Signaling mechanisms were identified by which hesperetin stimulates endothelial nitric oxide production that informed this translational study, demonstrating that oral hesperidin treatment improves endothelial dysfunction.
Abstract
Context:
Hesperidin, a citrus flavonoid, and its metabolite hesperetin may have vascular actions relevant to their health benefits. Molecular and physiological mechanisms of hesperetin actions are unknown.
Objective:
We tested whether hesperetin stimulates production of nitric oxide (NO) from vascular endothelium and evaluated endothelial function in subjects with metabolic syndrome on oral hesperidin therapy.
Design, Setting, and Interventions:
Cellular mechanisms of action of hesperetin were evaluated in bovine aortic endothelial cells (BAEC) in primary culture. A randomized, placebo-controlled, double-blind, crossover trial examined whether oral hesperidin administration (500 mg once daily for 3 wk) improves endothelial function in individuals with metabolic syndrome (n = 24).
Main Outcome Measure:
We measured the difference in brachial artery flow-mediated dilation between placebo and hesperidin treatment periods.
Results:
Treatment of BAEC with hesperetin acutely stimulated phosphorylation of Src, Akt, AMP kinase, and endothelial NO synthase to produce NO; this required generation of H2O2. Increased adhesion of monocytes to BAEC and expression of vascular cell adhesion molecule-1 in response to TNF-α treatment was reduced by pretreatment with hesperetin. In the clinical study, when compared with placebo, hesperidin treatment increased flow-mediated dilation (10.26 ± 1.19 vs. 7.78 ± 0.76%; P = 0.02) and reduced concentrations of circulating inflammatory biomarkers (high-sensitivity C-reactive protein, serum amyloid A protein, soluble E-selectin).
Conclusions:
Novel mechanisms for hesperetin action in endothelial cells inform effects of oral hesperidin treatment to improve endothelial dysfunction and reduce circulating markers of inflammation in our exploratory clinical trial. Hesperetin has vasculoprotective actions that may explain beneficial cardiovascular effects of citrus consumption.
Diabetes, obesity, the metabolic syndrome, and their cardiovascular complications cluster together due, in part, to reciprocal relationships between insulin resistance and endothelial dysfunction (1, 2). Indeed, therapeutic interventions in animal models and humans that reduce endothelial dysfunction often simultaneously ameliorate insulin resistance (and vice versa) (1–3). Flavonoids (flavones, flavonols, flavanones, and isoflavones) are polyphenols present in many foods of plant origin including citrus fruits, green tea, red wine, and cocoa (4). Large epidemiological studies link increased consumption of flavonoid-rich foods with reduced cardiovascular morbidity and mortality (5–7). However, molecular and physiological mechanisms underlying potential cardiovascular health benefits of flavonoid consumption are poorly understood.
The flavanone glycosides hesperidin and naringin are present in citrus fruits. Hesperidin (hesperetin-7-O-rutinoside) is deglycosylated by intestinal microflora in the colon to produce the active aglycone hesperetin, which is then absorbed in the gut and subsequently glucuronidated to hesperetin glucuronide that circulates in plasma (8, 9). Hesperidin and naringin may have antiinflammatory, hypolipidemic, and vasoprotective properties (10–14). Similar to insulin, naringenin reduces apolipoprotein B (apoB)-100 secretion in HepG2 hepatoma cells by activating both phosphatidylinositol 3-kinase (PI3K)- and MAPK-dependent signaling pathways (15–17). Dyslipidemias contribute to endothelial dysfunction and accelerated atherosclerosis, in part, by promoting imbalances between endothelial-derived vasoconstrictors and vasodilators, growth factors, and pro- and anticoagulant factors (18). Endothelial dysfunction often manifests as impaired endothelium-dependent vasodilator actions secondary to decreased production and/or bioavailability of nitric oxide (NO). We recently demonstrated that the green tea polyphenol epigallocatechin gallate (EGCG; a flavan-3-ol) acutely stimulates production of NO from vascular endothelium by activating signaling pathways involving Fyn/PI3K/Akt/endothelial NO synthase (eNOS) (19). Moreover, 3-wk treatment of spontaneously hypertensive rats (SHR; a model of human metabolic syndrome) with EGCG lowers blood pressure, reduces endothelial dysfunction and insulin resistance, and protects against myocardial ischemia/reperfusion injury (20). In human studies, EGCG or polyphenol-rich cocoa intake improves endothelial dysfunction (21–23). Therefore, in the present study, we hypothesized that hesperetin, a flavonoid related to EGCG, acutely stimulates production of NO from vascular endothelium to mediate beneficial vascular and antiinflammatory actions in humans.
Subjects and Methods
In vitro experiments
Cell culture
Bovine aortic endothelial cells (BAEC) in primary culture were used between passages 3 and 5 as previously described (24). BAEC were serum-starved overnight with endothelial basal medium before experimental procedures.
Evaluation of NO production in fixed cells
Production of NO in BAEC was assessed using the NO-specific fluorescent dye 4, 5-diaminofluorescein diacetate (Cayman Chemical, Ann Arbor, MI) as described (19, 24).
Monocyte adhesion assay
U937 cells (6 × 105) labeled with calcein-AM were incubated with confluent BAEC pretreated with hesperetin (10 μm for 5 h) and/or TNF-α (10 ng/ml for 5 h). Cocultured cells were washed three times with PBS, and then images of monocytes adhering to BAEC were obtained.
Supplemental Data providing further details on cell culture, evaluation of NO production, immunoblotting, and monocyte adhesion assay have been published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Clinical study
Study design and study subjects
We conducted a randomized, placebo-controlled, double-blind, crossover trial of hesperidin (500 mg/d orally for 3 wk) in individuals with metabolic syndrome. This study was conducted exclusively at the Clinical Center for Atherosclerosis at the University of Rome “Tor Vergata” (Rome, Italy) (www.ClinicalTrials.gov Identifier: NCT00914251). The study protocol was approved by the Institutional Ethics Board, and all procedures followed were in accordance with institutional guidelines. Adults between 21 and 65 yr of age with metabolic syndrome [according to National Cholesterol Education Program Adult Treatment Panel III criteria (25), detailed in Supplemental Table 1] were recruited from the local community through newspaper advertisements. Specific exclusion criteria are detailed in the Supplemental Data.
