Plasma 25-hydroxyvitamin D is associated inversely with adiposity and positively with HDL cholesterol in children.
Abstract
Objective:
The aim of the study was to examine the relationship between vitamin D status, total and abdominal adiposity, and lipids in black and white children.
Methods:
Plasma 25-hydroxyvitamin D [25(OH)D], adiposity [body mass index (BMI), percentage of total body fat, visceral adipose tissue (VAT), sc adipose tissue (SAT)], and fasting lipids were assessed in healthy obese and nonobese 8- to 18-yr-old black and white children.
Results:
We studied 237 children (mean ± sd age, 12.7 ± 2.2 yr; 47% black, 47% obese, and 43% male). Mean 25(OH)D concentration for the entire cohort was 19.4 ± 7.4 ng/ml. The majority of the children were vitamin D deficient [25(OH)D < 20 ng/ml; 73% blacks, 40% whites]. Plasma 25(OH)D was associated inversely with BMI, BMI percentile, percentage of total body fat, VAT, and SAT and positively with HDL cholesterol in the entire cohort. VAT was higher in vitamin D-deficient whites, and SAT was higher in vitamin D-deficient blacks compared with their respective vitamin D-nondeficient counterparts. Race, season, pubertal status, and VAT were independent significant predictors of 25(OH)D status.
Conclusions:
In black and white youth examined together, lower levels of 25(OH)D are associated with higher adiposity measures and lower HDL. Furthermore, vitamin D deficiency is associated with higher VAT in whites and greater SAT in blacks. Besides therapeutic interventions to correct the high rates of vitamin D deficiency in youth, benefits of vitamin D optimization on adiposity measures and lipid profile need to be explored.
Studies in adults and children have shown a link between obesity and vitamin D status (1–6). Serum 25-hydroxyvitamin D [25(OH)D], the recognized biomarker of vitamin D status, is inversely associated with clinical and laboratory measures of adiposity such as body mass index (BMI), waist circumference, and percentage of total body fat in adults and adolescents (1, 2, 4, 7–10). Sequestration of vitamin D in body fat stores and its consequent reduced bioavailability offer a plausible explanation for this association (5, 11). Racial and ethnic differences in the degree of adiposity and distribution of body fat are well recognized (12–16). For a given BMI, African-American children have lower levels of adiposity than Caucasian children (12). Furthermore, distribution of abdominal adipose tissue varies by race—compared with Caucasian children, African-American children have lower visceral adipose tissue (VAT) and higher sc adipose tissue (SAT) (13). Racial differences in abdominal fat topography may explain the racial variations in the metabolic risks of abdominal adiposity, such as higher diabetogenic risk and better lipid profile in African-Americans compared with Caucasians (13, 17).
Data characterizing the racial differences in the relationship between vitamin D status and adiposity, particularly abdominal adipose tissue distribution, are limited. Because adiposity is a risk factor for hypovitaminosis D and dyslipidemia and abdominal fat topography (distribution of SAT and VAT) influences the metabolic risks of adiposity, we examined the racial differences in the relationship between vitamin D status, BMI, total body adiposity, intraabdominal SAT and VAT, and lipid levels in healthy obese and nonobese 8- to 18-yr-old black and white children.
Subjects and Methods
Participants
Subjects were 237 normal-weight and overweight 8- to 18-yr-old black and white otherwise healthy children residing at a latitude of 40.4° N in Pittsburgh, Pennsylvania, of whom 28 had a diagnosis of polycystic ovary syndrome without any treatment. Subjects were participants in an ongoing National Institutes of Health-funded RO1 grant “Childhood Metabolic Markers of Adult Morbidity in Blacks” and a K24 grant “Childhood Insulin Resistance.” Subjects were enrolled between November 1995 and April 2009. Data from some of these participants have been reported previously (18–20). The studies were approved by the University of Pittsburgh Institutional Review Board. Signed parental informed consent and participant assent were obtained before enrollment. Healthy research volunteers were recruited through newspaper advertisements, fliers posted on bus routes, youth recreational locations, and the university campus. Children with diabetes or on medications that influence glucose or lipid metabolism or blood pressure were excluded. Medical history and physical examination, with assessment of pubertal development using Tanner staging, and routine hematological and biochemical tests were performed in all participants to ensure that subjects who entered into the protocol were healthy. Race was determined by self-identity of the parents as being black or white for at least three generations.
Study design
All study procedures were completed at the Pediatric Clinical and Translational Research Center at the Children's Hospital of Pittsburgh. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a weighing balance and a wall-mounted stadiometer. Body composition [total body fat and percentage body fat (%BF)] was assessed by dual-energy x-ray absorptiometry (DXA) as before (13). Abdominal SAT and VAT were measured in 216 subjects by a 10-mm single axial computed tomography scan at L4–5 intervertebral space as previously reported (13, 21) and in 11 subjects with magnetic resonance imaging (22). Data are not available in 10 subjects, in eight the weight exceeded the limit for computed tomography scan or magnetic resonance imaging, and in two electronic data were lost. Blood samples were obtained after a 10- to 12-h overnight fast for determination of plasma 25(OH)D and fasting lipid levels.
