This review summarizes evidence supporting a genetic contribution to bone loss, and current progress towards identifying bone loss-related genes.
Abstract
Context:
A strong genetic influence on bone mineral density has been long established, and modern genotyping technologies have generated a flurry of new discoveries about the genetic determinants of bone mineral density (BMD) measured at a single time point. However, much less is known about the genetics of age-related bone loss. Identifying bone loss-related genes may provide new routes for therapeutic intervention and osteoporosis prevention.
Evidence Acquisition:
A review of published peer-reviewed literature on the genetics of bone loss was performed. Relevant studies were summarized, most of which were drawn from the period 1990–2010.
Evidence Synthesis:
Although bone loss is a challenging phenotype, available evidence supports a substantial genetic contribution. Some of the genes identified from recent genome-wide association studies of cross-sectional BMD are attractive candidate genes for bone loss, most notably genes in the nuclear factor κB and estrogen endocrine pathways. New insights into the biology of skeletal development and regulation of bone turnover have inspired new hypotheses about genetic regulation of bone loss and may provide new directions for identifying genes associated with bone loss.
Conclusions:
Although recent genome-wide association and candidate gene studies have begun to identify genes that influence BMD, efforts to identify susceptibility genes specific for bone loss have proceeded more slowly. Nevertheless, clues are beginning to emerge on where to look, and as population studies accumulate, there is hope that important bone loss susceptibility genes will soon be identified.
Osteoporotic fractures are a significant public health burden. Excess mortality and morbidity including pain, disability, kyphosis, social isolation, and negative psychological sequelae after fracture are well documented (1–3). Hip, spine, and wrist fractures are the most common osteoporotic fractures with fractures at the hip and spine responsible for a disproportionate amount of osteoporosis morbidity (3). In the United States alone, over 3 million fractures are projected by 2025 at a cost of 25.3 billion dollars a year (4).
Given that low bone mineral density (BMD) is one of the strongest risk factors for osteoporotic fracture, a mainstay of fracture prevention is maintenance of BMD by lifestyle and/or pharmacological intervention. However, there is marked variation in the rate at which BMD declines with age, with known risk factors accounting for only a small portion of this variation. Heritability and family studies have consistently demonstrated a substantial genetic contribution to BMD. This has spurred efforts to identify genes contributing to BMD and the rate of bone loss, and these efforts have accelerated in recent years with advances in genomics. Finding genes for bone loss is significant because the pathways uncovered may lead to new pharmacotherapeutic targets and insights into osteoporosis prevention.
In this review, we provide an overview of current efforts to identify genes related to age-related bone loss. We begin by summarizing the lines of evidence implicating a genetic contribution to bone loss and then describe the progress made in identifying individual genes involved and what they might do. There have been numerous excellent reviews recently on genetics of BMD and hip fracture (5–8); our goal is not to summarize these, but rather to review more specifically current information on genetics of bone loss. Throughout this review, the terms BMD change and bone loss are sometimes used interchangeably, although they are not exactly the same thing.
Bone Loss Is a Challenging Phenotype
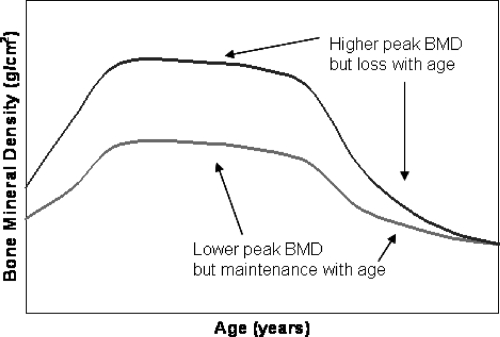
Bone strength cannot be directly measured in vivo, but BMD is highly correlated with bone strength and is commonly used in the clinic to assess fracture risk (9). There is a large and growing literature on the genetics of BMD that is based largely on a one-time measure of BMD. One-time measures, of course, convey limited information on the rate of bone loss. For example, as illustrated in Fig. 1, a high rate of bone loss can occur despite acquisition of high peak BMD. Thus, serial measures of BMD are required to more directly measure change in BMD, although it must be appreciated that the inherent variation in BMD change will be larger than the variation in BMD measured at a single time point because BMD change requires two separate measurements, each having variation associated with it. Measurement errors may be further compounded if there are equipment changes over time and multiple measures were not obtained using the same scanning technology, or if adequate measures are not taken to standardize different instruments. Thus, BMD change is a noisier phenotype than BMD alone, and the interval of follow-up must be long enough to ensure that inter-individual variation exceeds the measurement error.
Fig. 1.
Cross-sectional measures of BMD are influenced by both peak BMD and rate of BMD loss with age.
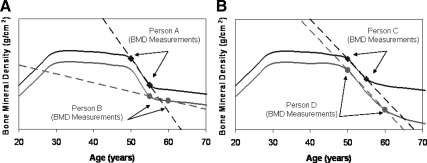
Although change phenotypes defined by estimating the slope from two serial measurements typically incorporate error from both time points, measurement of BMD change is further complicated by the fact that the rate of BMD change for a given individual is not constant, but rather is a function of age, including elapsed time since menopause in women. Consequently, comparisons of BMD change can be problematic if study subjects are not the same age or if the interval between baseline and follow-up BMD measures differs markedly among subjects so that the rate of bone loss is not constant within the interval. Consider persons A and B in Fig. 2A, who have similar rates of bone loss but whose rates of bone loss (slope shown as dotted line) appear strikingly different as a consequence of their being measured at different ages. Although in practice one can adjust for differences in age between subjects to allow for different rates of bone loss at different ages, the more relevant variable (at least in women) is probably time since menopause, and this may not always be available. Persons C and D (Fig. 2B), on the other hand, appear to have similar measures of bone loss, but in reality do not. Their baseline BMD was measured at the same age, but person D's follow-up BMD was measured 5 yr after person C's.
Fig. 2.
Comparability of BMD change between individuals depends on age (A) and duration of follow-up (B).
Epidemiology of Bone Loss
Prospective studies have revealed that increased rates of bone loss are associated with increased mortality (10) and conversely that maintenance of hip BMD is associated with lower rates of fracture, disability, and mortality (11–13). BMD increases through young adulthood until achievement of peak BMD and then declines beginning in the fifth and sixth decades (14–16). The rate of change in BMD does not occur uniformly across all sites, and in some studies, no significant decline in BMD is observed at the lumbar spine, although it is possible that real changes are obscured due to coexistent osteoarthritis or vascular calcification (16, 17). Despite these overall trends, there is substantial variability in the rate of decline of BMD after young adulthood. For example, not all individuals experience loss of BMD with age at the hip. There are significant gender differences in the rate of BMD decline, with females on average experiencing accelerated decline in BMD at the menopause transition and then continuing to experience greater rates of BMD decline than men after menopause (1, 18, 19).
Other notable risk factors for BMD change include low body mass index (BMI), smoking, and weight loss (18–20). Body composition is a particularly important risk factor for bone loss with low BMI and weight loss associated with higher rates of BMD loss (11, 20, 21). There is some indication that weight loss is a more important predictor of bone loss than body weight. In a study of older Caucasian women, weight change accounted for twice as much variance in bone loss as baseline body weight (20). Another study of men aged 60–75 yr noted that 4-yr BMD change was more closely associated with change in BMI than baseline BMI (21). Reduced mechanical loading of bone due to lower body weight is implicated as one reason for bone loss; however, weight loss or low BMI may also be marking loss of lean body mass and/or compromised nutritional status, which would have negative consequences on bone.