Of the 35 individuals screened, 28 were deemed eligible for the study (Supplemental Fig. 4). Informed consent was obtained from each subject. Enrolled subjects were randomly assigned in a double-blind fashion (block randomization by Clinical Center Pharmacy) to the initial arm of the study of either hesperidin (500 mg/d) or matching placebo for 3 wk. This was followed by a 3-d washout period. Subjects were then crossed over to the other treatment arm for an additional 3 wk. Endothelial function, metabolic parameters, and markers of inflammation were assessed at baseline and after each 3-wk treatment period. Compliance with treatment was assessed by capsule counts at the end of the study. Study investigators and participants were blinded to treatment assignment, and assignment codes were not available to investigators until the entire study and the database had been completed and secured. Participants were explicitly counseled before initiation of the study to maintain their usual physical activity and dietary habits, including avoiding foods rich in hesperidin. No additional medications (apart from medications at baseline) were allowed during the study period to avoid confounding effects of other drugs.
Hesperidin/placebo description
Hesperidin (98% pure) was a generous gift from Blue California (Rancho Santa Margarita, CA). Capsules containing 500 mg hesperidin and matching placebo capsules containing cellulose were formulated by the pharmacy at Clinical Center for Atherosclerosis at the University of Rome “Tor Vergata” (Rome, Italy). The dose (500 mg once a day) and duration (3 wk) of hesperidin treatment is based on available safety data in humans (12, 26).
Endothelial function testing using flow-mediated dilation (FMD)
Assessment of endothelial function was conducted in the fasting state (24 h after the last dose of hesperidin or placebo) using a standardized procedure at approximately the same point in time and before all of the scheduled metabolic tests. Endothelium-dependent and -independent vasodilator functions were assessed as previously reported (27, 28).
Details on endothelial function testing using flow-mediated dilation, measurement of circulating endothelial adhesion molecules and proinflammatory markers, and other laboratory assays are provided in the Supplemental Data.
Statistical analyses
Data from participants who completed all phases of the protocol (n = 24) were analyzed according to a preestablished statistical analysis plan. The primary outcome measure for this study was prospectively designated as the difference in FMD between placebo and hesperidin treatment periods. All other comparisons were considered secondary. Therefore, power analysis was calculated for this outcome in this two-treatment crossover study. A sample size of n = 20 was deemed sufficient to provide 80% power in detecting a difference of 2.0% or greater in FMD of the brachial artery between placebo and hesperidin, with α = 0.05 based on our previous studies (27). The presence of skewed data was evaluated by visual inspection of Q-Q plots, stem and leaf plots, or box plots and was verified by the Shapiro-Wilk test for normal distribution. After subtracting baseline observations, comparison of various posttreatment parameters was performed using crossover ANOVA (29). This analysis takes into account specific treatment arm, treatment order, and treatment effects. Thus, we explicitly tested for carryover effects in our crossover study. Because of the exploratory nature of this clinical study, no adjustments were made for multiple comparisons. The statistical software StatsDirect version 2.7.2 (StatsDirect Ltd., Chershire, UK) was used for data analysis.
Results
Hesperetin acutely stimulates phosphorylation of Akt, AMP kinase (AMPK), and eNOS to mediate NO production in vascular endothelial cells
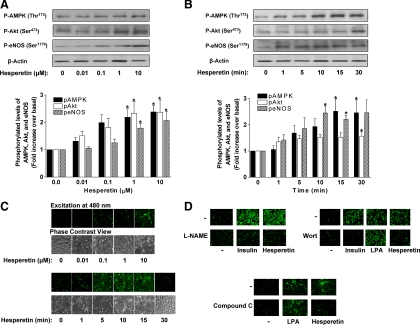
Treatment of BAEC in primary culture with hesperetin acutely increased cellular levels of phosphorylated (p) AMPK (Thr172) and Akt (Ser473) (indicative of enzyme activation) in a concentration- and time-dependent fashion (Fig. 1, A and B). Preliminary studies suggested that there was no time-dependent increase in pAMPK, pAKT, or peNOS levels in unstimulated cells (data not shown). Both AMPK and Akt regulate activity of eNOS by phosphorylating Ser1179, resulting in increased production of NO (30, 31). Accordingly, hesperetin treatment of BAEC also increased p-eNOS (Ser1179) with a corresponding concentration- and time-dependent increase in hesperetin-stimulated NO production in BAEC (Fig. 1C). Both the concentration- and time-dependent increases in pAMPK, pAkt, and peNOS were analyzed by one-way ANOVA and a Dunnett's post hoc test. In addition, individual t tests comparing 1 μm, 10 μm, or the 10 min to baseline conditions suggest that hesperetin significantly (P < 0.05) activated pAMPK, pAKT, and peNOS acutely and at low concentrations. Peak production of NO paralleled eNOS phosphorylation and occurred after 5–10 min of hesperetin treatment. As expected, pretreatment of cells with the NO synthase inhibitor L-Nitro-Arginine Methyl Ester blocked NO production in response to insulin (positive control) or hesperetin (Fig. 1D). Insulin phosphorylates and activates eNOS through a PI3K/Akt-dependent pathway (32). By contrast, lyso phosphatidic acid (LPA) (a phospholipid growth factor that mobilizes intracellular calcium) activates eNOS without phosphorylating eNOS (33). Importantly, pretreatment of BAEC with wortmannin (an inhibitor of PI3K) inhibited insulin- (positive control) and hesperetin-stimulated, but not LPA-stimulated (negative control), production of NO. Compound C, an inhibitor of AMPK, substantially inhibited production of NO in response to hesperetin but not to LPA (negative control) (Fig. 1D). Thus, similar to the green tea polyphenol EGCG, the citrus polyphenol hesperetin stimulates PI3K, which results in activation of downstream serine kinases Akt and AMPK to phosphorylate and activate eNOS to produce NO in vascular endothelium (19).
Fig. 1.