Biochemical measurements
Plasma lipid levels [total, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and very low-density lipoprotein (VLDL) cholesterol, and triglycerides] were measured using the standards recommended by the Centers for Disease Control and Prevention (23, 24). Plasma 25(OH)D was measured with competitive protein binding assay using vitamin D-binding protein as described by Chen et al. (25). The competitive protein binding assay identifies 25(OH)D2 and 25(OH)D3 equally well and has been validated by liquid chromatography tandem mass spectrophotometry assay, which measures the contributions of serum 25(OH)D2 and 25(OH)D3 (26). The intraassay and interassay coefficients of variation were 8 and 10%, respectively. The lower limit of detection was 4 ng/ml.
Statistical analysis
Differences in the means for the various adiposity measures (BMI, BMI percentile, VAT, SAT, %BF) and lipid parameters (total, LDL, HDL, and VLDL cholesterol, and triglycerides) were compared between vitamin D-deficient [25(OH)D <20 ng/ml] and nondeficient [25(OH)D ≥20 ng/ml] groups in the entire cohort and the racially stratified subgroups (blacks and whites) using Student t test or Mann-Whitney U test, depending on data distribution. Differences in the mean of 25(OH)D between black and white children and demographic characteristics [obese (BMI ≥95th percentile for age and sex) vs. nonobese (BMI <95th percentile), and pubertal vs. prepubertal] were also assessed by t tests or Mann-Whitney U tests. Racial differences in the rates of vitamin D deficiency [defined as plasma 25(OH)D less than 20 ng/ml] and insufficiency (from 20 to less than 30 ng/ml) were assessed using χ2. The association between plasma 25(OH)D and the different study variables was examined by bivariate Pearson or Spearman correlations depending on data distribution. Multiple linear regression analysis models were performed to determine the independent effects of race, season, pubertal status, and each of the adiposity measures (BMI, %BF, VAT, and SAT) separately on plasma 25(OH)D after adjusting for age and sex. Logistic regression was done to determine the influence of these variables on vitamin D deficiency [plasma 25(OH)D <20 ng/ml—as a categorical outcome]. A two-way ANOVA analysis was conducted to examine the independent relationship of obesity [obese (BMI ≥ 95th percentile) vs. nonobese (BMI <95th percentile)], race, and the interaction term of obesity*race with 25(OH)D concentration.
All statistical assumptions were met. Data are presented as mean ± sem unless otherwise indicated. Statistical significance was set at P < 0.05. The statistical analysis was done using PASW Statistics (version 18; SPSS Inc., Chicago, IL).
Results
A total of 237 healthy 8- to 18-yr-old obese [112 (47%)] and nonobese [125 (53%)] black [112 (47%)] and white [125 (53%)] children were studied during either winter/spring [110 (46%)] or summer/fall [127 (54%)]. The study population had a mean age (±sd) of 12.7 ± 2.2 yr, 43% were males, and 18% were Tanner I, prepubertal.
Vitamin D status
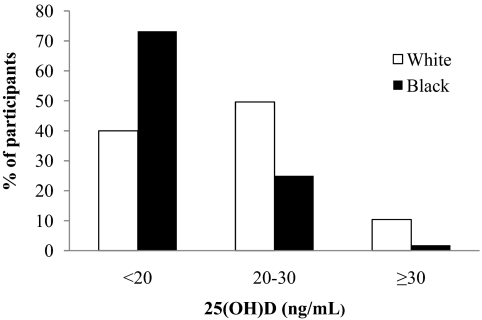
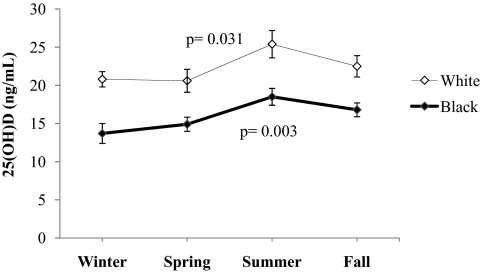
Mean (±sd) plasma 25(OH)D for the entire cohort was 19.4 ± 7.4 ng/ml. Mean plasma 25(OH)D levels were lower in black vs. white children (16.4 ± 5.8 vs. 22.0 ± 7.7 ng/ml; P < 0.001), or obese vs. nonobese (17.6 ± 7.1 vs. 20.9 ± 7.4 ng/ml; P < 0.001), or pubertal vs. prepubertal (18.5 ± 6.9 vs. 23.3 ± 8.4 ng/ml; P < 0.001). There were significant differences (P < 0.001) between the two racial groups in the proportion of children classified as vitamin D deficient [25(OH)D, <20 ng/ml], insufficient [25(OH)D, 20 to <30 ng/ml], or sufficient [25(OH)D, ≥30 ng/ml] (Fig. 1). The racial disparities in the circulating levels of 25(OH)D persisted throughout the year irrespective of season (Fig. 2). Within each racial group there was significant seasonal variation in vitamin D status. The mean plasma 25(OH)D levels during summer/fall were higher than during winter/spring (whites, 23.8 ± 8.2 vs. 20.7 ± 7.0 ng/ml, P = 0.031; blacks, 17.5 ± 5.9 vs. 14.2 ± 5.0 ng/ml, P = 0.003).
