Dietary methionine restriction increased fat oxidation and decreased intrahepatic lipid content in individuals with metabolic syndrome.
Abstract
Objective:
In preclinical reports, restriction of dietary methionine intake was shown to enhance metabolic flexibility, improve lipid profiles, and reduce fat deposition. The present report is the outcome of a “proof of concept” study to evaluate the efficacy of dietary methionine restriction (MR) in humans with metabolic syndrome.
Methods:
Twenty-six obese subjects (six male and 20 female) meeting criteria for metabolic syndrome were randomized to a diet restricted to 2 mg methionine/kg body weight per day and were provided capsules containing either placebo (n = 12) or 33 mg methionine/kg body weight per day (n = 14). Energy expenditure, body composition, insulin sensitivity, and biomarkers of metabolic syndrome were measured before and after 16 wk on the respective diets.
Results:
Insulin sensitivity and biomarkers of metabolic syndrome improved comparably in both dietary groups. Rates of energy expenditure were unaffected by the diets, but dietary MR produced a significant increase in fat oxidation (MR, 12.1 ± 6.0% increase; control, 8.1 ± 3.3% decrease) and reduction in intrahepatic lipid content (MR liver/spleen attenuation ratio, 8.1 ± 3.3% increase; control ratio, 2.2 ± 2.1% increase) that was independent of the comparable reduction in weight and adiposity that occurred in both groups.
Conclusions:
Sixteen weeks of dietary MR in subjects with metabolic syndrome produced a shift in fuel oxidation that was independent of the weight loss, decreased adiposity, and improved insulin sensitivity that was common to both diets.
Metabolic syndrome represents a clinical state characterized by a cluster of pathologies that includes obesity, insulin resistance, and dysregulation of carbohydrate and lipid metabolism. Lifestyle modifications producing weight reduction improve biomarkers of metabolic syndrome (1–3), but the high rate of recidivism with strategies based on calorie restriction has prompted evaluation of alternative nutritional strategies. For example, postweaning methionine restriction in rodents reduced circulating lipids, increased metabolic flexibility, enhanced insulin sensitivity, and limited fat deposition by increasing total daily energy expenditure (EE) (4–10). Initiation of dietary methionine restriction (MR) after physical maturity also increased EE and limited fat accretion (9), but its efficacy in reducing preexisting adiposity and insulin resistance has not been evaluated. Therefore, our goal was to evaluate the short-term (16 wk) efficacy of dietary MR in a human cohort meeting the criteria for metabolic syndrome.
Subjects and Methods
Subjects
Twenty-six subjects (six male and 20 female; age, 50 ± 2 yr) completed this randomized, double-blind, placebo-controlled clinical trial. Inclusion criteria included males (waist circumference > 101.6 cm) and females (waist circumference > 88.9 cm) with a stable body weight (BW) (±2.27 kg) for the last 6 months and any two additional criteria from the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (11). Exclusion criteria included a history of diabetes, myocardial infarction, stroke, cancer, illness requiring regular medication, or pregnancy/breast feeding. Institutional Review Board approval was obtained, and study subjects gave verbal and written informed consent.
Nutritional intervention
The goal was to examine metabolic responses to limiting dietary methionine from approximately 35 mg/kg BW/d (control group) to approximately 2 mg/kg BW/d in the MR group (12). This required elimination of dietary meat, poultry, dairy, and grains, and was achieved using Hominex-2 medical food (Abbott Nutrition, Columbus, OH). This semisynthetic diet is a mixture of l-amino acids lacking methionine, but it provides 0.9 g of methionine sparing cystine/100 g of diet (Supplemental Tables 1 and 2, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Based on a target protein intake of 0.8 g/kg BW/d, methionine requirements of 12.6 mg/kg BW/d (13), and the methionine-sparing cystine content of Hominex-2, the diet would limit methionine to target levels of 2 mg/kg BW/d (12). Twice a day capsules supplemented methionine in the control group to 35 mg/kg BW/d, whereas placebo capsules were given to the MR group. Subjects were directed to consume Hominex-2 at a rate that provided 100% and approximately 75% of daily protein and energy requirements, respectively, with remaining energy made up by unlimited fruit and vegetable intake and limited intake of grains. After baseline measurements, subjects were balanced between treatments by race and gender.
Baseline assessment, nutritional instruction, and clinical analyses
A detailed description of the baseline assessment of study subjects, nutritional instruction, study protocol, and clinical analyses is provided in Supplemental Data.