A number of medications (e.g. glucocorticoids, androgen deprivation therapy, and aromatase inhibitors) (22) and comorbidities are associated with bone loss and secondary osteoporosis. A variety of organ system dysfunctions, including but not limited to endocrine disorders (e.g. hyperparathyroidism), gastrointestinal disease (e.g. malabsorption), and nutritional deficits (e.g. calcium), can also lead to bone loss (23).
Heritability and Family Studies
Although numerous studies have documented the high heritability of BMD, far fewer data are available on the heritability of BMD change. Although genes account for 40–90% of variation in BMD overall (24–26), most studies find heritability to be higher in younger compared with older populations. In one multigenerational family study, heritability of BMD was estimated to peak at age 26, although the confidence interval around this estimate was large (27). One interpretation of these data is that variability in BMD is relatively lower at younger vs. older ages with genes accounting for a larger proportion of this variation and that the higher variation in BMD at older ages (documented explicitly in Ref. 28) is a consequence of age-related variation attributed to environmental and possibly genetic sources.
Table 1 summarizes studies estimating heritability of BMD change. The first of these were twin studies that included small sample sizes (46 and 40 twin pairs). In the first study, 16-yr changes in BMD at the forearm was estimated in veterans of World War II, and the correlations in BMD change were found to be similar between monozygotic (MZ) and dizygotic (DZ) twin pairs (29), thus providing no evidence for genetic influences on this trait in men. Reanalysis with an expanded sample size (111 total twin pairs) found the correlations in forearm BMD change to be only modestly higher in MZ twin pairs compared with DZ twin pairs, but virtually identical after adjusting for smoking and alcohol use, thus eliminating any evidence for heritability once accounting for the fact that MZ twins are more likely to share these environmental exposures (29–31).
Table 1.
Studies estimating heritability of BMD change
| Sample | Key results | Author (Ref.) |
|---|---|---|
| 21 MZ and 19 DZ twin pairs aged 24–65 yr; mostly women | h2 of 3-yr BMD Δ = 20% (femoral neck, NS), 76% (spine) | Kelly (31) |
| 327 Mexican-Americans aged 25–45 yr | h2 of 5.5-yr BMD Δ = 34% (hip), 27–34% (forearm), 22% (spine, NS) | Shaffer (33) |
| 300 Mexican-Americans aged 45 yr and older | h2 of 5.5-yr BMD Δ = 44% (hip), 31% (forearm), 42% (spine) | Shaffer (34) |
| 230 premenopausal sister pairs (338 total women) from Indiana (mean age 35 yr) | h2 of 5.7-yr BMD Δ = 40% (femoral neck) | Hui (32) |
| 25 MZ and 21 DZ male twin pairs (World War II veterans) aged 45–55 yr; sample later expanded to 57 MZ and 54 DZ pairs and reanalyzed | h2 of 16-yr BMD Δ ∼ 0% (forearm) | Christian (29); Slemenda (30) |
| 177 MZ and 185 DZ twin pairs, all peri- or postmenopausal women, aged 45–82 yr | h2 of 4.9-yr BMD Δ = 49% (forearm), 38% (spine). h2 of BMD Δ at hip not significant | Makovey (35) |
| 152 MZ and 204 DZ twin pairs, all postmenopausal women, aged 40 yr and older | h2 of 8-yr BMD Δ = 47% (femoral neck), 56% (forearm), 44% (spine, NS) | Zhai (36) |
BMD Δ, BMD change; h2, heritability; NS, Not statistically significant.
In contrast, Kelly et al. (31) studied predominantly female twins and reported substantially higher correlations in 3-yr changes in BMD of femoral neck and lumbar spine in MZ compared with DZ twin pairs, yielding heritability estimates of 60–80%. However, the confidence intervals around these estimates were large due to a relatively small sample size (40 total twin pairs), and only the heritability of BMD change at the lumbar spine was statistically greater than zero. Moreover, this sample included twins whose ages ranged widely (mean age ∼45 yr).
Several more recent studies have estimated heritability of BMD change in homogeneous populations of either younger or older adults. For example, Hui et al. (32) estimated the heritability of BMD change at the femoral neck over a 5- to 6-yr period in 230 premenopausal sister pairs from Indiana (mean age 35 yr). On average, bone area at the femoral neck increased and BMD decreased during this period. Heritability of femoral neck was estimated at 35–40% (32). Similar estimates were obtained from a family-based sample of Mexican-American men and women aged 25–45 yr, in whom BMD change over 5–6 yr was estimated to be 34% at the hip (33). Heritability of BMD change was also estimated to be 27 and 34% at two different forearm sites, although only 22% at the spine. A linkage analysis of BMD change at the femoral neck (heritability = 22%) revealed significant evidence for linkage (LOD = 3.6) to a gene-rich region on chromosome 1q23 containing 429 known or hypothetical genes, including BGLAP/osteocalcin (33, 34).
Several studies have estimated heritability of BMD change in populations of older adults/postmenopausal women. Makovey et al. (35) studied 362 peri- and postmenopausal female twin pairs and observed significantly higher correlations in 5-yr BMD change at the lumbar spine and forearm in MZ than in DZ twins, with heritability estimated at 38 and 49% at these sites. Similar estimates were obtained by Zhai et al. (36), whose sample included 152 MZ and 204 DZ pairs of postmenopausal women followed for an average of 8 yr. After adjustment for age at baseline and weight change during the follow-up, heritability of BMD change ranged from 44–56% for bone loss at the lumbar spine, femoral neck, and forearm. Also consistent with these estimates are those published by Shaffer et al. (34) from their study of large Mexican-American families, in whom heritabilities of 5-yr change in BMD were estimated to be 31% at the forearm, 44% at the hip, and 42% at the spine in family members aged 45 yr or older at baseline.
There is indirect evidence that some of the genetic influences on bone loss may be distinct from those on peak BMD. Brown and colleagues (28) performed an analysis of pre- and postmenopausal women from large families and used bivariate statistical techniques to estimate the genetic variance and heritability of BMD in each group of women and the correlation between these genetic variances, the latter representing the degree of pleiotropy, or shared genetic variance, between the two groups. Their analyses revealed that genes accounted for 58–88% of the total variation in BMD at the femoral neck, total hip, and spine in premenopausal women compared with 37–54% of the total variation in postmenopausal women. The genetic correlation in total hip BMD between pre- and postmenopausal women was high (0.81), but significantly less than one, implying that at least some genetic effects in the two groups for BMD did not overlap. They thus concluded that there is a substantial genetic contribution to BMD in both pre- and postmenopausal women and that many, but not all, of the genetic factors influencing variation in BMD are common to both groups of women.
How Might Genes Influence Bone Loss?