Fig. 1.
Vitamin D status (deficient, insufficient, and sufficient) by race.
Fig. 2.
Seasonal variation in plasma 25(OH)D by race. Mann-Whitney U test for 25(OH)D values in winter/spring vs. summer/fall in whites and blacks.
25(OH)D, adiposity, and fasting lipid relationships
Differences in adiposity and lipid measures between vitamin D deficient [25(OH)D <20 ng/ml] and nondeficient [25(OH)D ≥20 ng/ml] groups in the entire cohort and the racially stratified subgroups are depicted in Table 1. In the entire cohort, vitamin D-deficient subjects had higher BMI, BMI percentile, %BF, VAT, SAT, and proportion of obesity than nondeficient subjects. Results were similar in whites when analyzed separately, except that there was no difference in SAT between vitamin D-deficient and nondeficient subjects. In blacks, however, except for SAT, there were no differences in BMI, BMI percentile, %BF, VAT, and rates of obesity between vitamin D-deficient and nondeficient subjects. Because of a black outlier with high plasma 25(OH)D concentration, differences in adiposity indices between vitamin D-deficient and nondeficient blacks were examined after excluding this outlier. The results were similar, except for the difference in BMI, which became significant (27.7 ± 1.0 vs. 24.0 ± 1.5 kg/m2; P = 0.037) (Table 1, see footnote). There were no differences in lipid parameters (total, LDL, HDL, and VLDL cholesterol, and triglycerides) between the vitamin D-deficient and nondeficient groups in the entire cohort and in whites and blacks. Examination of the data separately for boys and girls in each racial group revealed significant differences in adiposity indices in vitamin D-deficient vs. nondeficient white females, but not white males, black males, or black females (data not shown). However, our numbers for such an analysis remain relatively limited. The ANOVA statistics of the independent relationship of obesity, race, and the interaction term of obesity*race with 25(OH)D concentration showed that obesity (P = 0.001) and race (P < 0.001) have an independent relationship to circulating 25(OH)D, but not the interaction term (P = 0.366).
Table 1.
Clinical characteristics of vitamin D-deficient and -nondeficient subjects by race
| 25(OH)D (ng/ml) | All subjects |
Whites |
Blacks |
||||||
|---|---|---|---|---|---|---|---|---|---|
| <20 | ≥20 | P | <20 | ≥20 | P | <20 | ≥20 | P | |
| n | 132 | 105 | 50 | 75 | 82 | 30 | |||
| Sex (males/females) | 46/86 | 55/50 | 0.007 | 17/33 | 40/35 | 0.033 | 29/53 | 15/15 | NS |
| Age (yr) | 12.9 ± 0.2 | 12.5 ± 0.2 | NS | 13.1 ± 0.3 | 12.9 ± 0.3 | NS | 12.8 ± 0.2 | 11.6 ± 0.3 | 0.006 |
| Race (white/black) | 50/82 | 75/30 | <0.001 | ||||||
| White (%) | 37.9 | 71.4 | |||||||
| Black (%) | 62.1 | 28.6 | |||||||
| Pubertal status (PP/P) | 14/118 | 28/77 | 0.001 | 6/44 | 15/60 | NS | 8/74 | 13/17 | <0.001 |
| Tanner I (%) | 10.6 | 26.7 | 12 | 20 | 9.8 | 43.3 | |||
