surgical pain awareness despite seemingly adequate general anesthesia or in 5% to 19% PTSD incidence surges at the 6 to 12 months follow up points in injured accidents’ survivors 145.
Additionally, increased central opiodergic tone 146, 147 along with robust elevations of endogenous opiates concentrations in the cerebral spinal fluid 148 and in plasma 149, but 150 is a relatively consistent clinical finding in PTSD. Therefore, similar to chronic users of opioid analgesics (Figure 3C), PTSD-related exaggerated CNS opioidergic activity could contribute to the sensitized pain phenomenon potentially mediated via the amplification of the excitatory (e.g., glutamtergic) neurotransmission 151–153. The proposed mechanism of excessive pain occurrence could have treatment implications, as it implies prophylactic use of opioid receptor antagonists. Indeed, on the basis of the hypothesis that opioidergic mechanisms are involved in the pathophysiology and symptoms of PTSD 146, 154, 155, opioid antagonists have been evaluated for a potential clinical efficacy in a number of clinical trials 147, 156, 157. On the whole, they were safe and well tolerated and resulted in significant improvements of various aspects of PTSD symtomatology such as emotional numbing, startle response, nightmares, flashbacks, intrusive thoughts and comorbid alcoholism 147, 156, 158.
Pain Training in Psychiatry
Pain themes are only marginally addressed by the respective training and certification requirements set forth by the Accreditation Council for Graduate Medical Education (ACGME) 159 and the American Board of Psychiatry and Neurology 159. The former includes Intractable Pain among Medical Knowledge items but not among Patient Care competencies, while the latter only lists Pain Medicine in the section on Psychiatric Subspecialties and Other Areas of Psychiatric Endeavors. Psychiatrists appear to be inadequately trained in pain medicine and to consequently perceive their work with pain patients as ungratifying 160, 161 so the present psychiatric training requirements may be suboptimal for the physicians that are uniquely poised to evaluate and treat pain patients whilst sharing responsibility with their medical colleagues for the management of mental health issues 162.
Empirical data on potential benefits of increased pain training for psychiatric residents are missing. This line of inquiry may be limited by educators’ concerns that any training add-ons may encroach on the time honored intensive and rigorous curriculum of the critical clinical skills and knowledge base 160. In our opinion, far from being adverse for psychiatric education, participation in pain training may be beneficial for psychiatric residents, their patients and medical field as whole.
Pain curriculum could build upon the residents’ proficiency in neurobiology and upon the existing IASP guidelines 163. Since there are countless ways in how the training can be operationalized, a consensus concerning the adoption of various training components perhaps needs to be developed by a Committee comprised of Psychiatric Residency Training Directors and/or through the American Psychiatric Association Task Force. Following is a brief outline of possible components that may be included in the training activities.
The main objective of the first post-graduate year (PGY-1) is to shape the physician identity of new medical school graduates. Recent graduates accordingly spend at least four months in primary care clinical settings and so that part of their caseload requirements may include treatment and follow up of chronic pain patient(s). This clinical experience can be reinforced via didactics and direct patient care during the two months mandatory neurology rotation. The PGY-2, focused on the foundations of physicians’ identities as psychiatrists may include assessments of dually diagnosed pain/psychiatric patients in conjunction with psychotherapeutic 164 and psychopharmacological 49 strategies in the treatment of pain patients. The PGY-3 provides an opportunity to refine the previously acquired skills in more specialized settings including inpatient detoxification unit and outpatient addiction clinic. Consultation-liaison rotation in the PGY-4 could emphasize the integrative nature of pain treatment within the context of medicine at large and the distinctive role of psychiatrists in identifying and handing individual and interpersonal issues within the medical team arising in the context of challenges inherent in the care for multi-problem and at times non compliant and treatment resistant pain patients.
Why is it Important to Teach Pain to Psychiatrists?
Rather than viewing psychosocial and biological interventions as distinct therapeutic modalities, psychiatrists are exceptionally trained to conceptualize patients’ care on the psychosocial-biological continuum and to shift flexibly across various parts of this spectrum among patients and treatment junctions. Thus, wide-ranging involvement of psychiatrists will invigorate essential elements of evaluation and treatment armamentarium that may be underutilized by their medical colleagues. This is a timely effort because majority of neuropathic pain is only partially responsive to opioids 27, 165 so innovative approaches are essential for the pain field.
