Physiological variations in insulin levels do not alter bone turnover, undercarboxylated osteocalcin, or osteoprotegerin levels in humans.
Abstract
Context:
Recent studies in mice have demonstrated that insulin signaling in osteoblasts stimulates bone formation and reduces osteoprotegerin production; the latter results in an increase in bone resorption, which then leads to the release of undercarboxylated osteocalcin from bone. Undercarboxylated osteocalcin, in turn, enhances insulin sensitivity.
Objective:
The objective of the study was to test whether physiological changes in insulin levels regulate bone metabolism in humans.
Design:
This investigation was an analysis of samples from a prospective study.
Setting:
The study was conducted at a clinical research unit.
Participants and Interventions:
Fourteen subjects underwent a 7-h stepped insulin infusion accompanied by a glucose clamp and somatostatin infusion along with replacement infusions of GH and glucagon, thus isolating possible effects of insulin on bone. Insulin was infused at rates achieving low (∼150 pmol/liter), intermediate (∼350 pmol/liter), or high (∼700 pmol/liter) plasma insulin levels.
Main Outcome Measures:
Bone turnover markers, undercarboxylated osteocalcin, and osteoprotegerin levels at the end of the low, intermediate, and high dose insulin infusions were measured.
Results:
Values for the outcome measures at the end of the intermediate- and high-dose insulin infusions were no different from values at the end of the low-dose insulin infusion. However, measures of insulin sensitivity (glucose infusion and disappearance rates) correlated positively with C-terminal telopeptide of type I collagen levels.
Conclusions:
Acute changes in insulin levels, as occur during meals, do not regulate bone turnover, undercarboxylated osteocalcin, or osteoprotegerin levels. However, the correlation of measures of insulin sensitivity with bone resorption suggests the need for further studies in humans on the possible regulation of bone metabolism by insulin.
Recent provocative studies in rodents have demonstrated novel links between bone and energy metabolism. Lee et al. (1) initially provided evidence that the undercarboxylated form of osteocalcin improved glucose tolerance by increasing the production of adiponectin by osteoblasts as well as CyclinD1 and insulin expression in β-cells. Subsequently, Ferron et al. (2) showed that long-term treatment of mice with bacterially produced undercarboxylated osteocalcin significantly attenuated the deleterious effects on body mass and glucose tolerance of gold thioglucose-induced hyperphagia and high-fat diet.
Whereas these studies demonstrated a role for bone (through the production of undercarboxylated osteocalcin) in regulating insulin secretion and sensitivity, the potential role of insulin in regulating bone metabolism in mice was examined in two very recent studies. Thus, Fulzele et al. (3) found that mice lacking the insulin receptor in osteoblasts had low circulating undercarboxylated osteocalcin levels and reduced bone acquisition due to decreased bone formation and deficient numbers of osteoblasts. Interestingly, these mice developed marked peripheral adiposity, glucose intolerance, and insulin resistance with aging. In an accompanying paper, Ferron et al. (4) showed that activation of the insulin receptor in osteoblasts resulted in an increase in undercarboxylated osteocalcin and decrease in osteoprotegerin (OPG) production, the latter leading to an increase in osteoclastic bone resorption. Furthermore, the acidic pH in the bone resorption lacunae was found to result in decarboxylation of osteocalcin, which would then, in turn, lead to an increase in insulin secretion and sensitivity through the mechanisms described above, essentially a feed-forward loop between insulin signaling and bone.
These studies in rodents raised the key question of whether physiological changes in insulin levels, as occur during the course of the day, regulate bone metabolism in humans. Based on the series of studies in mice described above, the prediction would be that increasing insulin levels would lead to increases in markers of bone formation, a decrease in OPG, and an increase in bone resorption markers and undercarboxylated osteocalcin levels. To test this model in humans, we used plasma samples from a previously published study that used a prolonged (7 h) insulin infusion accompanied by a glucose clamp in the presence of matched levels of GH and glucagon (5), thus isolating the possible effects of insulin on bone, independent of changes in glucose, GH, or glucagon levels. Insulin was infused at a rate so as to achieve low (∼150 pmol/liter), intermediate (∼350 pmol/liter), or high (∼700 pmol/liter) plasma insulin levels, thereby spanning the physiological range of insulin concentrations that may be present during the course of the day, pre- and postprandially. Our end points included the amino terminal propeptide of type I collagen (PINP), osteocalcin, and undercarboxylated osteocalcin (bone formation markers); C-terminal telopeptide of type I collagen (CTX; a bone resorption marker); and OPG. Because our study design also provided data on the glucose infusion rate needed to maintain constant glucose levels for each insulin infusion rate as well as the glucose disappearance rate (both are measures of insulin sensitivity), in further analyses we also assessed whether these insulin sensitivity measures were related to any of the bone metabolism parameters.