| Tanner II–V (%) | 89.4 | 73.3 | 88 | 80 | 90.2 | 56.7 | |||
| Season (WS/SF) | 66/66 | 44/61 | NS | 32/18 | 39/36 | NS | 34/48 | 5/25 | 0.015 |
| BMI (kg/m2) | 28.1 ± 0.8 | 24.7 ± 0.8 | 0.003 | 28.7 ± 1.4 | 24.7 ± 0.9 | 0.031 | 27.7 ± 1.0 | 24.5 ± 1.5 | 0.066a |
| BMI percentile (%) | 82.0 ± 2.1 | 72.5 ± 2.8 | 0.003 | 81.1 ± 3.6 | 72.2 ± 3.5 | 0.020 | 82.5 ± 2.6 | 73.3 ± 4.8 | NS |
| Obese (yes/no) | 73/59 | 39/66 | 0.005 | 29/21 | 27/48 | 0.015 | 44/38 | 12/18 | NS |
| VAT (cm2) | 49.4 ± 3.9 | 38.4 ± 3.0 | 0.027 | 65.1 ± 7.5 | 40.8 ± 3.6 | 0.026 | 39.7 ± 3.9 | 32.7 ± 5.5 | NS |
| SAT (cm2) | 315.7 ± 21.2 | 243.1 ± 22.5 | 0.014 | 341.1 ± 35.1 | 253.1 ± 27.2 | 0.060 | 300.1 ± 26.6 | 219.5 ± 39.9 | 0.047 |
| %BF | 32.8 ± 1.2 | 28.0 ± 1.3 | 0.008 | 33.7 ± 1.9 | 28.0 ± 1.5 | 0.021 | 32.3 ± 1.5 | 28.2 ± 2.7 | NS |
| Cholesterol (mg/dl) | 161.1 ± 2.8 | 161.3 ± 3.1 | NS | 166.7 ± 4.6 | 164.1 ± 3.8 | NS | 157.7 ± 3.5 | 154.0 ± 4.9 | NS |
| Triglycerides (mg/dl) | 96.0 ± 4.6 | 102.6 ± 6.8 | NS | 121.4 ± 9.4 | 109.9 ± 8.1 | NS | 80.6 ± 3.9 | 84.0 ± 12.1 | NS |
| LDL (mg/dl) | 96.1 ± 2.5 | 94.3 ± 2.8 | NS | 97.8 ± 4.1 | 96.7 ± 3.3 | NS | 95.2 ± 3.1 | 88.2 ± 4.8 | NS |
| HDL (mg/dl) | 45.8 ± 1.0 | 47.6 ± 1.9 | NS | 44.6 ± 1.7 | 45.2 ± 1.1 | NS | 46.6 ± 1.2 | 53.8 ± 5.9 | NS |
| VLDL (mg/dl) | 19.0 ± 0.9 | 20.9 ± 1.4 | NS | 23.8 ± 1.9 | 22.0 ± 1.6 | NS | 16.0 ± 0.8 | 17.9 ± 2.5 | NS |
Data were available for VAT and SAT in 227 participants, DEXA in 224 participants, and lipids in 233 participants. PP, Prepubertal (Tanner I); P, pubertal (Tanner II–V); WS, winter/spring; SF, summer/fall; obese, BMI percentile ≥95. Student t test or Mann-Whitney U test was used in quantitative variables and χ2 in qualitative variables.
After excluding the outlier with high plasma 25(OH)D concentration, differences in adiposity indices between vitamin D-deficient and nondeficient blacks did not change, except for the BMI (27.7 ± 1.0 vs. 24.0 ± 1.5 kg/m2; P = 0.037).
Correlates of plasma 25(OH)D
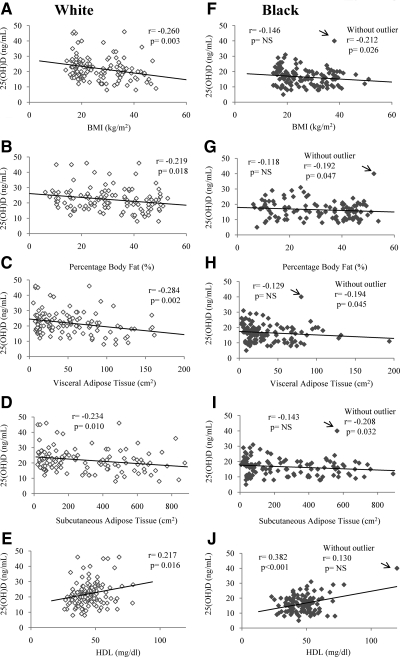
For the entire cohort, plasma 25(OH)D was associated inversely with BMI (r = −0.209; P = 0.001), BMI percentile (r = −0.187; P = 0.004), %BF (r = −0.172; P = 0.010), VAT (r = −0.149; P = 0.024), and SAT (r = −0.174; P = 0.009); and positively with HDL cholesterol (r = 0.214; P = 0.001). Plasma 25(OH)D was inversely associated with BMI, VAT, SAT, and %BF only in white children, and positively with HDL cholesterol in both black (r = 0.382; P < 0.001) and white children (r = 0.217; P = 0.016) (Fig. 3). In blacks, the relationships shown in Fig. 3 change when the analyses are performed excluding the outlier with the highest plasma 25(OH)D. Without the outlier, plasma 25(OH)D becomes inversely associated with BMI (r = −0.212; P = 0.026), VAT (r = −0.194; P = 0.045), SAT (r = −0.208; P = 0.032) and %BF (r = −0.192; P = 0.047) with no association with HDL (r = 0.130; P = 0.179).