Psychiatrists are understandably suited to recognize and manage subtle psychological processes including expression of feelings via somatic pain complaints 166, defense mechanisms e.g., denial and repression vs. lie and malingering along with conscious and unconscious motivations 167 such as self reported pain in the face of adequate analgesia due to unwarranted anxiety about the opioid dose reduction (i.e., pseudo-opioid resistance; 168) or drug craving vs. pseudo-addiction 84 or therapeutic dependence 85. Additional pertinent function is the motivational enhancement 169 fostering compliance and active participation in pain treatment plans 80.
While multiple cognitive and behavioral strategies (e.g., cognitive restructuring, stress management and systemic desensitization) were reportedly helpful for chronic pain 164, 170, National Institute of Health Technology Panel assigned the highest score (i.e., strong) to the evidence regarding the effectiveness muscle relaxation 171. Psychiatrists can become strong advocates for the utilization of cognitive and behavioral techniques by the pain field as they routinely apply them to the care of psychiatric patients. Moreover, being undisputed experts in psychopharmacology, psychiatrists can promulgate viable and non-addictive alternatives to opioids with substantial analgesic properties such as antidepressant, anticonvulsant and neuroleptic agents (49, however see 172 suggesting only minor pain effect of neuroleptics). Also, psychiatrists are the most logical physicians to diagnose and treat suicidal tendencies 1, 173 as well as mood, anxiety, psychotic and personality disorders exerting pivotal impacts on pain intensity and treatment outcomes 16.
The psychiatrists’ dialectical perspective 174 on pain alleviation while preventing, diagnosing and treating addiction to prescription opioid pain killers is addressed in the preceding sections. Additional contribution from the addiction psychiatry field may involve empirically driven treatment matching algorithms allowing provision of individualized level care according to patients needs with regard to their medical status, employment/support, drug addiction, family situation and psychiatric condition. Patient Placement Criteria by American Society of Addiction Medicine 175, may be adapted to pain patients 176, 177 since they provide multidimensional assessment of severity of illness and level of function and treatment assignment based on needs for service and level of care determination within the available treatment options.
Hence, outweighing any personal benefits for psychiatric trainees per se, this may be of a broader public health interest to engage psychiatrists in the care for the pain patients. Such an addendum will not only generate additional clinical expertise to evaluate and treat a large spectrum of pain-related problems, but will also help expanding the spectrum of psychiatric field to include pain as an entity rooted in numerous other specialties (to name a few: neurology, medicine, surgery and anesthesiology) and will thus advance the integration psychiatry into the mainstream medical care 178 and underscore the significance of attending in concert to mental and physical problems notwithstanding physicians’ specialties and patients’ presenting problems.
Conclusions
Chronic pain is a major problem afflicting the modern world. The coalescence of epidemiological and clinical data suggests that psychiatric patients have a predilection for a development of various pain conditions. This is at least partially due to functionally impaired neural substrates that overlap emotion, reward, motivation and pain processing. Although psychiatry is only one of many appropriate settings were pain patients can be treated, this approach has several advantages. First, psychiatry is comprehensively focused on the etiopathophysiological mind-body interactions with direct relevance to the pain syndromes. Second, neurobiological research in animal models and more recently in humans confirms a substantial degree of overlap between pain regions and brain areas implicated in core psychiatric symptoms. Third, the relationship and co-morbidity between persistent pain and psychiatric disorders has been extensively documented.
If the interactions between pain and psychiatric illnesses can be further elucidated, they might be used to screen patients at risk for the development or worsening of psychopathology (i.e., primary prevention). It may be also discovered that psychiatric patients need to be targeted for early intervention even in the presence of mild pain problems (i.e., secondary prevention). Lastly, pain medicine training could speed the introduction of other essential ‘medical’ topics to psychiatric curriculum that have now become timely (e.g., metabolic disturbances 14). Filling in the missing “P” in elevating the psychiatrists’ new role in pain patient care, teaching and research may be a substantial clinical contribution in a domain that currently offers relatively little in terms of adequate therapeutic responses.
Acknowledgements
The authors gratefully acknowledge Ms. Lauren Nutile and Ms. Jeanette Cohan for their assistance with preparation of this manuscript.
This work was supported by grants DA017959 (IE); R01 DA 016423, R01 DA 022520, R01 AT 001415 (J-KZ); and K24NS064050 (DB).
Footnotes
The authors of this manuscript, Drs. Elman, Zubieta and Borsook, have no conflicts of interest to report.