Statistical analyses
The efficacy of dietary MR was assessed using a paired analysis to evaluate changes in biomarkers of metabolic syndrome between baseline (W0) and wk 16 (W16) for each subject in the two groups. This was accomplished by calculating the percentage change between W0 and W16 for each participant and comparing the group means of percentage change of each response variable using a t test. To test for diet effects on EE, a predictive equation was derived from measures of 24-h EE of all subjects at W0. Comparison of 24-h EE of the same subjects after 16 wk on the respective diets was conducted by calculating the residuals and testing for lack of fit relative to the predicted baseline regression equation as described previously (14). Protection from type I errors was set at 5% (α = 0.05).
Results
Means of the clinical and biochemical variables measured at W0 did not differ between groups (Table 1). An initial survey of the data suggested improvement in several clinical variables in each group over the course of the study, but analyses of means at W16 and W0 using standard t tests detected no change in any response for either group. This outcome reflects the inability of the Student's t test to properly account for the sources of variance when testing for treatment effects and the high variability among study subjects with metabolic syndrome in our sample. However, the current study was designed to be analyzed using a paired model, which effectively isolates within-group variation of individuals from the variance term used to test treatment effects and focuses on changes in responses of individuals within each group between W0 and W16.
Table 1.
Physiological characteristics before and after 16 wk of dietary methionine restriction (MR)
| Control |
MR |
|||
|---|---|---|---|---|
| W0 | W16a | W0 | W16a | |
| Age (yr) | 47 ± 3 | 51 ± 2 | ||
| Gender (M/F) | 3/9 | 3/11 | ||
| Race (n) | ||||
| Caucasian | 6 | 9 | ||
| African-American | 6 | 5 | ||
| Body weight (kg) | 100.0 ± 4.8 | 95.8 ± 4.8 | 104.1 ± 4.0 | 101.2 ± 4.3 |
| BMI (kg/m2) | 34.4 ± 1.5 | 33.0 ± 1.6 | 37.6 ± 1.3 | 36.6 ± 1.2 |
| Waist circumference (cm) | 106.7 ± 3.5 | 112.4 ± 2.6 | ||
| % Fat | 38 ± 2 | 37 ± 2 | 41 ± 1 | 40 ± 1 |
| FFM (kg) | 62.4 ± 3.9 | 60.7 ± 3.7 | 62.3 ± 2.8 | 60.9 ± 2.8 |
| SBP (mm Hg) | 125 ± 4 | 125 ± 3 | 128 ± 5 | 125 ± 4 |
| DBP (mm Hg) | 83 ± 3 | 81 ± 2 | 81 ± 3 | 78 ± 3 |
| Liver HU/spleen HUb | 1.16 ± 0.32 | 1.18 ± 0.03 | 1.05 ± 0.03 | 1.11 ± 0.04 |
| TAG (mmol/liter) | 2.37 ± 0.44 | 2.12 ± 0.44 | 2.29 ± 0.20 | 2.08 ± 0.17 |
| TC (mmol/liter) | 5.26 ± 0.41 | 5.15 ± 0.36 | 5.26 ± 0.26 | 5.10 ± .21 |
| LDL-C (mmol/liter) | 3.00 ± 0.36 | 3.21 ± 0.36 | 3.08 ± 0.29 | 3.03 ± 0.17 |
| HDL-C (mmol/liter) | 1.14 ± 0.06 | 1.13 ± 0.05 | 1.11 ± 0.03 | 1.14 ± 0.03 |
| FFA (mmol/liter) | 0.81 ± 0.09 | 0.63 ± 0.07 | 0.79 ± 0.06 | 0.68 ± 0.07 |
| Insulin (pmol/liter) | 136.2 ± 24.0 | 108.6 ± 17.4 | 125.4 ± 12.0 | 115.2 ± 15.6 |
| Glucose (mmol/liter) | 5.56 ± 0.11 | 5.39 ± 0.15 | 5.61 ± 0.15 | 5.39 ± .22 |
| Leptin (ng/ml) | 27.5 ± 5.2 | 25.3 ± 5.0 | 38.6 ± 4.1 | 37.2 ± 4.4 |
| Adiponectin (μg/ml) | 6.9 ± 1.2 | 8.0 ± 1.4 | 7.2 ± 1.4 | 8.8 ± 1.7 |
| Glucose pre-clamp (mmol/liter) | 5.57 ± 0.11 | 5.40 ± 0.14 | 5.63 ± 0.15 | 5.38 ± 0.12 |
| Fasting insulin pre-clamp (pmol/liter) | 131.6 ± 23.4 | 104.8 ± 16.6 | 121.6 ± 11.6 | 111.3 ± 14.6 |
| Glucose disposal (mg/min) | 461.5 ± 48.4 | 586.8 ± 41.8 | 416.6 ± 45.6 | 550.0 ± 53.1 |
| Insulin sensitivity (mg/kg FFM/min) | 5.87 ± 0.65 | 7.80 ± 0.67 | 5.71 ± 0.70 | 6.96 ± 0.84 |
| Plasma FFA during clamp (mmol/liter) | 0.077 ± 0.012 | 0.061 ± 0.008 | 0.081 ± 0.019 | 0.060 ± 0.012 |