Fig. 3.
Correlations between plasma 25(OH)D levels and BMI, %BF, VAT, SAT, and HDL cholesterol in white (A–E) and black (F–J) youth. In blacks, the “r” and “P” values for the correlation analyses with and without an outlier with high plasma 25(OH)D (arrow) are shown.
Predictors of plasma 25(OH)D levels and vitamin D deficiency
Multiple linear regression analysis models assessing the independent effects of race, season, pubertal status, and each of the adiposity measures (BMI, %BF, VAT, and SAT) separately on plasma 25(OH)D levels after controlling for age and sex are shown in Table 2. Race, season, pubertal status, and VAT were significant independent predictors of plasma 25(OH)D, explaining 28.3% of variance in plasma 25(OH)D concentrations, the majority of which was attributable to the strong influence of race, pubertal status, and season. In a logistic regression model adjusted for age, the odds of vitamin D deficiency were highest in blacks compared with whites [odds ratio (OR), 95% confidence interval (CI): 6.00, 3.14 to 11.47; P < 0.001], higher in females compared with males (OR, 2.01; 95% CI, 1.10 to 3.66; P = 0.023), pubertal compared with prepubertal children (OR, 2.88; 95% CI, 1.27 to 6.57; P = 0.012), and winter/spring season compared with summer/fall (OR, 2.29; 95% CI, 1.22 to 4.29; P = 0.010). Furthermore, for each 10-cm2 increase in VAT, the odds of vitamin D deficiency multiplied by 1.087 (95% CI, 1.002 to 1.180; P = 0.042).
Table 2.
Multiple linear regression analysis for plasma 25(OH)D (ng/ml) adjusted for age and sex
| Estimate | 95% CI | P | R2 | |
|---|---|---|---|---|
| Model 1 | ||||
| Race | −6.31 | −8.01 to −4.61 | <0.001 | 0.275 |
| Pubertal status | −4.44 | −7.24 to −1.63 | 0.002 | |
| Season | −3.34 | −5.02 to −1.65 | <0.001 | |
| BMI | −0.08 | −0.19 to 0.03 | 0.168 | |
| Model 2 | ||||
| Race | −6.62 | −8.34 to −4.89 | <0.001 | 0.279 |
| Pubertal status | −4.69 | −7.64 to −1.73 | 0.002 | |
| Season | −3.45 | −5.19 to −1.72 | <0.001 | |
| %BF | −0.03 | −0.10 to 0.04 | 0.420 | |
| Model 3 | ||||
| Race | −6.48 | −8.20 to −4.76 | <0.001 | 0.283 |
| Pubertal status | −4.45 | −7.28 to −1.62 | 0.002 | |
| Season | −3.51 | −5.22 to −1.81 | <0.001 | |
| VAT (by every 10 cm2) | −0.25 | −0.48 to −0.01 | 0.037 | |
| Model 4 | ||||
| Race | −6.22 | −7.94 to −4.50 | <0.001 | 0.268 |
| Pubertal status | −4.66 | −7.51 to −1.80 | <0.001 | |
| Season | −3.56 | −5.28 to −1.84 | <0.001 | |
| SAT (by every 10 cm2) | −0.02 | −0.06 to 0.02 | 0.275 |
P < 0.05 is indicated in bold. Race: 0 = white, 1 = black; pubertal status: 0 = prepubertal, 1 = pubertal; season: 0 = summer/fall, 1 = winter/spring.
Discussion
We found in healthy obese and nonobese 8- to 18-yr-old black and white youth, when examined together, an inverse gradient in the relationship between plasma 25(OH)D levels and clinical and laboratory measures of adiposity. BMI, BMI percentile, %BF, and abdominal adiposity (SAT and VAT) were significantly higher in vitamin D-deficient than nondeficient children. Vitamin D deficiency was associated with higher levels of BMI, %BF, and VAT in whites but not in blacks, and higher levels of SAT in blacks but not in whites. Removal of an outlier with high plasma 25(OH)D in blacks altered the observed racial dimorphism in the correlations between vitamin D status and adiposity (Fig. 3).
Our findings compare and contrast with previously published research on vitamin D and adiposity relationships in children and adolescents (27–29). Dong et al. (27) observed inverse correlations between serum 25(OH)D and BMI, waist circumference, and total and regional measures of adiposity in 14- to 18-yr-old black and white adolescents (n = 559) residing in Atlanta, Georgia (33° N). Lenders et al. (2) noted an independent inverse association between serum 25(OH)D and total body fat (fat mass assessed by DXA) in a cross-sectional cohort of 58 obese adolescents enrolled from five geographic areas in the United States. Foo et al. (28) found no association between plasma 25(OH)D and total body adiposity assessed by DXA in 15-yr-old adolescent girls (n = 323) residing in Beijing, China (40° N). In a cross-sectional study of 6- to 21-yr-old children (n = 384; 4% obese) residing in Philadelphia, Pennsylvania (40° N), significant bivariate associations between low serum 25(OH)D status (<30 ng/ml) and BMI, BMI z-score, and fat mass assessed by DXA were not significant in multivariable regression analysis (29). Lack of similarity of body composition, growth, and pubertal status among the various study cohorts may explain the differences in the 25(OH)D-adiposity relationships in these studies.