References
- 1.Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005 Jun;7(3):213–219. doi: 10.1007/s11920-005-0056-x. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2006; with Chartbook on Trends in the Health of Americans. http://www.cdc.gov/nchs/data/hus/hus06.pdf. [PubMed]
- 3.Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984 Mar;18(3):299–314. doi: 10.1016/0304-3959(84)90824-8. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. New Directions in Pain Research: 1. http://grants.nih.gov/grants/guide/pa-files/PA-98-102.html.
- 5.Vaupel JW. Biodemography of human ageing. Nature. 2010 Mar 25;464(7288):536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breuer B, Pappagallo M, Tai JY, Portenoy RKUS. board-certified pain physician practices: uniformity and census data of their locations. J Pain. 2007 Mar;8(3):244–250. doi: 10.1016/j.jpain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Gianni W, Ceci M, Bustacchini S, et al. Opioids for the treatment of chronic non-cancer pain in older people. Drugs Aging. 2009 Dec 1;26 Suppl 1:63–73. doi: 10.2165/11534670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Gibson SJ. IASP global year against pain in older persons: highlighting the current status and future perspectives in geriatric pain. Expert Rev Neurother. 2007 Jun;7(6):627–635. doi: 10.1586/14737175.7.6.627. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Census Bureau. U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin: 2000–2050. http://www.census.gov/population/www/projections/usinterimproj/.
- 10.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998 Jul 8;280(2):147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 11.Hyman SE. Mental health in an aging population: the NIMH perspective. Am J Geriatr Psychiatry. Fall. 2001;9(4):330–339. [PubMed] [Google Scholar]
- 12.Leo RJ, Pristach CA, Streltzer J. Incorporating pain management training into the psychiatry residency curriculum. Acad Psychiatry. 2003 Spring;27(1):1–11. doi: 10.1176/appi.ap.27.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008 Nov;65(11):1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006 Oct;31(10):2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- 15.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008 May-Jun;9(4):444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 16.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom Med. 2002 Sep-Oct;64(5):773–786. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- 17.Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004 Mar;108(1–2):129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004 Nov 17;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009 Feb;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kringelbach ML, Berridge KC. The functional neuroanatomy of pleasure and happiness. Discov Med. 2010 Jun;9(49):579–587. [PMC free article] [PubMed] [Google Scholar]
- 21.Merskey H, Boduk N. Classification of Chronic Pain. 2nd ed. 1994. [Google Scholar]
- 22.Association AP. Diagnostic and Statistical Manual of Mental Disorders, Fourth Editioin, Text Revision (DSM-IV-TR) 4 ed. 2000. [Google Scholar]
- 23.Boland RJ. How could the validity of the DSM-IV pain disorder be improved in reference to the concept that it is supposed to identify? Curr Pain Headache Rep. 2002 Feb;6(1):23–29. doi: 10.1007/s11916-002-0020-y. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science. 2009 Feb 13;323(5916):937–939. doi: 10.1126/science.1165604. [DOI] [PubMed] [Google Scholar]
- 25.Treede RD. Peripheral acute pain mechanisms. Ann Med. 1995 Apr;27(2):213–216. doi: 10.3109/07853899509031961. [DOI] [PubMed] [Google Scholar]
- 26.Breitbart W, Gibson CA. Psychiatric Aspects of Cancer Pain Management. Primary Psychiatry. 2007;14(9):81–91. [Google Scholar]
- 27.Katz WA, Barkin RL. Dilemmas in chronic/persistent pain management. Am J Ther. 2008 May-Jun;15(3):256–264. doi: 10.1097/MJT.0b013e3181671c5a. [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract. 2005 Jun;5(2):85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- 29.Melzack R. The future of pain. Nat Rev Drug Discov. 2008 Aug;7(8):629. doi: 10.1038/nrd2640. [DOI] [PubMed] [Google Scholar]
- 30.Smith MB. Chronic Pain and Psychopathology: One Psychiatrist's View. Primary Psychiatry. 2007;14(9):55–68. [Google Scholar]
- 31.Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain's reward center. Neuroimage. 2008 Aug 15;42(2):969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borsook D, Becerra L, Carlezon WA, Jr, et al. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007 Jan;11(1):7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003 Sep;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 35.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006 Oct 18;26(42):10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010 Apr 15;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003 Aug;79(3):351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 38.Turk DC. A diathesis-stress model of chronic pain and disability following traumatic injury. Pain Res Manag. 2002 Spring;7(1):9–19. doi: 10.1155/2002/252904. [DOI] [PubMed] [Google Scholar]