| Total 24-h EE (kcal/d) | 2450 ± 122 | 2386 ± 113 | 2591 ± 86 | 2499 ± 107 |
| Fat oxidation (kcal/d) | 584 ± 109 | 351 ± 117 | 426 ± 129 | 752 ± 179 |
| CHO oxidation (kcal/d) | 1387 ± 122 | 1587 ± 147 | 1640 ± 130 | 1294 ± 96 |
| Protein oxidation (kcal/d) | 350 ± 58 | 327 ± 51 | 393 ± 29 | 323 ± 31 |
| 24-h RQ | 0.89 ± 0.01 | 0.92 ± 0.01 | 0.91 ± 0.02 | 0.88 ± 0.01 |
Values are expressed as overall means ± sem for subjects randomized to the control and MR groups at W0 and W16 on the respective diets. FFA concentrations were measured during the last 30 min of the clamp. As described in Subjects and Methods, a two-sample t test was used to determine differences between groups at W0. There were no statistically significant differences between groups for any of the characteristics at W0. M, Males; F, females; BMI, body mass index; FFM, fat-free mass; SBP, systolic blood pressure; DBP, diastolic blood pressure; TAG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FFA, free fatty acids; CHO, carbohydrate.
W16 values are provided here for comparison, but group differences were analyzed using two-sample t tests after calculating the percentage change between W0 and W16 characteristics for each subject (see Results and Fig. 2).
Interorgan liver fat was assessed by the ratio of attenuation in Hounsfield units (HU) measured by computed tomography scan in liver and spleen.
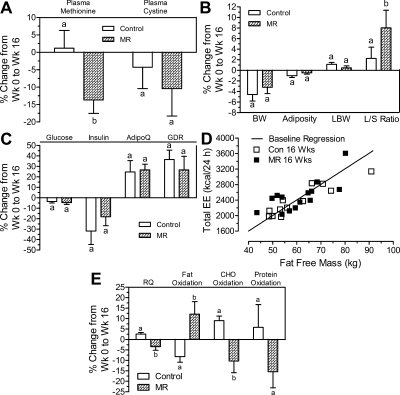
Dietary MR reduced plasma methionine by 13.8 ± 3.8% compared with a 1.2 ± 5.1% increase in the control group (P < 0.05; Fig. 1A). Plasma cystine was also reduced (∼10%) in the MR group, but this change did not differ from controls (Fig. 1A). Both groups lost weight and realized comparable improvements in several biomarkers of metabolic syndrome (Table 1 and Fig. 1, B and C). Fasting insulin decreased 10–15% in both groups, and plasma adiponectin increased approximately 25% in both groups (Fig. 1C). Plasma triglyceride levels decreased 20–25%, and modest decreases in free fatty acids and total cholesterol occurred in both groups (Table 1). In contrast, the percentage change in liver to spleen (L/S) attenuation from computed tomography scans was higher in the MR group (P < 0.05) relative to controls, indicative of a greater reduction in intrahepatic lipid content in the MR group (Fig. 1B).
Fig. 1.
A, Percentage differences between plasma methionine and cystine concentrations at W0 and W16. B and C, Percentage differences between body composition, L/S ratio, and markers of glucose metabolism at W0 and W16. L/S attenuations were calculated as previously described (14, 15). Glucose disposal rate was calculated as the mean rate of exogenous glucose infusion during steady-state insulin infusion during the last 30 min of the hyperinsulinemic-euglycemic clamp. D, Group differences in EE were evaluated by deriving a prediction equation relating lean body mass to EE using all 26 subjects at W0. The sum of the squared residuals of the W16 observations were then calculated relative to the regression line to test for evidence of a systematic change in EE within the groups. E, RQ was calculated as the ratio of volume of CO2 produced to volume of O2 consumed. Changes in the percentage of fat, carbohydrate, and protein oxidation were estimated using the change in RQ from W0 in both groups. The percentage change between W0 and W16 characteristics were calculated for each subject in the Control and methionine-restricted (MR) groups, analyzed using a paired t test to compare group changes, and means annotated with different letters in A–E differ at P < 0.05. LBW, Lean body weight; AdipoQ, adiponectin; CHO, carbohydrate.