Although a flurry of genes have recently been associated with variation in BMD, no genes have yet been associated specifically with age-related bone loss. Among the reported associations are 29 loci that have been robustly associated with variation in BMD in genome-wide association studies (GWAS) (37–46) (see Table 2). These loci could act wholly through their effects on acquisition of peak bone mass or might also have effects mediated through shifts in bone turnover activity at later ages. Given the very small effect sizes of these loci (e.g. the collective set of single nucleotide polymorphisms account for only 2–3% of the variance in spine and femoral neck BMD (39), a very large sample size that included individuals with repeat measures of BMD would be required to detect an effect on bone loss. Studies of this magnitude have not yet been carried out.
Table 2.
Genes robustly associated with BMD on GWAS meta-analyses
| Gene | Location | Full name |
|---|---|---|
| TGFBR3 | 1p22 | TGF, β-receptor III |
| ZBTB40 | 1p36 | Zinc finger and BTB domain containing 40 |
| GPR177 | 1p31.3 | G protein-coupled receptor 177 (aka WNTLESS homolog) |
| SPTBN1 | 2p21 | Spectrin, β, nonerythrocytic 1 |
| CTNNB1 | 3p22 | catenin (cadherin-associated protein) β1 |
| MEPE | 4q21.1 | matrix, extracellular phosphoglycoprotein; aka osteoblast/osteocyte factor 45 |
| MEF2C | 5q14 | MADS box transcription enhancer factor 2, polypeptide C |
| ESR1 | 6q25 | Estrogen receptor 1 |
| C6orf97 | 6q25 | Chromosome 6 open reading frame 97 |
| STARD3NL | 7p14 | STARD3 N-terminal like, encodes a cholesterol endosomal transporter |
| SFRP4 | 7p14 | Secreted frizzled-related protein 4 |
| FLJ42280 | 7q21.3 | Unknown function |
| FAM3C | 7q31 | Family with sequence similarity 3, member C |
| VPS13B | 8q22 | Vacuolar protein sorting-associated protein 13B |
| TNFRSF11B | 8q24 | TNF receptor superfamily, member 11b (encodes Opg) |
| ARHGAP1 | 11p11.2 | ρ-GTPase activating protein 1 |
| LRP5 | 11q13.4 | low density lipoprotein receptor-related protein 5 |
| DCDC5 | 11p14.1 | doublecortin domain containing 1 |
| SOX6 | 11p15 | SRY (sex determining region Y)-box 6 (encodes a SOX family transcription factor defined by a conserved high-mobility group DNA-binding domain) |
| SP7 | 12q13 | Osterix; Sp7 transcription factor |
| AKAP11 | 13q14 | A kinase (PRKA) anchor protein 11 |
| TNFSF11 | 13q14 | TNF (ligand) superfamily, member 11 (RANKL) |
| ADAMTS18 | 16q23 | ADAM metallopeptidase with thrombospondin type 1 motif, 18 |
| FOXL1, FOXC2 | 16q24.3 | Forkhead gene family, expressed in the gastrointestinal mucosa (FOXL1) or involved in adipocyte metabolism and early-stage chondrogenic differentiation (FOXC2) |
| SOST | 17q11.2 | Sclerostin |
| CRHR1 | 17q12-q22 | CRF receptor |
| HDAC5 | 17q21 | Histone deacetylase 5 |
| TNFRSF11A | 18q21 | TNF receptor superfamily, member 11a, NFKB activator (encodes RANK) |
| JAG1 | 20p12 | Protein jagged-1 precursor |
Many of the known genes implicated by the 29 loci identified through GWAS participate in the Wnt/β-catenin signaling pathway, one of four signaling pathways through which members of the Wnt family can signal (e.g. GPR177, CTNNB1, LRP5, and SFRP4). The importance of this pathway in bone metabolism first came to light 10 yr ago with the discovery of a mutation in LRP5 (low-density lipoprotein receptor-related protein 5) as the cause of osteoporosis pseudoglioma (47). Shortly thereafter, a gain-of-function mutation in this gene was found to be associated with high bone mass (48, 49), followed by the discovery that common variants in this gene are also associated with variation in BMD at the population level (39, 46, 50, 51). Much research implicates an important role for this pathway and Lrp5 in bone cell differentiation, proliferation, and apoptosis. Lrp5 may play an especially important role in bone mass accrual and also appears to be needed for the bone to respond to mechanical stress [see recent review by Johnson et al. (52)]. The degree to which this pathway influences bone loss at older ages is unclear.
The RANKL/RANK/OPG pathway has also prominently emerged from candidate gene and GWAS of BMD (e.g. TNFSF11 and TNFRSF11B). This pathway is involved in regulation of osteoclast formation and differentiation by osteoblasts and stromal stem cells through a signaling system that involves receptor activation of nuclear factor-κB (RANK) by its ligand (RANKL), which is regulated by the soluble decoy receptor osteoprotegerin (OPG) (53). The importance of this pathway in osteoclast regulation makes it an attractive candidate for genetic regulation of BMD change. Additionally, the multiple soluble factors that interact with this system, including sex hormones like estradiol, make this pathway even more appealing. For example, OPG production is shown to be reduced in ovariectomized animals and postmenopausal women (54). Estradiol is known to increase OPG expression in vitro, which would, in turn, reduce RANKL/RANK binding and ultimately osteoclast activity (55). These facts taken together make this pathway particularly interesting for the study of age-related bone loss, and in fact, the development of denosumab, an anti-RANKL antibody, for treatment of postmenopausal osteoporosis has capitalized on this (56).
The pivotal role that sex hormones play in the maintenance of skeletal health makes any gene involved in their regulation a potential candidate gene for variation in BMD change. For example, early menopause (either naturally occurring or surgical) has been associated with increased risk of osteoporotic fracture (57) and lower BMD at both the axial and appendicular skeleton (58). Estrogen interacts with receptors in bone and other tissues, and its importance to bone health is well known. One action of estrogen is to regulate osteoclast apoptosis; in the estrogen-deficient state, longer-living osteoclasts promote bone resorption. Drugs that block the synthesis of estrogen, such as aromatase inhibitors, are associated with bone loss. Age at natural menopause is heritable (31–53%), and recent GWAS have identified a number of loci associated with age at menopause, two of which (chromosome 19 near BRSK1 and chromosome 20 near MCM8) have been reported in two separate GWAS analyses (59, 60). Delayed menarche is also a risk factor for low BMD and fragility fractures (61–64). A recent GWAS and metaanalysis of age at menarche have identified two genes, LIN28B and TMEM38B, consistently associated with age at menarche and have implicated 30 additional loci (59, 65–67). Numerous candidate genes (reviewed in Ref. 68) as well as GWAS (37, 39) have documented associations of polymorphisms in the estrogen receptor 1 gene (ESR1) with BMD, and associations of ESR1 variants have now been reported with bone loss (69, 70). Other genes in the estrogen endocrine pathway are also logical candidate genes for bone loss.
In men, androgens are crucial for maintenance of bone (71) and in older men low total testosterone is associated with BMD (72) and with subsequent rapid bone loss (73). Estrogen levels may also be important for maintenance of BMD in men (74). Associations have been reported between BMD and polymorphisms in the androgen receptor gene (AR) in some (75, 76), but not all, (77, 78) studies. Yang et al. (79) conducted a genome-wide analysis of copy number variants in elderly patients with osteoporotic hip fractures and age-matched controls in which they identified a copy number variant in UGT2B17 at 4q13.2 that was associated with both hip fracture and BMD, with replication of the fracture association in an independent population. UGT2B17 was an attractive candidate gene for bone loss because it encodes enzyme-catabolizing steroid hormones. These investigators then measured concentrations of serum testosterone and estradiol in a population of younger males and found the deletion to be associated with significantly higher concentrations of testosterone and estradiol.