Hesperetin acutely stimulates phosphorylation of Akt, AMPK, and eNOS to produce NO in a concentration- and time-dependent manner in vascular endothelial cells. A, BAEC were serum-starved overnight and then treated with hesperetin for 10 min at the indicated concentrations. Cell lysates were immunoblotted with anti-phospho-AMPK (Thr172), anti-phospho-Akt (Ser473), anti-phospho-eNOS (Ser1179), and anti-β actin (loading control) antibodies. Representative blots are shown for experiments that were repeated independently five to six times (top). Scanning densitometry was used to quantify results of multiple independent experiments represented in panel A normalized to loading control (mean ± sem) (bottom). Significant concentration-dependent effects of hesperetin to increase pAMPK, pAkt, and peNOS were observed (P < 0.05; one-way ANOVA); *, P < 0.05 for Dunnett's comparisons to control (0 μm, hesperetin) for pAMPK, pAkt, and peNOS. B, BAEC were serum-starved overnight and then treated with hesperetin (10 μm) for the indicated durations. Cell lysates were immunoblotted as in panel A. Representative blots are shown for experiments that were repeated independently five to six times (top). Scanning densitometry was used to quantify results of multiple independent experiments represented in panel B normalized to loading control (mean ± sem) (bottom). Significant time-dependent effects of hesperetin to increase pAMPK, pAkt, and peNOS were observed (P < 0.05; one-way ANOVA); *, P < 0.05 for Dunnett's comparisons to control (0 min) for pAMPK, pAkt, or peNOS. C, BAEC were loaded with DAF-2-DA as described in Subjects and Methods before treatment with hesperetin for 10 min at the indicated concentrations (top) or with hesperetin (10 μm) for the indicated durations (bottom). Cells were then fixed and viewed as described in Subjects and Methods. Emission of green light (510 nm) from cells excited by light at 480 nm is indicative of NO production. Phase contrast views of cells corresponding to images in the upper panels are also shown. A representative experiment is shown for experiments that were repeated independently three times. D, BAEC prepared as in panel C were treated without or with insulin (100 nm, 5 min), hesperetin (10 μm, 10 min), or LPA (5 μm, 5 min). In some groups of cells, the NO synthase inhibitor L-Nitro-Arginine Methyl Ester (100 μm), PI3K inhibitor wortmannin (100 nm), or AMPK inhibitor compound C was added 30 min before loading cells with DAF-2-DA. A representative set of experiments is shown for experiments that were repeated independently five times.
Additional signaling mechanisms determining hesperetin-stimulated production of NO
We previously found that EGCG stimulates production of reactive oxygen species (ROS) leading to activation of Fyn upstream of PI3K/Akt/eNOS in endothelial cells (19). Therefore, we next demonstrated that hesperetin (and the related citrus polyphenol naringenin) also stimulated production of H2O2 in BAEC (Supplemental Fig. 1A). Moreover, pretreatment of BAEC with N-acetylcysteine (NAC) scavenged the production of ROS and inhibited hesperetin and naringenin-stimulated phosphorylation of Src (Tyr418), Akt (Ser473), and eNOS (Ser1179) (Supplemental Fig. 1B). Similar to EGCG (positive control), hesperetin-stimulated phosphorylation of Src, Akt, and eNOS and production of NO were all inhibited by pretreatment of BAEC with the Src inhibitor PP2, further supporting the role of Src family kinases (Supplemental Fig. 2). By contrast, PP2 pretreatment failed to inhibit insulin-stimulated NO production [negative control; insulin activates PI3K/Akt/eNOS in a src-independent fashion (19)]. Thus, signaling mechanisms by which hesperetin/naringenin activate eNOS to regulate production of NO in endothelial cells are similar to those we previously reported for EGCG (19) (Supplemental Fig. 3).
Effects of hesperetin on expression of adhesion molecules and monocyte adhesion to endothelium
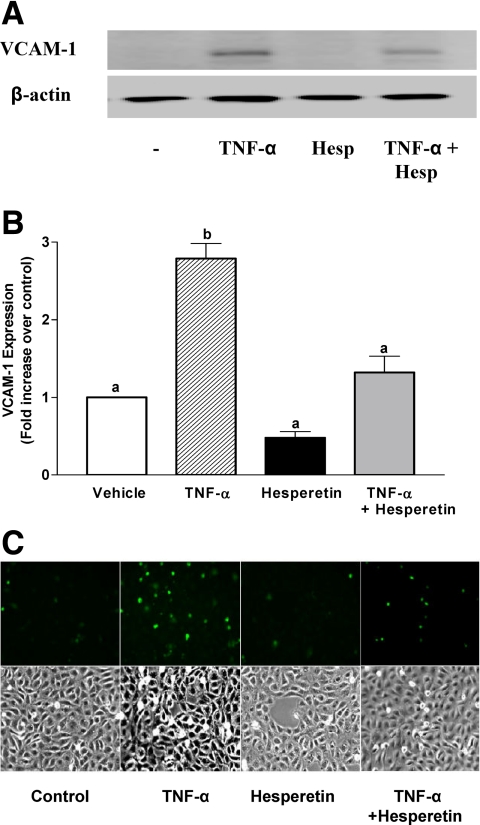
Endothelial production of NO exerts antiatherogenic, antithrombotic, and antiinflammatory actions (34). Therefore, we examined the potential of hesperetin pretreatment to reduce TNF-α-stimulated expression of vascular cell adhesion molecule-1 (VCAM-1) and adhesion of monocytes to endothelial cells (Fig. 2). As expected, when compared with vehicle-treated control cells, treatment of BAEC with the proinflammatory cytokine TNF-α increased expression of VCAM-1 with a corresponding increase in adhesion of labeled monocytes. Importantly, hesperetin pretreatment of BAEC substantially reduced these proinflammatory actions of TNF-α. Thus, in addition to stimulating acute production of NO from endothelium, hesperetin may help attenuate endothelial dysfunction by opposing atherogenic actions of proinflammatory cytokines.
Fig. 2.
TNF-α-stimulated expression of VCAM-1 and increased adhesion of monocytes to endothelial cells is diminished by hesperetin pretreatment. A, BAEC were serum-starved overnight and then treated without or with TNF-α (10 ng/ml, 5 h). In some groups, cells were pretreated with hesperetin (10 μm, 1 h) before treatment with TNF-α. Cell lysates were immunoblotted using antibodies against VCAM-1 or β-actin (loading control). Representative immunoblots are shown for experiments that were repeated independently four times. B, Scanning densitometry was used to quantify results of multiple independent experiments represented in panel A normalized to loading control (mean ± sem; n = 4). Bars labeled with different letters are significantly different from each other. *, P < 0.001, by one-way ANOVA and Bonferroni's posttest. C, BAEC were treated without or with TNF-α in the absence or presence of pretreatment with hesperetin as in panel A. Then, labeled U937 monocytes were cocultured with BAEC as described in Subjects and Methods. Adherent monocytes were visualized using an epifluorescent microscope (upper panel). Phase contrast view of cells is shown in lower panel. A representative set of experiments is shown for experiments that were repeated independently five times.
Translational clinical study with hesperidin
Based upon novel cellular mechanisms of action of hesperetin that we described in endothelial cells above, we designed an exploratory translational clinical intervention study to evaluate whether oral hesperidin administration improves endothelial dysfunction and circulating markers of inflammation in subjects with metabolic syndrome. From among 35 individuals screened, 28 were deemed eligible for study run-in. Among 28 subjects randomized, 24 subjects completed the entire study. One subject was lost to follow-up, and three subjects withdrew after recommendation by their personal physicians for reasons unrelated to the study (Supplemental Fig. 4). There were no adverse effects reported by the study participants during either the placebo or hesperidin treatment periods.