An inverse relationship between 25(OH)D status and regional adiposity was found in adults (7, 30), young women (31), and older adolescents (27), and our data confirm this relationship in 8- to 18-yr-old black and white children. We found VAT to be an independent predictor of plasma 25(OH)D levels and vitamin D deficiency in children. Similar to our findings, the inverse relationship between 25(OH)D and the individual indices of regional fat was much stronger for VAT than SAT in Caucasian adults examined as part of the third generation offspring cohort of the Framingham Heart Study (7).
Racial differences in the relationship between vitamin D status and adiposity have been observed in 12+-yr-old black (n = 2475) and white (n = 3567) adolescent and adult females examined in the National Health and Examination Nutrition Survey (NHANES) III (1988–94) cohort (32). Inverse association between body fat (assessed by bioelectrical impedance) and serum 25(OH)D varied by race and was stronger in whites compared with blacks. Our results extend those findings to children younger than 12 yr, in whom the inverse associations between 25(OH)D and adiposity measures were detected only in whites. Although these differences could stem from racial variation in the degree of sequestration of vitamin D in body fat, they are more likely to be due to the confounding effects of racial variations in skin color (32). In our cohort, blacks had significantly lower levels of 25(OH)D than whites throughout the year. Race-related differences in visceral adiposity could also play a role in the differing relationships of adiposity with vitamin D between the two racial groups (13, 33). However, our findings of lack of a significant association between 25(OH)D concentrations and adiposity in blacks should be interpreted with caution because this relationship was strongly influenced by one outlier with high plasma 25(OH)D, and the P values changed when analyzed without the outlier. Furthermore, we found no significant interaction terms between adiposity and race on their effects on circulating 25(OH)D, suggesting no racial differences in the relationship between adiposity and 25(OH)D. Therefore, additional data are needed regarding the relationship between 25(OH)D and adiposity in different racial groups.
Lipid profile parameters did not differ between vitamin D-deficient and nondeficient groups of children in the entire cohort or in the racial subgroups. However, there were positive correlations between plasma 25(OH)D and HDL cholesterol in black and white children (r = 0.214; P = 0.001), and this association was stronger in blacks (r = 0.382; P < 0.001) compared with whites (r = 0.217; P = 0.016); the relationship in blacks was strongly influenced by an outlier with high plasma 25(OH)D and became nonsignificant when examined without the outlier [r = 0.130; P = nonsignificant (NS)]. Similar to our findings, Johnson et al. (34) noted a positive association between 25(OH)D and HDL cholesterol (r = 0.41; P ≤ 0.001) and lower levels of HDL cholesterol in children with hypovitaminosis D in a retrospective record review of 2- to 18-yr-old children (70% Caucasian) seen at a pediatric specialty clinic at Rochester, Minnesota (44° N). These data lend support to the fact that vitamin D may play a role in HDL cholesterol levels in children.
We have documented excessive prevalence of vitamin D deficiency and insufficiency in a sample of healthy black and white children who volunteered to participate in research. Our combined prevalence rates of vitamin D deficiency and insufficiency in black children (98%) and white children (90%) are comparable to published rates of vitamin D deficiency and insufficiency in children examined in the 2001–2004 NHANES cohort (35). In the 2001–2004 NHANES cohort, 62% of white girls, 66% of white boys, 98% of black girls, and 96% of black boys who were 13–21 yr old; and 64% of white girls, 53% of white boys, 96% of black girls, and 93% of black boys who were 7–12 yr old were vitamin D deficient [serum 25(OH)D <15 ng/ml] or vitamin D insufficient (15–30 ng/ml). Our data confirm the relevance of season, race (surrogate indicator of skin color), pubertal status, BMI, and adiposity in the determination of vitamin D status of children residing in the Northeast (4, 35–40). Although plasma 25(OH)D concentrations were higher during summer/fall than during winter/spring in blacks and whites, the disparities in the vitamin D status between black and white children persisted throughout the year, and this can be explained by the racial differences in skin pigmentation. We found race, season, pubertal status, and visceral fat to be significant predictors of plasma 25(OH)D. These variables explained 28.3% of the variance in plasma 25(OH)D levels of our cohort. Black race, female gender, adolescence, winter/spring season, and excessive VAT were associated with higher odds of being vitamin D deficient.