The maintenance of calcium homeostasis by PTH makes the PTH pathway a strong candidate for genetic regulation of bone loss. In response to low serum calcium concentrations, the parathyroid gland secretes PTH, which acts to normalize calcium levels through several mechanisms, including promotion of bone resorption (80). Several studies have now reported polymorphisms in genes in the PTH pathway, including PTH, PTH-like hormone (PTHLH), and PTH1 receptor (PTHR1), to be associated with either fracture risk or BMD (81–84), although most of these associations have yet to be replicated.
Mice, which are commonly used to map quantitative trait loci influencing variation in BMD, are also an attractive choice for studying the genetics of growth rates and patterns in bone over time. Indeed, Buie et al. (85) recently characterized skeletal development patterns in mice and observed significant differences in bone mineralization patterns over time among three inbred strains, adding to the evidence that bone loss is genetic and providing the hope of eventually mapping genes for skeletal development through systematic backcross experiments.
Genes Associated with Bone Loss
Salamone et al. (86) examined the effect of APOE alleles on bone loss in a sample of pre- and postmenopausal women. In premenopausal women, they observed no significant differences in BMD or in the annualized percentage rate of change in BMD at the spine or hip when comparing women with and without the APOE*4 allele. However, in peri- and postmenopausal women, presence of the APOE*4 allele was strongly associated with spine bone loss. They further divided the peri- and postmenopausal sample into those currently using and not using hormone replacement therapy (HRT) and found the association of the APOE*4 allele to be restricted almost entirely to those not using HRT, raising the intriguing suggestion that estrogen modulates the effect of this locus on bone loss.
Several studies have evaluated the effect of the Sp1 polymorphism in collagen type I α1 (COL1A1) on bone loss. Type 1 collagen is a major protein of bone and the COL1A1 Sp1 polymorphism has been associated with osteoporotic fractures and BMD (reviewed in Ref. 68). Harris et al. (87) examined the association of this polymorphism with bone loss in a sample of subjects aged 65 yr and older. Baseline BMD did not differ by genotype, although the ss genotype at this locus was associated with significantly higher bone loss from the total body. There were no significant effects of genotype on bone loss at the spine or femoral neck and the study was limited by the small number of subjects with the ss genotype (n = 9). This association with bone loss could not be replicated in a subsequent study of postmenopausal women, although this study too was underpowered (88). A later study of 734 early postmenopausal women followed over a 5- to 7-yr period suggested that an effect of the COL1A1 Sp1 polymorphism on bone loss might be modulated by estrogen (89). In this study, there was no effect of the COL1A1 Sp1 genotype on bone loss in HRT users, but in nonusers, the s allele was associated with increased rate of bone loss at the lumbar spine and femoral neck. This locus accounted for 3% of the variation in spine BMD change after accounting for age, weight, and baseline BMD. In 6280 subjects 55 yr and older from the Rotterdam Study, neither this polymorphism nor the COL1A1 −1997 G/T promoter polymorphism was associated with 7-yr change in femoral neck BMD. Interestingly, however, women homozygous for the Sp1 T allele had a 2.3 times higher risk of fragility fracture, although subsequent adjustment for femoral neck BMD did not alter this association, suggesting that the increased fracture risk was independent of BMD change (90).
The vitamin D receptor (VDR) gene has been extensively studied for its potential role in bone metabolism, and numerous association studies have reported associations (or lack thereof) between polymorphisms in VDR and cross-sectional measures of BMD (reviewed in Ref. 91). Few studies have evaluated the relation of VDR polymorphisms to bone loss, although low 25-hydroxyvitamin D levels have been associated with higher rate of decline in total hip BMD in older men (92). Krall et al. (93) studied 229 healthy postmenopausal women and observed rates of bone loss over 2 yr to be greater at all sites in women having the Bsml BB genotype. Moreover, they reported that the extent of bone loss at the femoral neck was attenuated by calcium intake, although the modifying influence of calcium on the effect of the VDR BB genotype on bone loss was not significant. In contrast, Zmuda et al. (94) assessed the effects of the Bsml, TaqI, and ApaI polymorphisms at VDR in a sample of 156 older African-American women and found no strong evidence for association with 2-yr bone loss at the hip at any of these loci. However, women younger than age 70 with the TaqI tt genotype experienced greater decline in hip BMD than age-matched women with the TT genotype, although the sample size was small. The VDR Bsml polymorphism was not associated with bone loss in a study by Hansen of perimenopausal women (95), nor were the VDR Bsml, TaqI, and ApaI polymorphisms associated with bone loss in postmenopausal women in a study by Garnero et al. (96).
The well-known association of chronic inflammatory disease with bone loss (reviewed in Ref. 97) has prompted interest in investigating the relation of inflammation gene polymorphisms with bone loss. In the Study of Osteoporotic Fractures, an association was reported between a polymorphism in IL-6 and bone loss (98). This candidate gene was motivated because proinflammatory cytokines, including IL-6, are normally suppressed by estrogens, but production increases after menopause (99). IL-6 promotes osteoclast formation and bone resorption by promoting the differentiation of osteoclast precursor cells into mature osteoclasts (100). Investigators in this study assessed whether a common promoter variant (G-174C) in IL-6 that had previously been associated with decreased promoter activity and plasma IL-6 levels, was associated with bone loss at the hip in 3376 postmenopausal women. They found the G allele to be associated with significantly greater rate of BMD decline at the hip. Interestingly, this allele was also associated with weight loss during the follow-up interval, although the association with bone loss remained statistically significant even after adjustment for weight change.
The presence of adipocytes in the bone marrow, particularly in older individuals, has been recognized for many years but has recently prompted much debate as to whether fatty tissue infiltration in the bone marrow may precipitate or contribute to bone loss. Although adipocytes may merely migrate into vacated bone marrow space where they benignly fill space as osteoblast activity declines with age, it has also been proposed that this fatty infiltration may actively contribute to decreased bone formation, perhaps mediated through secretion of inflammatory cytokines (reviewed in Ref. 101). Osteoblasts and adipocytes arise from a common mesenchymal stem cell lineage, and their differentiation from mesenchymal stem cells is controlled by a variety of transcription and other factors, including peroxisome proliferator-activated receptor γ (PPARγ), a master regulator of adipogenesis. Recently, polymorphisms in PPARG, which encodes PPARγ, and ALOX12, which produces ligands for PPARγ, have been associated with BMD, fracture risk, and/or bone loss (102–105).
Summary
Despite the challenges of measuring bone loss, there is growing evidence for a substantial genetic contribution to this trait. Some of the genes identified from recent GWAS of cross-sectional BMD are attractive candidate genes for bone loss, most notably genes in the RANKL/RANK/OPG and estrogen endocrine pathways. New insights into the biology of skeletal development and regulation of bone turnover have inspired new hypotheses about genetic regulation of bone loss and may provide new directions for identifying genes associated with bone loss. Several recent candidate gene studies have raised the intriguing hypothesis that the effects of some genes on bone loss may be modulated by estrogen, and a more formal analysis of such effects may provide additional insight into the genetic architecture of bone loss. Uncovering these effects may provide new routes for therapeutic intervention and osteoporosis prevention.