Baseline clinical characteristics of study subjects
This study used a sequential crossover design where each participant received either placebo or hesperidin first in random order, followed by a washout period and then crossover to the other treatment arm. Baseline clinical characteristics of 24 subjects who completed our entire study are reported in Table 1. Baseline medications included oral antidiabetic therapy (71%; n = 17), antihypertensive therapy (42%; n = 10), statin therapy (4%; n = 1), and antiplatelet therapy (13%; n = 3) and were used in similar proportions in subjects assigned to each initial treatment arm. As expected for patients with metabolic syndrome, subjects were obese, hypertensive, and insulin resistant.
Table 1.
Baseline clinical characteristics of 24 study subjects who completed entire study
| Clinical parameters | Overall cohort | Placebo-hesperidin | Hesperidin-placebo |
|---|---|---|---|
| n | 24 | 12 | 12 |
| Age (yr) | 52 ± 2 | 50 ± 3 | 53 ± 1 |
| Gender (M/F) | 15/9 | 6/6 | 9/3 |
| Waist circumference (cm) | 112 ± 3 | 114 ± 4 | 110 ± 4 |
| Body mass index (kg/m2) | 34.7 ± 1.5 | 35.4 ± 2.0 | 33.9 ± 2.2 |
| Vascular parameters | |||
| Systolic blood pressure (mm Hg) | 138 ± 3 | 136 ± 4 | 139 ± 5 |
| Diastolic blood pressure (mm Hg) | 89 ± 2 | 90 ± 3 | 88 ± 3 |
| FMD (%) | 8.24 ± 0.88 | 8.61 ± 1.15 | 7.87 ± 1.40 |
| Nitroglycerin-mediated dilation (%) | 13.98 ± 1.32 | 15.75 ± 1.93 | 12.21 ± 1.73 |
| Metabolic parameters | |||
| Fasting plasma glucose (mg/dl) | 132 ± 12 | 116 ± 10 | 148 ± 21 |
| Fasting plasma insulin (μU/ml) | 21.3 ± 2.1 | 23.2 ± 2.8 | 19.3 ± 2.9 |
| QUICKI | 0.298 ± 0.005 | 0.296 ± 0.005 | 0.299 ± 0.008 |
| Hemoglobin A1c (%) | 6.6 ± 0.2 | 6.1 ± 0.3 | 7.1 ± 0.3 |
| Lipids (mg/dl) | |||
| Total cholesterol | 179 ± 8 | 194 ± 12 | 165 ± 11 |
| LDL | 119 ± 8 | 131 ± 10 | 107 ± 11 |
| ApoB | 90 ± 4 | 92 ± 6 | 89 ± 7 |
| Lipoprotein [a] | 9.4 (2.5–21.7) | 9.0 (3.7–19.9) | 11.6 (6.8–32.2) |
| HDL | 37 ± 2 | 42 ± 2 | 33 ± 3 |
| Apolipoprotein A-I | 134 ± 5 | 148 ± 5 | 121 ± 7 |
| Triglycerides | 158 ± 11 | 160 ± 17 | 157 ± 14 |
Results are shown for overall group and subgroups who were randomized to either placebo or hesperidin treatment arm first. Values represent mean ± sem or median (25th-75th percentile). M, Males; F, females.
Effects of hesperidin treatment on vascular parameters
When compared with placebo treatment, 3-wk treatment with hesperidin caused significant improvement in FMD (Table 2). Vasodilation of the brachial artery in response to sublingual nitroglycerin was not significantly different between placebo and hesperidin treatment (Table 2). When compared with baseline values, FMD values at the end of the placebo-treatment period were not significantly different (Table 2; P = 0.63) indicating no substantial deterioration of endothelial function. Moreover, a posteriori comparison of FMD values at baseline and at the end of the hesperidin-treatment period suggest that administration of hesperidin significantly improved endothelial function over baseline in our cohort (Table 2; P = 0.05). Thus, using an additional analysis that does not consider placebo treatment, hesperidin treatment also improved endothelial function. Using appropriate statistical analyses, we did not observe any significant carryover effects (i.e. treatment-period interactions were nonsignificant) between initial hesperidin or placebo treatment arms with respect to any of the measured vascular parameters in our crossover study.
Table 2.
Effect of hesperidin treatment (500 mg/d for 3 wk) on vascular function in study subjects who completed the entire study
| Vascular parameters (n = 24) | Baseline | Placebo | Hesperidin | Treatment effects | P value |
|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | 138 ± 3 | 132 ± 2 | 134 ± 3 | 2.7 (−1.25, 6.66) | 0.16 |
| Diastolic blood pressure (mm Hg) | 89 ± 2 | 90 ± 2 | 90 ± 2 | 0.6 (−2.1, 3.4) | 0.64 |
| FMD (%) | 8.24 ± 0.88 | 7.78 ± 0.76 | 10.26 ± 1.19 | 2.47 (0.39, 4.55) | 0.02 |
| Nitroglycerin-mediated dilation (%) | 13.98 ± 1.32 | 14.40 ± 1.02 | 14.04 ± 1.08 | −0.35 (−1.03, 0.32) | 0.30 |
Values shown at baseline and after treatment with placebo or hesperidin are expressed as mean ± sem or median (25th-75th percentile). After subtracting baseline observations, comparison of various posttreatment parameters was performed using crossover ANOVA. This analysis takes into account specific treatment arm, treatment order, and treatment effects. Treatment effects are expressed as mean (95% CI) or ratio (95% CI). P values are for posttreatment comparisons (placebo vs. hesperidin).
Effects of hesperidin treatment on circulating inflammatory markers and soluble adhesion molecules
Based upon our cellular studies, we explored mechanisms whereby hesperidin treatment may improve endothelial dysfunction by measuring circulating levels of inflammatory markers and soluble adhesion molecules in our study subjects (Table 3). When compared with placebo treatment, 3-wk treatment with hesperidin significantly reduced circulating concentrations of high-sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA) protein, and soluble E-selectin (sE-selectin). Post-placebo treatment levels of vascular adhesion molecules were not significantly different from baseline levels (P > 0.33). Similarly, post-placebo treatment levels of hsCRP and SAA protein were not significantly different from baseline levels (hsCRP, P = 0.24; SAA, P = 0.32). Direct comparisons of post-hesperidin treatment values with baseline levels indicate that hesperidin treatment reduces circulating levels of hsCRP, SAA, and sE-selectin (hsCRP, P = 0.007; SAA, P = 0.002; sE-selectin, P = 0.002).