Our data have few limitations for a better characterization of the racial differences in vitamin D levels of our cohort and assessment of the differences in adiposity measures between vitamin D-deficient and nondeficient blacks. Information regarding recognized confounders of vitamin D status such as dietary vitamin D, duration of sun exposure, usage of sunscreen, and quantification of UV radiation assessed by dosimeters and objective assessment of skin melanization with skin spectrophotometer would have helped. Lack of significant difference in BMI and adiposity measures (%BF and VAT) between vitamin D-deficient and nondeficient black children may be related to the small number of vitamin D-nondeficient children in this group and the associated limited variability in BMI categories in this small sample. Strengths of our study are accurate characterization of total body and abdominal adiposity in an appreciable number of obese and nonobese black and white healthy volunteer children.
Conclusions
We conclude that vitamin D deficiency and insufficiency are highly prevalent in 8- to 18-yr-old preadolescent and adolescent black and white children residing in the Northeast. Race, female gender, season, pubertal status, and visceral adiposity are independent predictors of plasma 25(OH)D status. Our data show that the relationship between vitamin D status and adiposity in children is complex and needs further exploration. Our observed racial differences in the relationship between plasma 25(OH)D concentrations and adiposity measures and HDL cholesterol should be interpreted with caution because the P values for our findings differ when analyzed with and without an outlier with high plasma 25(OH)D concentration. Lack of significant differences in %BF and VAT between vitamin D-deficient and nondeficient black children, even after excluding the outlier, could be due to the small sample size of vitamin D-nondeficient children and the limited variability in their BMI. Furthermore, there was no significant interaction between adiposity and race on their effects on circulating 25(OH)D, suggesting no differences in the influence of adiposity on 25(OH)D in whites and blacks. Therefore, additional studies are warranted to explore the relationship between 25(OH)D and adiposity in children of different ethnic groups. Plasma 25(OH)D is inversely associated with adiposity and positively associated with HDL cholesterol. Besides therapeutic interventions to correct the high rates of vitamin D deficiency in high-risk youth, studies are warranted to test the effects of vitamin D optimization on adiposity measures and lipid profile in children.
Acknowledgments
These studies would not have been possible without the devotion of nursing staff of the Pediatric Clinical and Translational Research Center and the research team, and most importantly, the commitment of the study participants and their parents.
This work was supported by U.S. Public Health Service Grant RO1 HD27503 (to S.A.A.), K24 HD01357 (to S.A.A.), the Richard L. Day Endowed Chair (to S.A.A.), K23 HD052550 (to K.R.), Río Hortega contract from the Instituto de Salud Carlos III of the Spanish Ministry of Health (CM07/00211) (to J.d.l.H.), and National Institutes of Health Grants 1UL1RR025771 CTSI and UL1 RR024153 CTSA (previously MO1 RR00084 GCRC).
Disclosure Summary: The authors have no financial relationships relevant to this article to disclose.
Footnotes
- %BF
- Percentage body fat
- BMI
- body mass index
- DXA
- dual-energy x-ray absorptiometry
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- NS
- nonsignificant
- 25(OH)D
- 25-hydroxyvitamin D
- OR
- odds ratio
- SAT
- sc adipose tissue
- VAT
- visceral adipose tissue
- VLDL
- very low-density lipoprotein.
References
- 1. Alemzadeh R, Kichler J, Babar G, Calhoun M. 2008. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 57:183–191 [DOI] [PubMed] [Google Scholar]
- 2. Lenders CM, Feldman HA, Von Scheven E, Merewood A, Sweeney C, Wilson DM, Lee PD, Abrams SH, Gitelman SE, Wertz MS, Klish WJ, Taylor GA, Chen TC, Holick MF. 2009. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am J Clin Nutr 90:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. 1988. Low circulating vitamin D in obesity. Calcif Tissue Int 43:199–201 [DOI] [PubMed] [Google Scholar]
- 4. Reis JP, von Mühlen D, Miller ER, 3rd, Michos ED, Appel LJ. 2009. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 124:e371–e379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. 2000. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693 [DOI] [PubMed] [Google Scholar]
- 6. Yanoff LB, Parikh SJ, Spitalnik A, Denkinger B, Sebring NG, Slaughter P, McHugh T, Remaley AT, Yanovski JA. 2006. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese black Americans. Clin Endocrinol (Oxf) 64:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O'Donnell CJ, Hoffmann U, Jacques PF, Booth SL, Vasan RS, Wolf M, Wang TJ. 2010. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 59:242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKinney K, Breitkopf CR, Berenson AB. 2008. Association of race, body fat and season with vitamin D status among young women: a cross-sectional study. Clin Endocrinol (Oxf) 69:535–541 [DOI] [PubMed] [Google Scholar]
- 9. Rodríguez-Rodríguez E, Navia-Lombán B, López-Sobaler AM, Ortega RM. 2010. Associations between abdominal fat and body mass index on vitamin D status in a group of Spanish schoolchildren. Eur J Clin Nutr 64:461–467 [DOI] [PubMed] [Google Scholar]