Acknowledgments
L.M.Y.-A. was supported by Training Grant AG000262 from the National Institutes of Aging and F32 AR059469 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- Bone mineral density
- BMI
- body mass index
- DZ
- dizygotic
- GWAS
- genome-wide association studies
- HRT
- hormone replacement therapy
- MZ
- monozygotic
- OPG
- osteoprotegerin
- PPARγ
- peroxisome proliferator-activated receptor γ
- RANK
- receptor activation of nuclear factor-κB
- RANKL
- RANK ligand.
References
- 1. Dennison E, Mohamed MA, Cooper C. 2006. Epidemiology of osteoporosis. Rheum Dis Clin North Am 32:617–629 [DOI] [PubMed] [Google Scholar]
- 2. Silverman SL. 2005. Quality-of-life issues in osteoporosis. Curr Rheumatol Rep 7:39–45 [DOI] [PubMed] [Google Scholar]
- 3. Cummings SR, Melton LJ. 2002. Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767 [DOI] [PubMed] [Google Scholar]
- 4. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 5. Ralston SH, Uitterlinden AG. 2010. Genetics of osteoporosis. Endocr Rev 31:629–662 [DOI] [PubMed] [Google Scholar]
- 6. Li WF, Hou SX, Yu B, Li MM, Férec C, Chen JM. 2010. Genetics of osteoporosis: accelerating pace in gene identification and validation. Hum Genet 127:249–285 [DOI] [PubMed] [Google Scholar]
- 7. Farber CR, Lusis AJ. 2009. Future of osteoporosis genetics: enhancing genome-wide association studies. J Bone Miner Res 24:1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duncan EL, Brown MA. 2010. Genetic determinants of bone density and fracture risk: state of the art and future directions. J Clin Endocrinol Metab 95:2576–2587 [DOI] [PubMed] [Google Scholar]
- 9. Adams J, Bishop N. ASMBR 2009. Chapter 29. DXA in adults and children. In: Primer on the metabolic bone diseases and disorders of mineral metabolism. Hoboken, NJ: John Wiley, Sons; 151–158 [Google Scholar]
- 10. Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. 2000. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res 15:1974–1980 [DOI] [PubMed] [Google Scholar]
- 11. Cauley JA, Lui LY, Barnes D, Ensrud KE, Zmuda JM, Hillier TA, Hochberg MC, Schwartz AV, Yaffe K, Cummings SR, Newman AB. 2009. Successful skeletal aging: a marker of low fracture risk and longevity. The Study of Osteoporotic Fractures (SOF). J Bone Miner Res 24:134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen TV, Center JR, Eisman JA. 2005. Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res 20:1195–1201 [DOI] [PubMed] [Google Scholar]
- 13. Nguyen ND, Center JR, Eisman JA, Nguyen TV. 2007. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res 22:1147–1154 [DOI] [PubMed] [Google Scholar]
- 14. Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. 1995. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res 10:1778–1787 [DOI] [PubMed] [Google Scholar]
- 15. Greenspan SL, Maitland LA, Myers ER, Krasnow MB, Kido TH. 1994. Femoral bone loss progresses with age: a longitudinal study in women over age 65. J Bone Miner Res 9:1959–1965 [DOI] [PubMed] [Google Scholar]
- 16. Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA. 1994. Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo Osteoporosis Epidemiology Study. BMJ 309:691–695 [PMC free article] [PubMed] [Google Scholar]
- 17. Smith JA, Vento JA, Spencer RP, Tendler BE. 1999. Aortic calcification contributing to bone densitometry measurement. J Clin Densitom 2:181–183 [DOI] [PubMed] [Google Scholar]
- 18. Burger H, de Laet CE, van Daele PL, Weel AE, Witteman JC, Hofman A, Pols HA. 1998. Risk factors for increased bone loss in an elderly population: the Rotterdam Study. Am J Epidemiol 147:871–879 [DOI] [PubMed] [Google Scholar]
- 19. Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. 2000. Risk factors for longitudinal bone loss in elderly men and women: The Framingham Osteoporosis Study. J Bone Miner Res 15:710–720 [DOI] [PubMed] [Google Scholar]
- 20. Nguyen TV, Sambrook PN, Eisman JA. 1998. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 13:1458–1467 [DOI] [PubMed] [Google Scholar]
- 21. Dennison E, Eastell R, Fall CH, Kellingray S, Wood PJ, Cooper C. 1999. Determinants of bone loss in elderly men and women: A prospective population-based study. Osteoporos Int 10:384–391 [DOI] [PubMed] [Google Scholar]
- 22. Weng MY, Lane NE. 2007. Medication-induced osteoporosis. Curr Osteoporos Rep 5:139–145 [DOI] [PubMed] [Google Scholar]
- 23. Stein E, Shane E. 2003. Secondary osteoporosis. Endocrinol Metab Clin North Am 32:115–134, vii [DOI] [PubMed] [Google Scholar]
- 24. Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. 1987. Genetic determinants of bone mass in adults: a twin study. J Clin Invest 80:706–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell BD, Kammerer CM, Schneider JL, Perez R, Bauer RL. 2003. Genetic and environmental determinants of bone mineral density in Mexican Americans: results from the San Antonio Family Osteoporosis Study. Bone 33:839–846 [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Kammerer CM, Wheeler VW, Patrick AL, Bunker CH, Zmuda JM. 2007. Genetic and environmental determinants of volumetric and areal BMD in multi-generational families of African ancestry: the Tobago Family Health Study. J Bone Miner Res 22:527–536 [DOI] [PubMed] [Google Scholar]
- 27. Guéguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G. 1995. Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res 10:2017–2022 [DOI] [PubMed] [Google Scholar]
- 28. Brown LB, Streeten EA, Shapiro JR, McBride D, Shuldiner AR, Peyser PA, Mitchell BD. 2005. Genetic and environmental influences on bone mineral density in pre- and post-menopausal women. Osteoporos Int 16:1849–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christian JC, Yu PL, Slemenda CW, Johnston CC., Jr 1989. Heritability of bone mass: a longitudinal study in aging male twins. Am J Hum Genet 44:429–433 [PMC free article] [PubMed] [Google Scholar]
- 30. Slemenda CW, Christian JC, Reed T, Reister TK, Williams CJ, Johnston CC., Jr 1992. Long-term bone loss in men: effects of genetic and environmental factors. Ann Intern Med 117:286–291 [DOI] [PubMed] [Google Scholar]
- 31. Kelly PJ, Nguyen T, Hopper J, Pocock N, Sambrook P, Eisman J. 1993. Changes in axial bone density with age: a twin study. J Bone Miner Res 8:11–17 [DOI] [PubMed] [Google Scholar]
- 32. Hui SL, Koller DL, Foroud TM, Econs MJ, Johnston CC, Peacock M. 2006. Heritability of changes in bone size and bone mass with age in premenopausal white sisters. J Bone Miner Res 21:1121–1125 [DOI] [PubMed] [Google Scholar]