- 39.Banks S, Kerns R. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull. 1996;119:95–110. [Google Scholar]
- 40.Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008 Aug 31;138(2):392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007 Sep;131(1–2):162–170. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010 Jun 1;67(11):1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Bair MJ, Robinson RL, Eckert GJ, Stang PE, Croghan TW, Kroenke K. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004 Jan-Feb;66(1):17–22. doi: 10.1097/01.psy.0000106883.94059.c5. [DOI] [PubMed] [Google Scholar]
- 44.Fava M, Mallinckrodt CH, Detke MJ, Watkin JG, Wohlreich MM. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry. 2004 Apr;65(4):521–530. doi: 10.4088/jcp.v65n0411. [DOI] [PubMed] [Google Scholar]
- 45.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001 Jul 13;293(5528):311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 46.Stohler CS, Kowalski CJ. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999 Feb;79(2–3):165–173. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 47.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997 Jun;13(2):116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Koike AK, Unutzer J, Wells KB. Improving the care for depression in patients with comorbid medical illness. Am J Psychiatry. 2002 Oct;159(10):1738–1745. doi: 10.1176/appi.ajp.159.10.1738. [DOI] [PubMed] [Google Scholar]
- 49.Clark M. Psychopharmacology of Chronic Pain. Primary Psychiatry. 2007;14(9):70–79. [Google Scholar]
- 50.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006 Nov;63(11):1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. Depression. http://www.who.int/mental_health/management/depression/definition/en/
- 52.Schmahl C, Bohus M, Esposito F, et al. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006 Jun;63(6):659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- 53.Ludascher P, Bohus M, Lieb K, Philipsen A, Jochims A, Schmahl C. Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Res. 2007 Jan 15;149(1–3):291–296. doi: 10.1016/j.psychres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of Regional Endogenous Opioid Function in Borderline Personality Disorder. Am J Psychiatry. 2010 May 13; doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zubieta JK, Smith YR, Bueller JM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 56.Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009 Oct;66(10):1124–1134. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown RL, Patterson JJ, Rounds LA, Papasouliotis O. Substance abuse among patients with chronic back pain. J Fam Pract. 1996 Aug;43(2):152–160. [PubMed] [Google Scholar]
- 58.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006 Feb 1;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009 Oct;11(5):377–384. doi: 10.1007/s11920-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 60.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003 Apr 10;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 61.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56 Suppl 1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010 Jan;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009 Oct;32(10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 65.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006 Jul 29;361(1471):1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz W. Subjective neuronal coding of reward: temporal value discounting and risk. Eur J Neurosci. 2010 Jun;31(12):2124–2135. doi: 10.1111/j.1460-9568.2010.07282.x. [DOI] [PubMed] [Google Scholar]
- 67.Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med. 2010 Jul;11(7):1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dayan P, Niv Y. Reinforcement learning: the good, the bad and the ugly. Curr Opin Neurobiol. 2008 Apr;18(2):185–196. doi: 10.1016/j.conb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009 Dec 15;66(12):1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010 Apr;90(4):385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Boutelle MG, Zetterstrom T, Pei Q, Svensson L, Fillenz M. In vivo neurochemical effects of tail pinch. J Neurosci Methods. 1990 Sep;34(1–3):151–157. doi: 10.1016/0165-0270(90)90053-i. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt BL, Tambeli CH, Barletta J, et al. Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci. 2002 Aug 1;22(15):6773–6780. doi: 10.1523/JNEUROSCI.22-15-06773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008 Nov 26;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience. 2009 Jul 7;161(3):671–679. doi: 10.1016/j.neuroscience.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974 Mar;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 78.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992 Oct 20;221(2–3):227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 79.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001 Feb;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 80.Karoly P, Ruehlman LS. Motivational implications of pain: chronicity, psychological distress, and work goal construal in a national sample of adults. Health Psychol. 1996 Sep;15(5):383–390. doi: 10.1037//0278-6133.15.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coscia L, Causa P, Giuliani E, Nunziata A. Pharmacological properties of new neuroleptic compounds. Arzneimittelforschung. 1975 Sep;25(9):1436–1442. [PubMed] [Google Scholar]