Subjects and Methods
Study subjects and experimental protocol
This study involved reanalysis of samples from a previous study examining insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production in nondiabetic and diabetic subjects (5). Details of the study design have previously been published (5). Briefly, after approval from the Mayo Institutional Review Board, 14 nondiabetic subjects and 12 subjects with type 2 diabetes gave informed written consent to participate in the study. For the present analysis, we selected plasma samples from seven nondiabetic and seven diabetic subjects in whom sufficient stored plasma was available for analysis. All subjects were in good health and at a stable weight. None regularly engaged in vigorous physical exercise, and all subjects had assessment of body composition, including percentage of fat and lean body mass, using dual-energy x-ray absorptiometry (DPX-IQ scanner, SmartScan version 4.6; Hologic, Bedford, MA). In the diabetic subjects, oral hypoglycemic agents were discontinued 3 wk before the study and long-acting insulin switched to regular insulin with meals, beginning with the midday meal 2 d before the study. All subjects were instructed to follow a weight-maintenance diet containing 55% carbohydrate, 30% fat, and 15% protein for at least 3 d before the day of the study.
As described in detail previously (5), subjects were admitted to the Mayo Inpatient Clinical Research Unit at 1700 h on the evening before the study. After a standard 10-cal/kg meal, an infusion of insulin was started in the diabetic subjects or saline in the nondiabetic subjects. The insulin infusion rate was adjusted to maintain glucose concentrations in the diabetic subjects at approximately 5 mmol/liter during the night. To eliminate endogenous pancreatic hormone secretion and to ensure equal levels of GH and glucagon in all subjects, at 0900 h (t = 0), infusions were started for somatostatin (60 ng/kg·min), GH (3 ng/kg·min), and glucagon (0.65 ng/kg·min) and continued until the end of the study. Insulin was infused at a rate of 0.78 mU/kg lean body weight/min (∼0.5 mU/kg total body weight per minute) from 0 to 180 min (low dose insulin), 1.56 (∼1.0) from 181 to 300 min (intermediate dose insulin), and 3.1 (∼2.0) from 301 to 420 min (high dose insulin). A glucose infusion was also begun and the rate adjusted to maintain plasma glucose concentrations at approximately 9.3 mmol/liter (∼165 mg/dl) over the next 7 h of the study. A primed-continuous infusion of [3-3H] glucose was also initiated (see Ref. 5 for details) to assess glucose dynamics. A hyperglycemic clamp was used in this study because the original intent was to define possible differences in splanchnic glucose uptake and suppression of endogenous glucose production in nondiabetic vs. diabetic subjects in the setting of modest hyperglycemia (5).
Analytical techniques
All samples were placed on ice, centrifuged at 4 C, and the plasma separated. The samples were stored long term at −20 C until analysis, and only previously unthawed samples were used in this study. Plasma glucose was measured by a glucose oxidase method using a YSI glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured using a chemiluminescence method with the Access Ultrasensitive Immunoenzymatic assay system (Beckman, Palo Alto, CA). C-peptide concentrations were assayed by RIA (Linco Research, St. Charles, MO). In the present analyses, we also used the steady-state glucose infusion rate (GIR; micromoles per kilogram per minute) and the glucose disappearance rate (Rd; micromoles per kilogram per minute) (see Ref. 5 for details of this calculation) as measures of insulin sensitivity.
Serum PINP was measured by RIA [interassay coefficient of variation (CV) < 10%; Immunodiagnostic Systems, Fountain Hills, AZ], and serum osteocalcin was measured using an enzyme immunoassay (Quidel, San Diego, CA; interassay CV < 8%; CIS-US, Bedford, MA). Serum undercarboxylated osteocalcin was measured using a validated enzyme immunoassay (6) with antibodies specific for the undercarboxylated form of osteocalcin (interassay CV < 10%; Takara Bio Inc., Shiga, Japan). Serum CTX was measured by ELISA (interassay CV < 8%; Immunodiagnostic Systems), as was serum OPG (interassay CV < 8%; ALPCO Immunoassays, Windham, NH).