- 33. Shaffer JR, Kammerer CM, Bruder JM, Cole SA, Dyer TD, Almasy L, Maccluer JW, Blangero J, Bauer RL, Mitchell BD. 2009. Quantitative trait locus on chromosome 1q influences bone loss in young Mexican American adults. Calcif Tissue Int 84:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaffer JR, Kammerer CM, Bruder JM, Cole SA, Dyer TD, Almasy L, MacCluer JW, Blangero J, Bauer RL, Mitchell BD. 2008. Genetic influences on bone loss in the San Antonio Family Osteoporosis study. Osteoporos Int 19:1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makovey J, Nguyen TV, Naganathan V, Wark JD, Sambrook PN. 2007. Genetic effects on bone loss in peri- and postmenopausal women: a longitudinal twin study. J Bone Miner Res 22:1773–1780 [DOI] [PubMed] [Google Scholar]
- 36. Zhai G, Andrew T, Kato BS, Blake GM, Spector TD. 2009. Genetic and environmental determinants on bone loss in postmenopausal Caucasian women: a 14-year longitudinal twin study. Osteoporos Int 20:949–953 [DOI] [PubMed] [Google Scholar]
- 37. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K. 2008. Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365 [DOI] [PubMed] [Google Scholar]
- 38. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Snorradóttir S, Center JR, Nguyen TV, Alexandersen P, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K. 2009. New sequence variants associated with bone mineral density. Nat Genet 41:15–17 [DOI] [PubMed] [Google Scholar]
- 39. Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG. 2009. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41:1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL. 2009. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 41:527–534 [DOI] [PubMed] [Google Scholar]
- 41. Kung AW, Xiao SM, Cherny S, Li GH, Gao Y, Tso G, Lau KS, Luk KD, Liu JM, Cui B, Zhang MJ, Zhang ZL, He JW, Yue H, Xia WB, Luo LM, He SL, Kiel DP, Karasik D, Hsu YH, Cupples LA, Demissie S, Styrkarsdottir U, Halldorsson BV, Sigurdsson G, Thorsteinsdottir U, Stefansson K, Richards JB, Zhai G, Soranzo N, Valdes A, Spector TD, Sham PC. 2010. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am J Hum Genet 86:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu YZ, Pei YF, Liu JF, Yang F, Guo Y, Zhang L, Liu XG, Yan H, Wang L, Zhang YP, Levy S, Recker RR, Deng HW. 2009. Powerful bivariate genome-wide association analyses suggest the SOX6 gene influencing both obesity and osteoporosis phenotypes in males. PLoS ONE 4:e6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Timpson NJ, Tobias JH, Richards JB, Soranzo N, Duncan EL, Sims AM, Whittaker P, Kumanduri V, Zhai G, Glaser B, Eisman J, Jones G, Nicholson G, Prince R, Seeman E, Spector TD, Brown MA, Peltonen L, Smith GD, Deloukas P, Evans DM. 2009. Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet 18:1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deng FY, Zhao LJ, Pei YF, Sha BY, Liu XG, Yan H, Wang L, Yang TL, Recker RR, Papasian CJ, Deng HW. 2010. Genome-wide copy number variation association study suggested VPS13B gene for osteoporosis in Caucasians. Osteoporos Int 21:579–587 [DOI] [PubMed] [Google Scholar]
- 45. Xiong DH, Liu XG, Guo YF, Tan LJ, Wang L, Sha BY, Tang ZH, Pan F, Yang TL, Chen XD, Lei SF, Yerges LM, Zhu XZ, Wheeler VW, Patrick AL, Bunker CH, Guo Y, Yan H, Pei YF, Zhang YP, Levy S, Papasian CJ, Xiao P, Lundberg YW, Recker RR, Liu YZ, Liu YJ, Zmuda JM, Deng HW. 2009. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am J Hum Genet 84:388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD. 2008. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371:1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed] [Google Scholar]
- 48. Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. 2002. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521 [DOI] [PubMed] [Google Scholar]
- 50. Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE. 2004. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet 74:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, Kiel DP, Langdahl BL, Lips P, Ljunggren O, Lorenc R, Obermayer-Pietsch B, Ohlsson C, Pettersson U, Reid DM, Rousseau F, Scollen S, Van Hul W, Agueda L, Akesson K, Benevolenskaya LI, Ferrari SL, Hallmans G, Hofman A, Husted LB, Kruk M, Kaptoge S, Karasik D, Karlsson MK, Lorentzon M, Masi L, McGuigan FE, Mellström D, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Weber K, Ioannidis JP, Uitterlinden AG. 2008. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299:1277–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson ML, Lara N, Kamel MA. 2009. How genomics has informed our understanding of the pathogenesis of osteoporosis. Genome Med 1:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leibbrandt A, Penninger JM. 2009. ESCI award lecture: from a little mouse to rationale medicine for bone loss. Eur J Clin Invest 39:842–850 [DOI] [PubMed] [Google Scholar]
- 54. Vega D, Maalouf NM, Sakhaee K. 2007. The role of receptor activator of nuclear factor-κB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab 92:4514–4521 [DOI] [PubMed] [Google Scholar]
- 55. Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. 1999. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 140:4367–4370 [DOI] [PubMed] [Google Scholar]
- 56. Iqbal J, Sun L, Zaidi M. 2010. Denosumab for the treatment of osteoporosis. Curr Osteoporos Rep 8:163–167 [DOI] [PubMed] [Google Scholar]
- 57. Gallagher JC. 2007. Effect of early menopause on bone mineral density and fractures. Menopause 14:567–571 [DOI] [PubMed] [Google Scholar]
- 58. Kritz-Silverstein D, Barrett-Connor E. 1993. Early menopause, number of reproductive years, and bone mineral density in postmenopausal women. Am J Public Health 83:983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. He C, Kraft P, Chen C, Buring JE, Paré G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. 2009. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 41:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stolk L, Zhai G, van Meurs JB, Verbiest MM, Visser JA, Estrada K, Rivadeneira F, Williams FM, Cherkas L, Deloukas P, Soranzo N, de Keyzer JJ, Pop VJ, Lips P, Lebrun CE, van der Schouw YT, Grobbee DE, Witteman J, Hofman A, Pols HA, Laven JS, Spector TD, Uitterlinden AG. 2009. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet 41:645–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fox KM, Magaziner J, Sherwin R, Scott JC, Plato CC, Nevitt M, Cummings S. 1993. Reproductive correlates of bone mass in elderly women. J Bone Miner Res 8:901–908 [DOI] [PubMed] [Google Scholar]