- 82.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005 Aug;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 83.Karoly P, Ruehlman LS. Psychosocial aspects of pain-related life task interference: an exploratory analysis in a general population sample. Pain Med. 2007 Oct-Nov;8(7):563–572. doi: 10.1111/j.1526-4637.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 84.Weissman DE, Haddox JD. Opioid pseudoaddiction--an iatrogenic syndrome. Pain. 1989 Mar;36(3):363–366. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- 85.Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986 May;25(2):171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 86.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998 Aug;1(4):304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 87.Karoly P, Lecci L. Motivational correlates of self-reported persistent pain in young adults. Clin J Pain. 1997 Jun;13(2):104–109. doi: 10.1097/00002508-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 88.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007 Jun;129(3):235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 89.Fields HL. Should we be reluctant to prescribe opioids for chronic non-malignant pain? Pain. 2007 Jun;129(3):233–234. doi: 10.1016/j.pain.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Carinci AJ, Mao J. Pain and opioid addiction: what is the connection? Curr Pain Headache Rep. 2010 Feb;14(1):17–21. doi: 10.1007/s11916-009-0086-x. [DOI] [PubMed] [Google Scholar]
- 91.Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010 Jul;133(Pt 7):2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Passik SD, Messina J, Golsorkhi A, Xie F. Aberrant Drug-Related Behavior Observed During Clinical Studies Involving Patients Taking Chronic Opioid Therapy for Persistent Pain and Fentanyl Buccal Tablet for Breakthrough Pain. J Pain Symptom Manage. 2010 Jun 24; doi: 10.1016/j.jpainsymman.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007 Feb;106(2):312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- 94.Gutstein HB, Akil H. Opioid Analgesics. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. Tenth ed. New York: McGraw-Hill; 2001. pp. 569–619. [Google Scholar]
- 95.Savage S, Covington E, Heit H, Hunt J, Joranson D, Schnoll S. Advocacy: Definitions Related to the Use of Opioids for the Treatment of Pain. [DOI] [PubMed] [Google Scholar]
- 96.Miotto K, Compton P, Ling W, Conolly M. Diagnosing addictive disease in chronic pain patients. Psychosomatics. 1996 May-Jun;37(3):223–235. doi: 10.1016/S0033-3182(96)71561-X. [DOI] [PubMed] [Google Scholar]
- 97.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006 Jun;83 Suppl 1:S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 98.De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci. 2002 May 1;22(9):3321–3325. doi: 10.1523/JNEUROSCI.22-09-03321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984 Jun;20(6):917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 100.Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009 Jul;34(8):1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rada P, Barson JR, Leibowitz SF, Hoebel BG. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res. 2010 Feb 2;1312:1–9. doi: 10.1016/j.brainres.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999 Aug 15;19(16):7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wood PB. Mesolimbic dopaminergic mechanisms and pain control. Pain. 2006 Feb;120(3):230–234. doi: 10.1016/j.pain.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 104.Clark JD. Opioid self-administration: a better way to evaluate analgesics in animal models? Anesthesiology. 2007 Feb;106(2):208–209. doi: 10.1097/00000542-200702000-00005. [DOI] [PubMed] [Google Scholar]