- 10. Vilarrasa N, Maravall J, Estepa A, Sánchez R, Masdevall C, Navarro MA, Alía P, Soler J, Gómez JM. 2007. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest 30:653–658 [DOI] [PubMed] [Google Scholar]
- 11. Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. 2008. Vitamin D(3) in fat tissue. Endocrine 33:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freedman DS, Wang J, Thornton JC, Mei Z, Pierson RN, Jr, Dietz WH, Horlick M. 2008. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 16:1105–1111 [DOI] [PubMed] [Google Scholar]
- 13. Lee S, Kuk JL, Hannon TS, Arslanian SA. 2008. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 16:1066–1071 [DOI] [PubMed] [Google Scholar]
- 14. Rush EC, Scragg R, Schaaf D, Juranovich G, Plank LD. 2009. Indices of fatness and relationships with age, ethnicity and lipids in New Zealand European, Maori and Pacific children. Eur J Clin Nutr 63:627–633 [DOI] [PubMed] [Google Scholar]
- 15. Sisson SB, Katzmarzyk PT, Srinivasan SR, Chen W, Freedman DS, Bouchard C, Berenson GS. 2009. Ethnic differences in subcutaneous adiposity and waist girth in children and adolescents. Obesity (Silver Spring) 17:2075–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tybor DJ, Lichtenstein AH, Dallal GE, Daniels SR, Must A. 2010. Racial differences in central adiposity in a longitudinal cohort of black and white adolescent females. BMC Pediatr 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goran MI, Gower BA. 1999. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr 70:149S–156S [DOI] [PubMed] [Google Scholar]
- 18. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. 2002. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 19. Burns SF, Arslanian SA. 2009. Waist circumference, atherogenic lipoproteins, and vascular smooth muscle biomarkers in children. J Clin Endocrinol Metab 94:4914–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S, Gungor N, Bacha F, Arslanian S. 2007. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30:2091–2097 [DOI] [PubMed] [Google Scholar]
- 21. Danadian K, Balasekaran G, Lewy V, Meza MP, Robertson R, Arslanian SA. 1999. Insulin sensitivity in African-American children with and without family history of type 2 diabetes. Diabetes Care 22:1325–1329 [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Guerra N, Arslanian S. 2010. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab 95:2426–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S, Bacha F, Arslanian SA. 2006. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr 149:809–816 [DOI] [PubMed] [Google Scholar]
- 24. Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. 1996. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol 143:971–978 [DOI] [PubMed] [Google Scholar]
- 25. Chen TC, Turner AK, Holick MF. 1990. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem 1:315–319 [DOI] [PubMed] [Google Scholar]
- 26. Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. 2005. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224 [DOI] [PubMed] [Google Scholar]
- 27. Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, Keeton D, Petty K, Holick MF, Zhu H. 2010. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics 125:1104–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foo LH, Zhang Q, Zhu K, Ma G, Trube A, Greenfield H, Fraser DR. 2009. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos Int 20:417–425 [DOI] [PubMed] [Google Scholar]
- 29. Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. 2007. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 86:150–158 [DOI] [PubMed] [Google Scholar]
- 30. Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM. 2009. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab 94:3306–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kremer R, Campbell PP, Reinhardt T, Gilsanz V. 2009. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 94:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Looker AC. 2005. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab 90:635–640 [DOI] [PubMed] [Google Scholar]
- 33. Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. 2003. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab 88:2534–2540 [DOI] [PubMed] [Google Scholar]
- 34. Johnson MD, Nader NS, Weaver AL, Singh R, Kumar S. 2010. Relationships between 25-hydroxyvitamin D levels and plasma glucose and lipid levels in pediatric outpatients. J Pediatr 156:444–449 [DOI] [PubMed] [Google Scholar]
- 35. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. 2009. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124:e362–e370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clemens TL, Adams JS, Henderson SL, Holick MF. 1982. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1:74–76 [DOI] [PubMed] [Google Scholar]
- 37. Harris SS, Dawson-Hughes B. 1998. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 67:1232–1236 [DOI] [PubMed] [Google Scholar]
- 38. Saintonge S, Bang H, Gerber LM. 2009. Implications of a new definition of vitamin D deficiency in a multiracial US adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics 123:797–803 [DOI] [PubMed] [Google Scholar]
- 39. Norman AW. 1998. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr 67:1108–1110 [DOI] [PubMed] [Google Scholar]
- 40. Webb AR. 2006. Who, what, where and when—influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol 92:17–25 [DOI] [PubMed] [Google Scholar]