Table 3.
Effect of hesperidin treatment (500 mg/d for 3 wk) on circulating inflammatory markers and vascular adhesion molecules in study subjects who completed the entire study
| Circulating biomarkers (n = 24) | Baseline | Placebo | Hesperidin | Treatment effect | P value |
|---|---|---|---|---|---|
| Inflammatory markers | |||||
| hsCRP (mg/liter) | 3.9 (2.0–6.5) | 4.9 (2.3–7.4) | 2.6 (1.1–5.7) | 0.68 (0.51, 0.91) | 0.01 |
| SAA protein (mg/liter) | 7.3 (5.6–6.1) | 8.0 (5.6–11.2) | 5.6 (3.2–7.8) | 0.62 (0.47, 0.82) | 0.001 |
| Fibrinogen (mg/dl) | 320 ± 14 | 330 ± 16 | 331 ± 15 | 0.60 (−19.9, 21.2) | 0.94 |
| Homocysteine (μm/liter) | 11.9 (10.3–14.9) | 13.6 (10.6–16.7) | 13.0 (10.2–15.5) | 0.95 (0.87, 1.05) | 0.30 |
| Vascular adhesion molecules (ng/ml) | |||||
| VCAM | 956 ± 29 | 976 ± 30 | 950 ± 27 | −28 (−71, 16) | 0.19 |
| Intercellular adhesion molecule | 291 ± 6 | 299 ± 7 | 294 ± 7 | −4 (−19, 11) | 0.59 |
| sE-selectin | 31 ± 2 | 31 ± 2 | 27 ± 2 | −4 (−6, −1) | 0.002 |
Values shown at baseline and after treatment with placebo or hesperidin are expressed as mean ± sem or median (25th-75th percentile). After subtracting baseline observations, comparison of various posttreatment parameters was performed using crossover ANOVA. This analysis takes into account specific treatment arm, treatment order, and treatment effects. Treatment effects are expressed as mean (95% CI) or ratio (95% CI). P values are for posttreatment comparisons (placebo vs. hesperidin).
Effects of hesperidin treatment on metabolic parameters
When compared with placebo treatment, 3-wk treatment with hesperidin caused significant decreases in circulating concentrations of total cholesterol and apoB and increased high-density lipoprotein (HDL) (Table 4). No significant differences were observed in low-density lipoprotein (LDL), lipoprotein [a], apolipoprotein A-I, triglycerides, fasting plasma glucose, or fasting plasma insulin concentrations. Interestingly, hesperidin treatment caused a trend toward improving insulin resistance as assessed by quantitative insulin-sensitivity check index (QUICKI) (P = 0.06).
Table 4.
Effect of hesperidin treatment (500 mg/d for 3 wk) on metabolic parameters in study subjects who completed the entire study
| Metabolic parameters (n = 24) | Baseline | Placebo | Hesperidin | Treatment effect | P value |
|---|---|---|---|---|---|
| Body mass index (kg/m2) | 34.7 ± 1.5 | 34.7 ± 1.5 | 34.7 ± 1.5 | 0.004 (−0.2, 0.2) | 0.95 |
| Waist circumference (cm) | 112 ± 3 | 112 ± 3 | 112 ± 3 | 0.42 (−0.15, 0.98) | 0.14 |
| Fasting plasma glucose (mg/dl) | 132 ± 12 | 129 ± 7 | 126 ± 6 | −3.66 (−9.39, 2.05) | 0.19 |
| Fasting plasma insulin (μU/ml) | 21.3 ± 2.1 | 21.1 ± 1.9 | 20.2 ± 2.1 | −0.86 (−2.29, 0.56) | 0.22 |
| QUICKI | 0.298 ± 0.004 | 0.297 ± 0.003 | 0.300 ± 0.004 | 0.003 (−0.0002, 0.006) | 0.06 |
| Hemoglobin A1c (%) | 6.61 ± 0.24 | 6.63 ± 0.24 | 6.59 ± 0.24 | −0.04 (−0.09, 0.01) | 0.12 |
| Lipids (mg/dl) | |||||
| Total cholesterol | 179 ± 8 | 185 ± 9 | 174 ± 8 | −11.4 (−21.6, −1.1) | 0.03 |
| LDL | 119 ± 8 | 122 ± 6 | 115 ± 6 | −7 (−16, 2) | 0.11 |
| ApoB | 90 ± 4 | 93 ± 4 | 88 ± 4 | −4.79 (−9.36, −0.22) | 0.04 |
| Lipoprotein [a] | 9.4 (2.5–21.7) | 12.0 (5.0–24.7) | 10.5 (5.0–26.5) | 0.87 (0.70, 1.08) | 0.20 |
| HDL | 37 ± 2 | 34 ± 2 | 35 ± 2 | 1.3 (−0.01, 2.51) | 0.05 |
| Apolipoprotein A-I | 134 ± 5 | 136 ± 7 | 137 ± 6 | 0.33 (−4.94, 5.61) | 0.89 |
| Triglycerides | 158 ± 11 | 179 ± 17 | 164 ± 10 | −15 (−44, 14) | 0.28 |
Values shown at baseline and after treatment with placebo or hesperidin are expressed as mean ± sem or median (25th-75th percentile). After subtracting baseline observations, comparison of various posttreatment parameters was performed using crossover ANOVA. This analysis takes into account specific treatment arm, treatment order, and treatment effects. Treatment effects are expressed as mean (95% CI) or ratio (95% CI). P values are for posttreatment comparisons (placebo vs. hesperidin).
Discussion
In the present study, we elucidated mechanisms of action for hesperetin to stimulate production of NO from endothelial cells to inform our translational clinical study demonstrating that oral hesperidin treatment significantly improved endothelial function in subjects with the metabolic syndrome.
Hesperetin acutely stimulates production of NO in vascular endothelial cells
Treatment of BAEC with hesperetin acutely stimulated production of NO, requiring generation of ROS and subsequent activation of Src, Akt, AMPK, and eNOS. The H2O2-sensitive fluorescent dye CM-H2DCFDA we used is specific but semiquantitative at best. Hesperetin-stimulated H2O2 production was substantially blunted by treatment of BAEC with the ROS scavenger NAC (Supplemental Fig. 1A). Intracellular H2O2 is an upstream activator of Src family kinases that promotes endothelium-dependent vasorelaxation (35–37). Pretreating cells with NAC inhibited hesperetin-stimulated phosphorylation of Src family kinases, Akt, AMPK, and eNOS (Supplemental Fig. 1B). Thus, H2O2 is likely upstream from activation of Src family kinases, Akt, AMPK, and eNOS. In endothelium, enzymatic sources of ROS include reduced NADPH oxidase, xanthine oxidase, cytochrome P450, and the mitochondrial respiratory chain. Other ROS in addition to H2O2 may be involved in hesperetin-induced activation of eNOS. However, studies of the related green tea polyphenol EGCG suggest that activation of Akt, eNOS, and NO-dependent vasorelaxation are critically dependent on hydroxyl groups in EGCG and not on other enzymatic sources of ROS (38). Hesperetin is a polyphenol containing hydroxyl groups that may generate ROS through autooxidation similar to EGCG (38).