- 105.George O, Koob GF. Individual Differences in Prefrontal Cortex Function and the Transition from Drug Use to Drug Dependence. Neurosci Biobehav Rev. 2010 May 18; doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999 Sep;15(3):184–191. doi: 10.1097/00002508-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008 Apr;9(4):360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain. 2010 Mar 23; doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Felice M, Porreca F. Opiate-induced persistent pronociceptive trigeminal neural adaptations: potential relevance to opiate-induced medication overuse headache. Cephalalgia. 2009 Dec;29(12):1277–1284. doi: 10.1111/j.1468-2982.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okada-Ogawa A, Porreca F, Meng ID. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J Neurosci. 2009 Dec 16;29(50):15828–15835. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strakowski SM, McElroy SL, Keck PE, Jr, West SA. The effects of antecedent substance abuse on the development of first-episode psychotic mania. J Psychiatr Res. 1996 Jan-Feb;30(1):59–68. doi: 10.1016/0022-3956(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 112.Satel SL, Seibyl JP, Charney DS. Prolonged cocaine psychosis implies underlying major psychopathology. J Clin Psychiatry. 1991 Aug;52(8):349–350. [PubMed] [Google Scholar]
- 113.Post RM, Contel NR, Gold P. Impaired behavioral sensitization to cocaine in vasopressin deficient rats. Life Sci. 1982 Dec 13;31(24):2745–2750. doi: 10.1016/0024-3205(82)90720-2. [DOI] [PubMed] [Google Scholar]
- 114.Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003 Dec;13(6):435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 115.Southwick SM, Morgan CA, 3rd, Charney DS, High JR. Yohimbine use in a natural setting: effects on posttraumatic stress disorder. Biol Psychiatry. 1999 Aug 1;46(3):442–444. doi: 10.1016/s0006-3223(99)00107-9. [DOI] [PubMed] [Google Scholar]
- 116.Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993 Apr 1;33(7):479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- 117.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002 Dec;100(3):213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 118.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006 Mar;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 119.Mitra S. Opioid-induced hyperalgesia: pathophysiology and clinical implications. J Opioid Manag. 2008 May-Jun;4(3):123–130. doi: 10.5055/jom.2008.0017. [DOI] [PubMed] [Google Scholar]
- 120.Fishbain DA, Cole B, Lewis JE, Gao J, Rosomoff RS. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med. 2009 Jul-Aug;10(5):829–839. doi: 10.1111/j.1526-4637.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 121.Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain. 1986 Aug;26(2):181–197. doi: 10.1016/0304-3959(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 122.Ingvar M. Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci. 1999 Jul 29;354(1387):1347–1358. doi: 10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001 Dec 6;32(5):927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 124.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008 Feb;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 125.Commons KG. Neuronal pathways linking substance P to drug addiction and stress. Brain Res. 2010 Feb 16;1314:175–182. doi: 10.1016/j.brainres.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006 Aug 15;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 127.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 128.Liberzon I, Taylor SF, Amdur R, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999 Apr 1;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 129.Pavic L, Gregurek R, Petrovic R, et al. Alterations in brain activation in posttraumatic stress disorder patients with severe hyperarousal symptoms and impulsive aggressiveness. Eur Arch Psychiatry Clin Neurosci. 2003 Apr;253(2):80–83. doi: 10.1007/s00406-003-0411-z. [DOI] [PubMed] [Google Scholar]
- 130.Norman SB, Stein MB, Dimsdale JE, Hoyt DB. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychol Med. 2008 Apr;38(4):533–542. doi: 10.1017/S0033291707001389. [DOI] [PubMed] [Google Scholar]
- 131.Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry. 2001 Aug;40(8):915–921. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 132.Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009 Mar 1;65(5):438–440. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 133.Stoddard FJ, Jr, Sorrentino EA, Ceranoglu TA, et al. Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. J Burn Care Res. 2009 Sep-Oct;30(5):836–843. doi: 10.1097/BCR.0b013e3181b48102. [DOI] [PubMed] [Google Scholar]
- 134.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010 Jan 14;362(2):110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 135.Sherman JJ, Turk DC, Okifuji A. Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. Clin J Pain. 2000 Jun;16(2):127–134. doi: 10.1097/00002508-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 136.Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. 2003 Sep-Oct;40(5):397–405. doi: 10.1682/jrrd.2003.09.0397. [DOI] [PubMed] [Google Scholar]
- 137.Villano CL, Rosenblum A, Magura S, Fong C, Cleland C, Betzler TF. Prevalence and correlates of posttraumatic stress disorder and chronic severe pain in psychiatric outpatients. J Rehabil Res Dev. 2007;44(2):167–178. doi: 10.1682/jrrd.2006.05.0052. [DOI] [PubMed] [Google Scholar]