Statistical methods
Data are reported as mean ± sem. Glucose turnover measurements were determined by averaging the results between 150 and 180, 270 and 300, and 390 and 420 min for the three insulin doses. Paired t tests were used to compare parameters at the end of the intermediate- and high-dose insulin infusions to values at the end of the low-dose infusion. Unadjusted and adjusted [for body mass index (BMI) or gender] Pearson correlation coefficients were used to describe the relationships between the various bone turnover markers and OPG levels to GIR and Rd. P < 0.05 was considered significant.
Results
Table 1 shows the clinical characteristics of the subjects in whom samples were analyzed for this study; these subjects were similar in all of the parameters listed in Table 1 to the larger group of subjects from which this subset was obtained (5). Data are shown separately for the nondiabetic and diabetic subjects and also for the subjects combined. The nondiabetic and diabetic subjects were similar in age, gender distribution, and height. The diabetic subjects did have higher weights and BMIs, but these differences were not statistically significant. As expected, fasting plasma glucose and hemoglobin A1c levels were significantly higher in the diabetic compared with the nondiabetic subjects. In all subsequent analyses, the pattern of changes in bone turnover markers and OPG levels was virtually identical in the nondiabetic and diabetic subjects. Thus, to increase the power of the study, analysis of the findings is presented for the combined group of 14 subjects.
Table 1.
Clinical characteristics of the study subjects
| Nondiabetic | Diabetic | Combined | |
|---|---|---|---|
| n | 7 | 7 | 14 |
| Age (yr) | 57.9 ± 4.0 | 62.0 ± 1.8 | 59.9 ± 2.2 |
| Male/female | 4/3 | 4/3 | 8/6 |
| Height (m) | 1.71 ± 0.04 | 1.70 ± 0.04 | 1.70 ± 0.03 |
| Weight (kg) | 80.6 ± 4.3 | 93.0 ± 5.4 | 86.8 ± 3.7 |
| BMI (kg/m2) | 27.8 ± 1.8 | 32.4 ± 2.0 | 30.1 ± 1.4 |
| Fasting plasma glucose (mmol/liter) | 5.40 ± 0.18 | 7.96 ± 0.24a | 6.68 ± 0.38 |
| Hemoglobin A1c (%) | 5.25 ± 0.11 | 6.97 ± 0.52b | 6.18 ± 0.36 |
Data are mean ± sem.
P < 0.001 vs. nondiabetic subjects.
P < 0.05 vs. nondiabetic subjects.
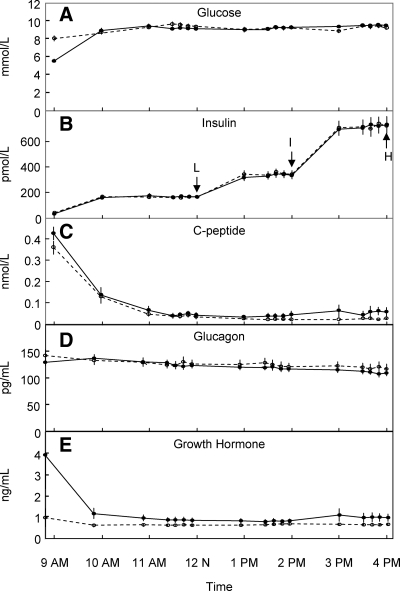
Figure 1 shows the glucose, insulin, C-peptide, glucagon, and GH levels in the nondiabetic and diabetic subjects over the course of the study. For the subsequent analysis, bone turnover markers and OPG levels were analyzed at the 1200, 1400, and 1600 h time points, corresponding to the low-, intermediate-, and high-dose insulin infusions, respectively. The respective mean plasma insulin levels were approximately 150, approximately 350, and approximately 700 pmol/liter. As is evident, plasma glucagon and GH concentrations remained stable through the course of the variable insulin infusions due to the concomitant somatostatin infusion accompanied by the glucagon and GH infusions.
Fig. 1.