We and others have previously linked Src kinases to activation of PI3K/Akt/eNOS and production of NO in endothelial cells (19, 39). Our studies do not delineate the relative importance of Akt or AMPK signaling pathways in mediating the NO-stimulatory actions of hesperetin. However, they both seem important because pretreatment of cells with either compound C (AMPK inhibitor) or wortmannin (PI3K inhibitor upstream of Akt) was sufficient to block hesperetin-stimulated production of NO. Our results are consistent with previous studies suggesting endothelial-dependent vasorelaxant actions of naringenin in rat aortic rings (40, 41) and reports where treatment of endothelial cells with naringenin or hesperetin for 24 h increases nitrite concentrations in conditioned media (42). However, previous studies did not provide detailed mechanisms of action for hesperetin.
In contrast to our findings of hesperetin as a prooxidant, Ghanim et al. (43) report that treatment of human mononuclear cells with hesperetin reduced ROS production in vitro. The apparent inconsistencies in the actions of hesperetin on ROS in these studies may reflect differences in cell type, the concentration of hesperetin used, assays used to measure ROS, specific ROS measured, and the relative levels of superoxide dismutase and catalases in the specific cell types. Consistent with this, it is well recognized that flavonoids and flavanones may be prooxidant or antioxidant depending on concentrations and structure of the polyphenol as well as cellular redox environment (44). Orange juice (a principal source of hesperidin) prevents postprandial ROS generation, induction of p47phox (a key component of reduced NADPH oxidase), and proinflammatory changes induced by a high-fat and high-carbohydrate meal (45). Thus, orange juice by reducing oxidative stress may increase NO bioavailability and endothelial function. However, other components of orange juice such as ascorbic acid may also contribute to the reduction in ROS production in addition to the actions of flavanones, hesperidin and naringin. Nevertheless, our finding of hesperetin stimulating H2O2 generation provides insights into vascular actions of citrus polyphenols we observed in our clinical study. Identifying putative specific cellular receptors for hesperidin or autooxidation to generate ROS is important for future investigation into vascular actions of hesperetin but is beyond the scope of the present study.
Hesperetin treatment opposes proatherogenic actions of TNF-α in endothelium
Production of NO by endothelium opposes effects of proinflammatory cytokines to mediate atherogenesis and endothelial dysfunction (34). Increased expression of VCAM-1 plays a role in recruiting monocytes to endothelium to promote atherogenesis (46). We demonstrated that pretreatment of BAEC with hesperetin protected against TNF-α-stimulated increases in expression of VCAM-1 and adhesion of monocytes. This may be related to hesperetin-stimulated production of NO. Our findings are consistent with a study demonstrating that naringenin (0.1% supplement in diet for 8 wk) reduces VCAM-1 expression in aortas of rabbits fed a high-cholesterol diet (47).
Clinical study considerations
One strength of our clinical study is the crossover design that ensures that comparisons of placebo and hesperidin treatment are in perfectly matched groups. Primary sources of dietary hesperetin and naringenin are fruit juices [∼59 mg/d; orange juice contains 200–600 mg hesperidin/liter (48)]. Ingested flavanones are absorbed in the colon as active aglycones (hesperetin and naringenin) and subsequently metabolized to glucuronide forms (48). Based on results from in vitro studies by us and others, hesperetin, the aglycone, stimulates NO production and evokes vascular relaxation (42, 49). However, vasoactive properties of hesperetin conjugates both in vitro and in vivo are unknown. Administration of 130–220 mg of hesperetin (as orange juice) results in peak plasma hesperetin concentrations of 1.3–2.2 μm/liter with an elimination half-life of approximately 2.5 h (48). These concentrations are within the range used in our in vitro studies to activate eNOS (1–10 μm). Accordingly, our oral dose of 500 mg of hesperidin is likely sufficient to achieve plasma concentrations in the micromolar range. Previous clinical studies have demonstrated that oral administration of hesperidin at 500 mg/d for 6 wk is safe (12, 26). The primary prospectively designated outcome measure for this exploratory clinical study was difference in FMD between placebo and hesperidin treatment periods. Based on existing literature (12, 26, 48), we administered an oral dose of hesperidin that was both safe and sufficient to achieve plasma concentrations in the micromolar range. Nevertheless, to better interpret the physiological significance of in vitro study results, clinical studies measuring the steady-state plasma concentrations of hesperetin are needed and are planned for the future. Regarding safety parameters monitored, we evaluated liver function tests and routine biochemistry tests and did not observe any abnormalities in these tests for any of our subjects during the entire study. A dose-finding study is beyond the scope of this initial exploratory study and is prohibitively complicated.
Clinical endothelial function
When compared with a historical cohort of healthy, lean, normotensive individuals with normal glucose tolerance from our previous study (28), our subjects with metabolic syndrome manifested endothelial dysfunction (FMD %, 8.24 ± 0.88 vs. 15.10 ± 1.2; P < 0.0001). In individuals with low risk of coronary heart disease, a 1.4% increase in FMD decreases Framingham risk by 1% (50). Daily consumption of hesperidin (500 mg) for 3 wk improved FMD by approximately 2.5% in our subjects (when compared with placebo). An unexplained trend of the FMD in the placebo group to worsen (although not statistically significant per se) may potentially contribute to a larger effect size in our study. However, additional analyses comparing baseline values with post-hesperidin treatment values confirmed a significant effect of hesperidin to improve FMD. Thus, hesperidin therapy is likely to generate improvements in endothelial function that are clinically meaningful. Nitroglycerin-mediated vasodilation was similar after placebo and hesperidin treatment, suggesting that vascular smooth muscle function is unaffected by hesperidin treatment. Considering the short half-life of hesperetin and the fact that FMD was assessed at least 24 h after the last placebo or hesperidin dose, beneficial outcomes of hesperidin therapy on FMD are likely due to chronic effects (3-wk therapy) on endothelium. In rodent studies, sustained antihypertensive effects of chronic EGCG therapy are NO-dependent (20). Hesperetin and EGCG use similar signaling mechanisms to stimulate NO production. Thus, improvement in FMD after chronic hesperidin therapy in our subjects is likely linked to acute actions of hesperetin to stimulate NO production. Nevertheless, it remains possible that acute effects of hesperetin on endothelium may not be related to the beneficial outcomes in our clinical study.