- 138.Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 1998 Apr;75(2–3):187–198. doi: 10.1016/s0304-3959(97)00219-4. [DOI] [PubMed] [Google Scholar]
- 139.Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001 Dec 15;21(24):9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Asmundson GJ, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002 Dec;47(10):930–937. doi: 10.1177/070674370204701004. [DOI] [PubMed] [Google Scholar]
- 141.Liedl A, Knaevelsrud C. Chronic pain and PTSD: the Perpetual Avoidance Model and its treatment implications. Torture. 2008;18(2):69–76. [PubMed] [Google Scholar]
- 142.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26(10):888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 143.Liedl A, O'Donnell M, Creamer M, et al. Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychol Med. 2010 Jul;40(7):1215–1223. doi: 10.1017/S0033291709991310. [DOI] [PubMed] [Google Scholar]
- 144.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001 Aug;21(6):857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 145.Jenewein J, Wittmann L, Moergeli H, Creutzig J, Schnyder U. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: a longitudinal study. J Trauma Stress. 2009 Dec;22(6):540–548. doi: 10.1002/jts.20453. [DOI] [PubMed] [Google Scholar]
- 146.Pitman RK, van der Kolk BA, Orr SP, Greenberg MS. Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Arch Gen Psychiatry. 1990 Jun;47(6):541–544. doi: 10.1001/archpsyc.1990.01810180041007. [DOI] [PubMed] [Google Scholar]
- 147.Glover H. A preliminary trial of nalmefene for the treatment of emotional numbing in combat veterans with post-traumatic stress disorder. Isr J Psychiatry Relat Sci. 1993;30(4):255–263. [PubMed] [Google Scholar]
- 148.Baker DG, West SA, Orth DN, et al. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinology. 1997 Oct;22(7):517–529. doi: 10.1016/s0306-4530(97)00053-x. [DOI] [PubMed] [Google Scholar]
- 149.Hamner MB, Hitri A. Plasma beta-endorphin levels in post-traumatic stress disorder: a preliminary report on response to exercise-induced stress. J Neuropsychiatry Clin Neurosci. 1992 Winter;4(1):59–63. doi: 10.1176/jnp.4.1.59. [DOI] [PubMed] [Google Scholar]
- 150.Hoffman L, Burges Watson P, Wilson G, Montgomery J. Low plasma beta-endorphin in post-traumatic stress disorder. Aust N Z J Psychiatry. 1989 Jun;23(2):269–273. doi: 10.3109/00048678909062145. [DOI] [PubMed] [Google Scholar]
- 151.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993 Mar;52(3):259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 152.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995 Sep;62(3):259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 153.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002 Sep 15;22(18):8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ibarra P, Bruehl SP, McCubbin JA, et al. An unusual reaction to opioid blockade with naltrexone in a case of post-traumatic stress disorder. J Trauma Stress. 1994 Apr;7(2):303–309. doi: 10.1007/BF02102950. [DOI] [PubMed] [Google Scholar]
- 155.Liberzon I, Taylor SF, Phan KL, et al. Altered central micro-opioid receptor binding after psychological trauma. Biol Psychiatry. 2007 May 1;61(9):1030–1038. doi: 10.1016/j.biopsych.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 156.Bills LJ, Kreisler K. Treatment of flashbacks with naltrexone. Am J Psychiatry. 1993 Sep;150(9):1430. doi: 10.1176/ajp.150.9.1430a. [DOI] [PubMed] [Google Scholar]
- 157.Lubin G, Weizman A, Shmushkevitz M, Valevski A. Short-term treatment of post-traumatic stress disorder with naltrexone: an open-label preliminary study. Hum Psychopharmacol. 2002 Jun;17(4):181–185. doi: 10.1002/hup.395. [DOI] [PubMed] [Google Scholar]
- 158.Kozaric-Kovacic D. Pharmacotherapy treatment of PTSD and comorbid disorders. Psychiatr Danub. 2009 Sep;21(3):411–414. [PubMed] [Google Scholar]
- 159.Neurology ABoP. Psychiatry and Neurology Core Competencies: Version 4.1. 2009. [Google Scholar]
- 160.Wright MT. Training psychiatrists in nonpsychiatric medicine: what do our patients and our profession need? Acad Psychiatry. 2009 May-Jun;33(3):181–186. doi: 10.1176/appi.ap.33.3.181. [DOI] [PubMed] [Google Scholar]
- 161.Yanni L, McKinney-Ketchum J, Harrington S, et al. Preparation, Confidence, and Attitudes About Chronic Noncancer Pain in Graduate Medical Education. Journal of Graduate Medical Education. 2010;2(2):260–268. doi: 10.4300/JGME-D-10-00006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weissman MM, Neria Y, Gameroff MJ, et al. Positive screens for psychiatric disorders in primary care: a long-term follow-up of patients who were not in treatment. Psychiatr Serv. 2010 Feb;61(2):151–159. doi: 10.1176/appi.ps.61.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.International Association for the Study of Pain. IASP Guidelines. http://www.iasp-pain.org/AM/Template.cfm?Section=Guidelines1&Template=/CM/HTMLDisplay.cfm&ContentID=9113.