Glucose disposal rates increased by 25–30% between W0 and W16 in both groups (Fig. 1C), whereas 24-h EE was unchanged over the course of the study in both groups (Table 1). This conclusion is also supported by Fig. 1D, which shows that total EE for each group at W16, relative to the regression line at W0, is unchanged over the course of the study. In contrast, 24-h respiratory quotient (RQ) was differentially affected by the two diets (P < 0.05), with RQ increasing by approximately 3% in controls and decreasing by approximately 4% in the MR group (Fig. 1E). The changes in RQ reflected a 10% decrease in fat oxidation and 8% increase in carbohydrate oxidation in the control group. In contrast, fat (∼12% increase) and carbohydrate (∼10% decrease) oxidation were reciprocally altered by dietary MR (P < 0.05). Protein oxidation was unaltered in the control group and modestly decreased in the MR group (Fig. 1E). Lastly, a χ2 analysis was conducted to compare the number of study subject responders and nonresponders for each biomarker. The results parallel the outcome of the paired analysis (Supplemental Table 3).
Discussion
The present report describes the first clinical evaluation of dietary MR as a viable intervention for metabolic syndrome in humans. Comprehensive preclinical data show that dietary MR increases EE, reduces plasma lipids, limits fat accretion, enhances insulin sensitivity, and increases longevity in rodents and flies (4–10, 15–19), but its efficacy in obese individuals with metabolic disease is unknown. In the present report, dietary MR produced a significant increase in fat oxidation and decrease in hepatic lipid, but failed to decrease adiposity, increase EE, or enhance insulin sensitivity. The observed effects on fuel selection and hepatic lipid metabolism are consistent with preclinical data, where dietary MR enhanced the shift from carbohydrate to fat oxidation upon fasting and reduced hepatic and circulating triglycerides (9). Although the mechanisms are unknown, dietary MR also increases plasma adiponectin (9) and reduces lipogenic gene expression in the liver (Plaisance, E. P., A. Boudreau, K. L. Hill, and T. W. Gettys, unpublished observations). Although it is not possible to examine hepatic gene expression in the present study, it is interesting that plasma adiponectin increased by an average of 27% in all 14 study subjects on the MR diet. A differential diet effect was not detected because adiponectin was increased to a similar extent in the control group. It seems likely that the increases in adiponectin are linked to the comparable reductions in adiposity between groups and may be causative with respect to the observed 25–30% improvement in insulin sensitivity in both groups. Thus, with the exception of fuel selection and hepatic lipid content, the beneficial effects of dietary MR cannot be distinguished from the beneficial effects that accrued from the decrease in adiposity observed in the control group.
The experimental diet (Hominex-2) used to limit methionine is a commercial food designed to provide nutritional support for patients with pyridoxine-unresponsive homocystinuria or hypermethioninemia. Study subjects were directed to consume quantities of Hominex-2 that would provide 100% of their protein and 75% of their daily energy requirements. Subjects were not allowed meat, dairy, and most grains, but were allowed unlimited intake of fruits and vegetables to make up their daily energy deficit.
The comparable weight loss in both groups, without any change in EE, indicates that they were unsuccessful. This differs in two important ways from our preclinical studies (9), where initiation of MR after maturity produced a perfectly matched increase in energy intake (∼25%) and EE (∼25%) and BW homeostasis over 6 months. The failure of dietary MR to increase EE in the present study, relative to the implied increase observed by Epner et al. (12) and the robust increases in our preclinical studies (9, 10), raises several interesting questions. First, plasma methionine levels suggest that dietary compliance in our study (14% decrease) was less than that achieved by Epner et al. (12), who observed a 75% decrease. Given the certainty of compliance in rodent studies, a dosage effect of MR on EE seems possible. Indeed, the effect of dietary MR on longevity was not fully realized until methionine was restricted by approximately 80% (Orentreich Foundation, unpublished observations). It is unclear whether the effects of dietary MR on EE follow a similar dose-response relationship. However, the clear effects of dietary MR on liver fat and fuel selection argue that the degree of MR achieved herein produced significant metabolic responses. That these responses were independent of the decrease in BW and adiposity is supported by the opposite effects of the control diet on fuel selection and intrahepatic lipid content.