- 62. Tuppurainen M, Kröger H, Saarikoski S, Honkanen R, Alhava E. 1995. The effect of gynecological risk factors on lumbar and femoral bone mineral density in peri- and postmenopausal women. Maturitas 21:137–145 [DOI] [PubMed] [Google Scholar]
- 63. Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, Dilsen G, Gennari C, Lopes Vaz A, Lyritis G. 1995. Risk factors for hip fracture in European women: The MEDOS study. J Bone Miner Res 10:1802–1815 [DOI] [PubMed] [Google Scholar]
- 64. Jacobsen BK, Nilssen S, Heuch I, Kvåle G. 1998. Reproductive factors and fatal hip fractures. A Norwegian prospective study of 63,000 women. J Epidemiol Community Health 52:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perry JR, Stolk L, Franceschini N, Lunetta KL, Zhai G, McArdle PF, Smith AV, Aspelund T, Bandinelli S, Boerwinkle E, Cherkas L, Eiriksdottir G, Estrada K, Ferrucci L, Folsom AR, Garcia M, Gudnason V, Hofman A, Karasik D, Kiel DP, Launer LJ, van Meurs J, Nalls MA, Rivadeneira F, Shuldiner AR, Singleton A, Soranzo N, Tanaka T, Visser JA, Weedon MN, Wilson SG, Zhuang V, Streeten EA, Harris TB, Murray A, Spector TD, Demerath EW, Uitterlinden AG, Murabito JM. 2009. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet 41:648–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, Bingham SA, Brage S, Smith GD, Ekelund U, Gillson CJ, Glaser B, Golding J, Hardy R, Khaw KT, Kuh D, Luben R, Marcus M, McGeehin MA, Ness AR, Northstone K, Ring SM, Rubin C, Sims MA, Song K, Strachan DP, Vollenweider P, Waeber G, Waterworth DM, Wong A, Deloukas P, Barroso I, Mooser V, Loos RJ, Wareham NJ. 2009. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet 41:729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, Lunetta KL, Visser JA, Byrne EM, Cousminer DL, Gudbjartsson DF, Esko T, Feenstra B, Hottenga JJ, Koller DL, Kutalik Z, Lin P, Mangino M, Marongiu M, McArdle PF, Smith AV, Stolk L, van Wingerden SH, Zhao JH, Albrecht E, Corre T, Ingelsson E, Hayward C, Magnusson PK, Smith EN, Ulivi S, Warrington NM, Zgaga L, Alavere H, Amin N, Aspelund T, Bandinelli S, Barroso I, Berenson GS, Bergmann S, Blackburn H, Boerwinkle E, Buring JE, Busonero F, Campbell H, Chanock SJ, Chen W, Cornelis MC, Couper D, Coviello AD, d'Adamo P, de Faire U, de Geus EJ, Deloukas P, Döring A, Smith GD, Easton DF, Eiriksdottir G, Emilsson V, Eriksson J, Ferrucci L, Folsom AR, Foroud T, Garcia M, Gasparini P, Geller F, Gieger C, Gudnason V, Hall P, Hankinson SE, Ferreli L, Heath AC, Hernandez DG, Hofman A, Hu FB, Illig T, Järvelin MR, Johnson AD, Karasik D, Khaw KT, Kiel DP, Kilpeläinen TO, Kolcic I, Kraft P, Launer LJ, Laven JS, Li S, Liu J, Levy D, Martin NG, McArdle WL, Melbye M, Mooser V, Murray JC, Murray SS, Nalls MA, Navarro P, Nelis M, Ness AR, Northstone K, Oostra BA, Peacock M, Palmer LJ, Palotie A, Paré G, Parker AN, Pedersen NL, Peltonen L, Pennell CE, Pharoah P, Polasek O, Plump AS, Pouta A, Porcu E, Rafnar T, Rice JP, Ring SM, Rivadeneira F, Rudan I, Sala C, Salomaa V, Sanna S, Schlessinger D, Schork NJ, Scuteri A, Segrè AV, Shuldiner AR, Soranzo N, Sovio U, Srinivasan SR, Strachan DP, Tammesoo ML, Tikkanen E, Toniolo D, Tsui K, Tryggvadottir L, Tyrer J, Uda M, van Dam RM, van Meurs JB, Vollenweider P, Waeber G, Wareham NJ, Waterworth DM, Weedon MN, Wichmann HE, Willemsen G, Wilson JF, Wright AF, Young L, Zhai G, Zhuang WV, Bierut LJ, Boomsma DI, Boyd HA, Crisponi L, Demerath EW, van Duijn CM, Econs MJ, Harris TB, Hunter DJ, Loos RJ, Metspalu A, Montgomery GW, Ridker PM, Spector TD, Streeten EA, Stefansson K, Thorsteinsdottir U, Uitterlinden AG, Widen E, Murabito JM, Ong KK, Murray A. 2010. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet 42:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ralston SH. 2010. Genetics of osteoporosis. Ann NY Acad Sci 1192:181–189 [DOI] [PubMed] [Google Scholar]
- 69. Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, Honkanen R, Mäenpää PH. 2000. Early postmenopausal bone loss is associated with PvuII Estrogen Receptor gene polymorphism in Finnish women: effect of hormone replacement therapy. J Bone Miner Res 15:315–321 [DOI] [PubMed] [Google Scholar]
- 70. Albagha OM, Pettersson U, Stewart A, McGuigan FE, MacDonald HM, Reid DM, Ralston SH. 2005. Association of oestrogen receptor alpha gene polymorphisms with postmenopausal bone loss, bone mass, and quantitative ultrasound properties of bone. J Med Genet 42:240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ebeling PR. 2010. Androgens and osteoporosis. Curr Opin Endocrinol Diabetes Obes 17:284–292 [DOI] [PubMed] [Google Scholar]
- 72. Mellström D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C. 2006. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21:529–535 [DOI] [PubMed] [Google Scholar]
- 73. Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, Orwoll ES. 2006. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91:3908–3915 [DOI] [PubMed] [Google Scholar]
- 74. Greendale GA, Edelstein S, Barrett-Connor E. 1997. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12:1833–1843 [DOI] [PubMed] [Google Scholar]
- 75. Zitzmann M, Brune M, Kornmann B, Gromoll J, Junker R, Nieschlag E. 2001. The CAG repeat polymorphism in the androgen receptor gene affects bone density and bone metabolism in healthy males. Clin Endocrinol (Oxf) 55:649–657 [DOI] [PubMed] [Google Scholar]
- 76. Guadalupe-Grau A, Rodríguez-González FG, Ponce-González JG, Dorado C, Olmedillas H, Fuentes T, Pérez-Gómez J, Sanchís-Moysi J, Díaz-Chico BN, Calbet JA. 2010. Bone mass and the CAG and GGN androgen receptor polymorphisms in young men. PLoS ONE 5:e11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kenny AM, McGee D, Joseph C, Covault J, Abreu C, Raisz LG. 2005. Lack of association between androgen receptor polymorphisms and bone mineral density or physical function in older men. Endocr Res 31:285–293 [DOI] [PubMed] [Google Scholar]
- 78. Van Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM. 2001. Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin Endocrinol (Oxf) 55:659–666 [DOI] [PubMed] [Google Scholar]
- 79. Yang TL, Chen XD, Guo Y, Lei SF, Wang JT, Zhou Q, Pan F, Chen Y, Zhang ZX, Dong SS, Xu XH, Yan H, Liu X, Qiu C, Zhu XZ, Chen T, Li M, Zhang H, Zhang L, Drees BM, Hamilton JJ, Papasian CJ, Recker RR, Song XP, Cheng J, Deng HW. 2008. Genome-wide copy-number-variation study identified a susceptibility gene, UGT2B17, for osteoporosis. Am J Hum Genet 83:663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nissenson RA, Jüppner H. ASMBR 2009. Parathyroid hormone. In: Primer on the metabolic bone diseases and disorders of mineral metabolism. Hoboken, NJ: John Wiley, Sons; 123–127 [Google Scholar]