- 164.Lebovits A. Cognitive-Behavioral Approaches to Chronic Pain. Primary Psychiatry. 2007;14(9):48–54. [Google Scholar]
- 165.Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain. 1999 Apr;80(3):453–462. doi: 10.1016/S0304-3959(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 166.Blumer D, Heilbronn M. Chronic pain as a variant of depressive disease: the pain-prone disorder. J Nerv Ment Dis. 1982 Jul;170(7):381–406. doi: 10.1097/00005053-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 167.Geppert CMA. Navigating the Straits of Chronic Pain and Addiction. Psychiatric Times. 2007 [Google Scholar]
- 168.Evers GC. Pseudo-opioid-resistant pain. Support Care Cancer. 1997 Nov;5(6):457–460. doi: 10.1007/s005200050114. [DOI] [PubMed] [Google Scholar]
- 169.DiClemente CC, Haug N, Bellino L, Whyte S. Psychotherapy and motivational enhancement. Recent Dev Alcohol. 2003;16:115–132. doi: 10.1007/0-306-47939-7_10. [DOI] [PubMed] [Google Scholar]
- 170.Spiegel D. Facilitating emotional coping during treatment. Cancer. 1990 Sep 15;66(6 Suppl):1422–1426. doi: 10.1002/1097-0142(19900915)66:14+<1422::aid-cncr2820661419>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 171.Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. NIH Technology Assessment Panel on Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia. JAMA. 1996 Jul 24–31;276(4):313–318. doi: 10.1001/jama.1996.03540040057033. [DOI] [PubMed] [Google Scholar]
- 172.Potvin S, Marchand S. Hypoalgesia in schizophrenia is independent of antipsychotic drugs: a systematic quantitative review of experimental studies. Pain. 2008 Aug 15;138(1):70–78. doi: 10.1016/j.pain.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 173.Meier DE, Emmons CA, Litke A, Wallenstein S, Morrison RS. Characteristics of patients requesting and receiving physician-assisted death. Arch Intern Med. 2003 Jul 14;163(13):1537–1542. doi: 10.1001/archinte.163.13.1537. [DOI] [PubMed] [Google Scholar]
- 174.Geppert CMA. Between Pain and Addiction. Psychiatric Times. 2007;24(12) [Google Scholar]
- 175.Gastfriend DR, McLellan AT. Treatment matching. Theoretic basis and practical implications. Med Clin North Am. 1997 Jul;81(4):945–966. doi: 10.1016/s0025-7125(05)70557-5. [DOI] [PubMed] [Google Scholar]
- 176.Turk DC. The potential of treatment matching for subgroups of patients with chronic pain: lumping versus splitting. Clin J Pain. 2005 Jan-Feb;21(1):44–55. doi: 10.1097/00002508-200501000-00006. discussion 69–72. [DOI] [PubMed] [Google Scholar]
- 177.Lipchik GL, Nicholson RA, Penzien DB. Allocation of patients to conditions in headache clinical trials: randomization, stratification, and treatment matching. Headache. 2005 May;45(5):419–428. doi: 10.1111/j.1526-4610.2005.05093.x. [DOI] [PubMed] [Google Scholar]
- 178.Kupfer DJ, Regier DA. Why all of medicine should care about DSM-5. JAMA. May 19;303(19):1974–1975. doi: 10.1001/jama.2010.646. [DOI] [PubMed] [Google Scholar]
- 179.Flor H. Maladaptive plasticity, memory for pain and phantom limb pain: review and suggestions for new therapies. Expert Rev Neurother. 2008 May;8(5):809–818. doi: 10.1586/14737175.8.5.809. [DOI] [PubMed] [Google Scholar]
- 180.Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007 Jun;25(12):3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 181.Portenoy RK, Farrar JT, Backonja MM, et al. Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry study. Clin J Pain. 2007 May;23(4):287–299. doi: 10.1097/AJP.0b013e31802b582f. [DOI] [PubMed] [Google Scholar]
- 182.McPhie P. The origin of the alkaline inactivation of pepsinogen. Biochemistry. 1975 Dec 2;14(24):5253–5256. doi: 10.1021/bi00695a003. [DOI] [PubMed] [Google Scholar]
- 183.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999 Jun 29;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 184.Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999 Jun 29;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 185.Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003 Feb 14;963(1–2):203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- 186.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000 Apr 10;861(2):288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 187.Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000 Mar 15;47(6):526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 188.Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993 Apr;50(4):266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 189.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999 Nov 1;46(9):1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 190.Angrist BM, Gershon S. The phenomenology of experimentally induced amphetamine psychosis--preliminary observations. Biol Psychiatry. 1970 Apr;2(2):95–107. [PubMed] [Google Scholar]
- 191.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002 Feb;5(1):55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]