Collectively, the present results suggest that the experimental approach used here to limit methionine is suboptimal, resulting in a control group whose negative energy balance confounded the beneficial effects of weight loss with the metabolic effects of MR. The poor palatability resulted in high withdrawal rates and raised questions about compliance and achieving the intended degree of MR. Preclinical studies have established that dietary MR produces many beneficial metabolic responses in rodents (9, 10). To determine whether dietary MR can reproduce the full complement of these responses in humans, it will be necessary to develop palatable foods in which methionine has been selectively removed. Notwithstanding the limitations of the experimental approach employed herein, the present studies make a strong case that limitation of dietary methionine has metabolic effects that are beneficial in subjects with metabolic disease.
Acknowledgments
We thank Natalie Lenard, Tara Henagan, Aaron Adamson, Anne Gooch, Dawn Rachal, Jennifer Rood, and the clinical support staff of the Pennington Biomedical Research Center for their excellent support of this study. We also thank Eric Ravussin (Pennington Biomedical Research Center) for helpful discussions, and Barbara Marriage and Abbott Nutrition for providing the commercially available medical food, Hominex-2, used in this study.
This work was supported by the Orentreich Family Foundation, the National Center for Complementary and Alternative Medicine, and the Office of Dietary Supplements (P50AT002776-01), and in part by National Institutes of Health (NIH) Grant P20-RR021945 from the National Center for Research Resources and NIH Nutrition Obesity Research Center (NORC) Grant 1P30 DK072476.
Clinical Trial Registration: NCT00640757.
Disclosure Summary: The authors have no relevant conflicts of interest to disclose.
Footnotes
- BW
- Body weight
- EE
- energy expenditure
- L/S
- liver to spleen (ratio)
- MR
- methionine restriction
- RQ
- respiratory quotient
- W0
- baseline
- W16
- week 16.
References
- 1. Bloomgarden ZT. 2004. Definitions of the insulin resistance syndrome: the 1st World Congress on the Insulin Resistance Syndrome. Diabetes Care 27:824–830 [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. 2004. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol 24:e19–e24 [DOI] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richie JP, Jr, Komninou D, Leutzinger Y, Kleinman W, Orentreich N, Malloy V, Zimmerman JA. 2004. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition 20:800–805 [DOI] [PubMed] [Google Scholar]
- 5. Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8:1302–1307 [DOI] [PubMed] [Google Scholar]
- 6. Orentreich N, Matias JR, DeFelice A, Zimmerman JA. 1993. Low methionine ingestion by rats extends life span. J Nutr 123:269–274 [DOI] [PubMed] [Google Scholar]
- 7. Sun L, Sadighi Akha AA, Miller RA, Harper JM. 2009. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 64:711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. 2006. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5:305–314 [DOI] [PubMed] [Google Scholar]
- 9. Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, Shin J, Huypens P, Malloy VL, Plaisance EP, Krajcik RA, Orentreich N, Gettys TW. 2010. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol 299:R728–R739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plaisance EP, Henagan TM, Echlin H, Boudreau A, Hill KL, Lenard NR, Hasek BE, Orentreich N, Gettys TW. 2010. Role of β-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol 299:R740–R750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 12. Epner DE, Morrow S, Wilcox M, Houghton JL. 2002. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr Cancer 42:158–166 [DOI] [PubMed] [Google Scholar]
- 13. Di Buono M, Wykes LJ, Ball RO, Pencharz PB. 2001. Dietary cysteine reduces the methionine requirement in men. Am J Clin Nutr 74:761–766 [DOI] [PubMed] [Google Scholar]
- 14. Ravussin E, Burnand B, Schutz Y, Jéquier E. 1982. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr 35:35:566–573 [DOI] [PubMed] [Google Scholar]
- 15. Perrone CE, Mattocks DA, Hristopoulos G, Plummer JD, Krajcik RA, Orentreich N. 2008. Methionine restriction effects on 11β-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J Lipid Res 49:12–23 [DOI] [PubMed] [Google Scholar]
- 16. Komninou D, Leutzinger Y, Reddy BS, Richie JP., Jr 2006. Methionine restriction inhibits colon carcinogenesis. Nutr Cancer 54:202–208 [DOI] [PubMed] [Google Scholar]
- 17. Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. 2005. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmerman JA, Malloy V, Krajcik R, Orentreich N. 2003. Nutritional control of aging. Exp Gerontol 38:47–52 [DOI] [PubMed] [Google Scholar]
- 19. Grandison RC, Piper MD, Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462:1061–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]