- 81. Tenne M, McGuigan F, Jansson L, Gerdhem P, Obrant KJ, Luthman H, Akesson K. 2008. Genetic variation in the PTH pathway and bone phenotypes in elderly women: evaluation of PTH, PTHLH, PTHR1 and PTHR2 genes. Bone 42:719–727 [DOI] [PubMed] [Google Scholar]
- 82. Vilariño-Güell C, Miles LJ, Duncan EL, Ralston SH, Compston JE, Cooper C, Langdahl BL, Maclelland A, Pols HA, Reid DM, Uitterlinden AG, Steer CD, Tobias JH, Wass JA, Brown MA. 2007. PTHR1 polymorphisms influence BMD variation through effects on the growing skeleton. Calcif Tissue Int 81:270–278 [DOI] [PubMed] [Google Scholar]
- 83. Scillitani A, Jang C, Wong BY, Hendy GN, Cole DE. 2006. A functional polymorphism in the PTHR1 promoter region is associated with adult height and BMD measured at the femoral neck in a large cohort of young caucasian women. Hum Genet 119:416–421 [DOI] [PubMed] [Google Scholar]
- 84. Gupta A, Välimäki VV, J Välimäki M, Löyttyniemi E, Richard M, L Bukka P, Goltzman D, Karaplis AC. 2008. Variable number of tandem repeats polymorphism in parathyroid hormone-related protein as predictor of peak bone mass in young healthy Finnish males. Eur J Endocrinol 158:755–764 [DOI] [PubMed] [Google Scholar]
- 85. Buie HR, Moore CP, Boyd SK. 2008. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res 23:2048–2059 [DOI] [PubMed] [Google Scholar]
- 86. Salamone LM, Cauley JA, Zmuda J, Pasagian-Macaulay A, Epstein RS, Ferrell RE, Black DM, Kuller LH. 2000. Apolipoprotein E gene polymorphism and bone loss: Estrogen status modifies the influence of apolipoprotein E on bone loss. J Bone Miner Res 15:308–314 [DOI] [PubMed] [Google Scholar]
- 87. Harris SS, Patel MS, Cole DE, Dawson-Hughes B. 2000. Associations of the collagen type I α1 Sp1 polymorphism with five-year rates of bone loss in older adults. Calcif Tissue Int 66:268–271 [DOI] [PubMed] [Google Scholar]
- 88. Heegaard A, Jorgensen HL, Vestergaard AW, Hassager C, Ralston SH. 2000. Lack of influence of collagen type Iα1 Sp1 binding site polymorphism on the rate of bone loss in a cohort of postmenopausal Danish women followed for 18 years. Calcif Tissue Int 66:409–413 [DOI] [PubMed] [Google Scholar]
- 89. MacDonald HM, McGuigan FA, New SA, Campbell MK, Golden MH, Ralston SH, Reid DM. 2001. COL1A1 Sp1 polymorphism predicts perimenopausal and early postmenopausal spinal bone loss. J Bone Miner Res 16:1634–1641 [DOI] [PubMed] [Google Scholar]
- 90. Yazdanpanah N, Rivadeneira F, van Meurs JB, Zillikens MC, Arp P, Hofman A, van Duijn CM, Pols HA, Uitterlinden AG. 2007. The −1997 G/T and Sp1 polymorphisms in the collagen type I alpha1 (COLIA1) gene in relation to changes in femoral neck bone mineral density and the risk of fracture in the elderly: the Rotterdam study. Calcif Tissue Int 81:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cooper GS, Umbach DM. 1996. Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis J Bone Miner Res 11:1841–1849 [DOI] [PubMed] [Google Scholar]
- 92. Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM, Orwoll ES. for the Osteoporotic Fractures in Men Study Group 2009. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab 94:2773–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Krall EA, Parry P, Lichter JB, Dawson-Hughes B. 1995. Vitamin D receptor alleles and rates of bone loss: influences of years since menopause and calcium intake. J Bone Miner Res 10:978–984 [DOI] [PubMed] [Google Scholar]
- 94. Zmuda JM, Cauley JA, Danielson ME, Wolf RL, Ferrell RE. 1997. Vitamin D receptor gene polymorphisms, bone turnover, and rates of bone loss in older African-American women. J Bone Miner Res 12:1446–1452 [DOI] [PubMed] [Google Scholar]
- 95. Hansen TS, Abrahamsen B, Henriksen FL, Hermann AP, Jensen LB, Hørder M, Gram J. 1998. Vitamin D receptor alleles do not predict bone mineral density or bone loss in Danish perimenopausal women. Bone 22:571–575 [DOI] [PubMed] [Google Scholar]
- 96. Garnero P, Borel O, Sornay-Rendu E, Arlot ME, Delmas PD. 1996. Vitamin D receptor gene polymorphisms are not related to bone turnover, rate of bone loss, and bone mass in postmenopausal women: the OFELY Study. J Bone Miner Res 11:827–834 [DOI] [PubMed] [Google Scholar]
- 97. Hardy R, Cooper MS. 2009. Bone loss in inflammatory disorders. J Endocrinol 201:309–320 [DOI] [PubMed] [Google Scholar]
- 98. Moffett SP, Zmuda JM, Cauley JA, Stone KL, Nevitt MC, Ensrud KE, Hillier TA, Hochberg MC, Joslyn G, Morin P, Cummings SR. 2004. Association of the G-174C variant in the interleukin-6 promoter region with bone loss and fracture risk in older women. J Bone Miner Res 19:1612–1618 [DOI] [PubMed] [Google Scholar]
- 99. Manolagas SC. 1995. Role of cytokines in bone resorption. Bone 17:63S–67S [DOI] [PubMed] [Google Scholar]
- 100. Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T, et al. 1993. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA 90:11924–11928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rosen CJ, Bouxsein ML. 2006. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43 [DOI] [PubMed] [Google Scholar]
- 102. Ichikawa S, Koller DL, Johnson ML, Lai D, Xuei X, Edenberg HJ, Klein RF, Orwoll ES, Hui SL, Foroud TM, Peacock M, Econs MJ. 2006. Human ALOX12, but not ALOX15, is associated with BMD in white men and women. J Bone Miner Res 21:556–564 [DOI] [PubMed] [Google Scholar]
- 103. Mullin BH, Spector TD, Curtis CC, Ong GN, Hart DJ, Hakim AJ, Worthy T, Wilson SG. 2007. Polymorphisms in ALOX12, but not ALOX15, are significantly associated with BMD in postmenopausal women. Calcif Tissue Int 81:10–17 [DOI] [PubMed] [Google Scholar]
- 104. Harsløf T, Husted L, Nyegaard M, Carstens M, Stenkjær L, Brixen K, Eiken P, Jensen JE, Børglum A, Mosekilde L, Rejnmark L, Langdahl B. 23 November 2010. Polymorphisms in the ALOX12 gene and osteoporosis. Osteoporos Int 10.1007/s00198-010-1472-2 [DOI] [PubMed] [Google Scholar]
- 105. Harsløf T, Tofteng C, Husted L, Nyegaard M, Børglum A, Carstens M, Stenkjær L, Brixen K, Eiken P, Jensen JE, Mosekilde L, Rejnmark L, Langdahl B. 23 November 2010. Polymorphisms of the peroxisome proliferator-activated receptor γ (PPARγ) gene are associated with osteoporosis. Osteoporos Int 10.1007/s00198-010-1491-z [DOI] [PubMed] [Google Scholar]