Circulating biomarkers of inflammation and endothelial dysfunction
Both endothelial dysfunction and insulin resistance may be worsened by increased circulating levels of proinflammatory markers including TNF-α, hsCRP, and endothelial adhesion molecules (VCAM-1, sE-selectin) (1, 2, 51, 52). Consistent with endothelial-protective effects of hesperetin in BAEC, we observed significant decreases in hsCRP, SAA protein, and sE-selectin that may help explain improved endothelial function in hesperidin-treated subjects.
Clinical metabolic parameters
Although hesperidin treatment reduced total cholesterol and apoB and increased HDL levels in our subjects, the clinical relevance of these modest changes is unknown. These results are in keeping with cellular studies showing that hesperetin and naringenin have actions similar to insulin to inhibit assembly and secretion of apoB-100-containing lipoproteins from hepatoma cells (15–17). Hepatic overproduction of apoB-containing lipoproteins is characteristic of dyslipidemias associated with insulin resistance. In individuals with hypercholesterolemia, consumption of orange juice or hesperidin for 4–6 wk increases HDL and lowers triglycerides, respectively (12, 53). A more recent study found no effect of hesperidin (800 mg/d for 4 wk) on serum cholesterol or triglyceride levels (54). Unfortunately, no pharmacokinetic data are available in either our study or previously published studies. Consequently, differences among results of these various studies are not easily explained. Future studies designed to specifically examine effects of citrus flavanones on lipid metabolism are warranted to confirm our initial findings.
We observed a trend toward improvement in insulin sensitivity, a secondary outcome measure assessed by the surrogate index QUICKI (P = 0.06). Our study may be slightly underpowered to detect improvement in insulin sensitivity with QUICKI. We are planning studies to address a primary outcome of change in insulin sensitivity using the reference glucose clamp technique with adequate power and sample size to definitively follow up on this important issue. Hesperidin-induced improvement in endothelial function may augment the vasodilator actions of insulin to enhance glucose disposal. Orange juice reduces high-fat and high-carbohydrate meal-induced increases in the expression of suppressor of cytokine signaling-3, a negative modulator of insulin signaling (45). Thus, it is possible that hesperidin may increase glucose disposal by positively modulating insulin signaling and increasing muscle glucose delivery by its action on the endothelium.
Conclusions
We elucidated novel mechanisms of action for hesperetin to stimulate production of NO in vascular endothelial cells that may oppose atherogenic actions of proinflammatory cytokines. Our clinical translational study rigorously demonstrated daily oral consumption of hesperidin for 3-wk improved endothelial function, reduced circulating biomarkers of inflammation, and favorably altered lipid profiles in subjects with the metabolic syndrome. Cellular and physiological actions of hesperetin may help explain cardiovascular health benefits of citrus fruit consumption.
Acknowledgments
This work was supported in part by the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health (to M.J.Q.) and by a Fondi d'Ateneo grant from the University of Tor Vergata (to M.T.). Hesperidin was a generous gift from Blue California (Rancho Santa Margarita, CA).
www.ClinicalTrials.gov Identifier: NCT00914251
The corresponding author had full access to the data in the study and had final responsibility for the decision to submit for publication. All authors have read and agree to the manuscript as written.
Disclosure Summary: There are no potential conflicts of interest relevant to this article.
Footnotes
- AMPK
- AMP kinase
- apoB
- apolipoprotein B
- BAEC
- bovine aortic endothelial cells
- EGCG
- epigallocatechin gallate
- eNOS
- endothelial NO synthase
- FMD
- flow-mediated dilation
- HDL
- high-density lipoprotein
- hsCRP
- high-sensitivity C-reactive protein
- lpa
- lysophosphatidic acid
- NAC
- N-acetylcysteine
- NO
- nitric oxide
- p-
- phosphorylated
- PI3K
- phosphatidylinositol 3-kinase
- QUICKI
- quantitative insulin-sensitivity check index
- ROS
- reactive oxygen species
- SAA
- serum amyloid A
- sE-selectin
- soluble E-selectin
- VCAM-1
- vascular cell adhesion molecule-1.
References
- 1. Kim JA, Montagnani M, Koh KK, Quon MJ. 2006. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 2. Muniyappa R, Montagnani M, Koh KK, Quon MJ. 2007. Cardiovascular actions of insulin. Endocr Rev 28:463–491 [DOI] [PubMed] [Google Scholar]
- 3. Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. 2006. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55:3594–3603 [DOI] [PubMed] [Google Scholar]
- 4. Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. 2006. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem 54:9966–9977 [DOI] [PubMed] [Google Scholar]
- 5. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. 1993. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342:1007–1011 [DOI] [PubMed] [Google Scholar]
- 6. Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H, Feskens EJM, Hollman PCH, Katan MB. 1995. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155:381–386 [PubMed] [Google Scholar]
- 7. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr 2007. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 85:895–909 [DOI] [PubMed] [Google Scholar]
- 8. Williamson G, Manach C. 2005. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81:243S–255S [DOI] [PubMed] [Google Scholar]
- 9. Nielsen IL, Chee WS, Poulsen L, Offord-Cavin E, Rasmussen SE, Frederiksen H, Enslen M, Barron D, Horcajada MN, Williamson G. 2006. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr 136:404–408 [DOI] [PubMed] [Google Scholar]
- 10. Choe SC, Kim HS, Jeong TS, Bok SH, Park YB. 2001. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol 38:947–955 [DOI] [PubMed] [Google Scholar]
- 11. Kim JY, Jung KJ, Choi JS, Chung HY. 2006. Modulation of the age-related nuclear factor-κB (NF-κB) pathway by hesperetin. Aging Cell 5:401–411 [DOI] [PubMed] [Google Scholar]
- 12. Miwa Y, Yamada M, Sunayama T, Mitsuzumi H, Tsuzaki Y, Chaen H, Mishima Y, Kibata M. 2004. Effects of glucosyl hesperidin on serum lipids in hyperlipidemic subjects: preferential reduction in elevated serum triglyceride level. J Nutr Sci Vitaminol (Tokyo) 50:211–218 [DOI] [PubMed] [Google Scholar]
- 13. Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, Kim HK, Jeong TS, Choi MS. 2003. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr 22:561–568 [DOI] [PubMed] [Google Scholar]
- 14. Ohtsuki K, Abe A, Mitsuzuwi H, Kondo M, Uemura K, Iwasaki Y, Kondo Y. 2002. Effects of long-term administration of hesperidin and glucosyl hesperidin to spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 48:420–422 [DOI] [PubMed] [Google Scholar]
- 15. Allister EM, Borradaile NM, Edwards JY, Huff MW. 2005. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes 54:1676–1683 [DOI] [PubMed] [Google Scholar]
- 16. Borradaile NM, de Dreu LE, Huff MW. 2003. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes 52:2554–2561 [DOI] [PubMed] [Google Scholar]
- 17. Borradaile NM, Carroll KK, Kurowska EM. 1999. Regulation of HepG2 cell apolipoprotein B metabolism by the citrus flavanones hesperetin and naringenin. Lipids 34:591–598 [DOI] [PubMed] [Google Scholar]
- 18. Davignon J, Ganz P. 2004. Role of endothelial dysfunction in atherosclerosis. Circulation 109:III27–III32 [DOI] [PubMed] [Google Scholar]
- 19. Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. 2007. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282:13736–13745 [DOI] [PubMed] [Google Scholar]
- 20. Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. 2007. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292:E1378–E1387 [DOI] [PubMed] [Google Scholar]
- 21. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. 2008. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 88:38–50 [DOI] [PubMed] [Google Scholar]
- 22. Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. 2008. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr 88:1685–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, Keaney JF, Jr, Vita JA. 2007. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr 26:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. 2006. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol 20:1153–1163 [DOI] [PubMed] [Google Scholar]
- 25. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 24:e13–e18 [DOI] [PubMed] [Google Scholar]
- 26. Miwa Y, Mitsuzumi H, Sunayama T, Yamada M, Okada K, Kubota M, Chaen H, Mishima Y, Kibata M. 2005. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J Nutr Sci Vitaminol (Tokyo) 51:460–470 [DOI] [PubMed] [Google Scholar]
- 27. Rizza S, Tesauro M, Cardillo C, Galli A, Iantorno M, Gigli F, Sbraccia P, Federici M, Quon MJ, Lauro D. 2009. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis 206:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tesauro M, Rizza S, Iantorno M, Campia U, Cardillo C, Lauro D, Leo R, Turriziani M, Cocciolillo GC, Fusco A, Panza JA, Scuteri A, Federici M, Lauro R, Quon MJ. 2007. Vascular, metabolic, and inflammatory abnormalities in normoglycemic offspring of patients with type 2 diabetes mellitus. Metabolism 56:413–419 [DOI] [PubMed] [Google Scholar]
- 29. Armitage P, Berry G, Matthews J. 1994. Statistical methods in medical research. 4th ed Oxford, UK: Blackwell Science [Google Scholar]
- 30. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605 [DOI] [PubMed] [Google Scholar]
- 31. Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. 1999. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443:285–289 [DOI] [PubMed] [Google Scholar]
- 32. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. 2000. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101:1539–1545 [DOI] [PubMed] [Google Scholar]
- 33. Montagnani M, Chen H, Barr VA, Quon MJ. 2001. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276:30392–30398 [DOI] [PubMed] [Google Scholar]
- 34. Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. 2006. Nitric oxide and atherosclerosis: an update. Nitric Oxide 15:265–279 [DOI] [PubMed] [Google Scholar]
- 35. Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649–1652 [DOI] [PubMed] [Google Scholar]
- 36. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. 1995. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299 [DOI] [PubMed] [Google Scholar]
- 37. Rubanyi GM, Vanhoutte PM. 1986. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol 250:H815–H821 [DOI] [PubMed] [Google Scholar]
- 38. Auger C, Kim JH, Chabert P, Chaabi M, Anselm E, Lanciaux X, Lobstein A, Schini-Kerth VB. 2010. The EGCG-induced redox-sensitive activation of endothelial nitric oxide synthase and relaxation are critically dependent on hydroxyl moieties. Biochem Biophys Res Commun 393:162–167 [DOI] [PubMed] [Google Scholar]
- 39. Thomas SR, Chen K, Keaney JF., Jr 2002. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277:6017–6024 [DOI] [PubMed] [Google Scholar]
- 40. Orallo F, Alvarez E, Basaran H, Lugnier C. 2004. Comparative study of the vasorelaxant activity, superoxide-scavenging ability and cyclic nucleotide phosphodiesterase-inhibitory effects of hesperetin and hesperidin. Naunyn Schmiedebergs Arch Pharmacol 370:452–463 [DOI] [PubMed] [Google Scholar]
- 41. Orallo F, Camiña M, Alvarez E, Basaran H, Lugnier C. 2005. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (+/−)-naringenin. Planta Med 71:99–107 [DOI] [PubMed] [Google Scholar]
- 42. Liu L, Xu DM, Cheng YY. 2008. Distinct effects of naringenin and hesperetin on nitric oxide production from endothelial cells. J Agric Food Chem 56:824–829 [DOI] [PubMed] [Google Scholar]
- 43. Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Sia CL, Dandona P. 2007. Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care 30:1406–1411 [DOI] [PubMed] [Google Scholar]
- 44. Lambert JD, Elias RJ. 2010. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. 2010. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr 91:940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Price DT, Loscalzo J. 1999. Cellular adhesion molecules and atherogenesis. Am J Med 107:85–97 [DOI] [PubMed] [Google Scholar]
- 47. Lee CH, Jeong TS, Choi YK, Hyun BH, Oh GT, Kim EH, Kim JR, Han JI, Bok SH. 2001. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun 284:681–688 [DOI] [PubMed] [Google Scholar]
- 48. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. 2005. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto M, Suzuki A, Hase T. 2008. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 54:95–98 [DOI] [PubMed] [Google Scholar]
- 50. Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. 2005. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol 45:1987–1993 [DOI] [PubMed] [Google Scholar]
- 51. Fernández-Real JM, Ricart W. 2003. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 24:278–301 [DOI] [PubMed] [Google Scholar]
- 52. Tanigaki K, Mineo C, Yuhanna IS, Chambliss KL, Quon MJ, Bonvini E, Shaul PW. 2009. C-Reactive protein inhibits insulin activation of endothelial nitric oxide synthase via the immunoreceptor tyrosine-based inhibition motif of FcγRIIB and SHIP-1. Circ Res 104:1275–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piché LA, Serratore P. 2000. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr 72:1095–1100 [DOI] [PubMed] [Google Scholar]
- 54. Demonty I, Lin Y, Zebregs YE, Vermeer MA, van der Knaap HC, Jäkel M, Trautwein EA. 2010. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr 140:1615–1620 [DOI] [PubMed] [Google Scholar]