Plasma glucose (A), insulin (B), C-peptide (C), glucagon (D), and GH (E) concentrations observed in the diabetic (open circles, dashed lines) and nondiabetic (closed circles, solid lines) subjects before and during the insulin clamp study. L, I, and H indicate the time points corresponding to the end of the low, intermediate, and high insulin infusion rates, respectively, when samples were analyzed for the bone metabolism markers.
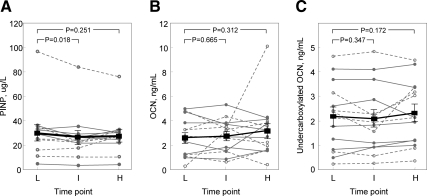
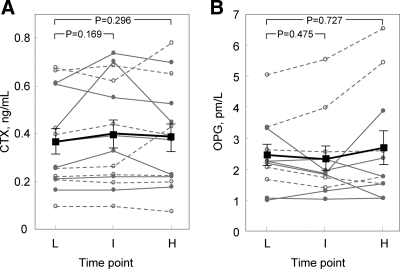
Compared with values at the end of the low insulin dose, serum PINP levels were slightly (but significantly) lower at the end of the intermediate dose interval but no different between the high and low time points (Fig. 2A). Serum osteocalcin (Fig. 2B) or undercarboxylated osteocalcin (Fig. 2C) did not differ across any of the insulin dose intervals. Similarly, serum CTX (Fig. 3A) or OPG (Fig. 3B) did not differ in the intermediate or high insulin dose intervals compared with the low-dose time point. Supplementary Figs. 1 and 2, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org, show the data for these bone turnover markers in the female and male subjects separately, and as is evident, the pattern of the changes in both sexes was very similar.
Fig. 2.
PINP (A), osteocalcin (OCN) (B), and undercarboxylated OCN (C) levels in the diabetic (open circles, dashed lines) and nondiabetic (closed circles, solid lines) subjects at the end of the low (L), intermediate (I), and high (H) doses. Because the changes in the diabetic and nondiabetic subjects were similar, the squares and dark solid lines indicate the mean values (± sem) in the two groups combined.
Fig. 3.
CTX (A) and OPG (B) levels in the diabetic (open circles, dashed lines) and nondiabetic (closed circles, solid lines) subjects at the end of the low (L), intermediate (I), and high (H) doses. Because the changes in the diabetic and nondiabetic subjects were similar, the squares and dark solid lines indicate the mean values (±sem) in the two groups combined.
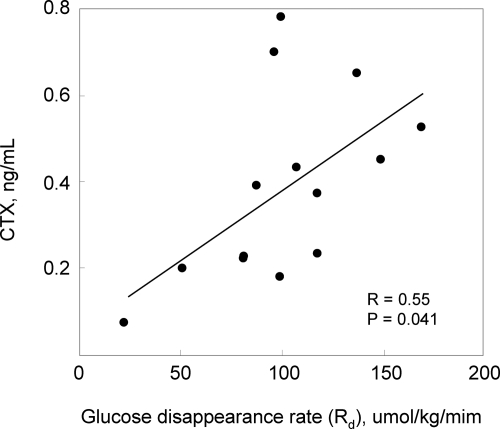
Whereas the above findings indicated that over the course of the study, acute changes (over hours) in insulin concentrations did not modulate bone turnover markers (including undercarboxylated osteocalcin) or circulating OPG levels, we next explored whether the specific measures of insulin sensitivity (GIR and Rd) were correlated with any of the bone turnover markers or OPG levels. For this analysis, we used the values of GIR and Rd derived from the high insulin infusion dose because preliminary analyses indicated that this dose provided the greatest dynamic range (i.e. the spread in values) for GIR and Rd to use in the correlation analysis. As shown in Table 2, serum CTX was significantly correlated with both measures of insulin sensitivity. Figure 4 shows in more detail the correlation between CTX and Rd (higher Rd reflects greater insulin sensitivity). These findings thus indicate that higher insulin sensitivity is associated with an increase in bone resorption. OPG tended to be inversely associated with measures of insulin sensitivity; conversely, PINP and undercarboxylated osteocalcin (but not total osteocalcin) levels showed positive associations with the insulin sensitivity measures, but these correlations did not achieve statistical significance (Table 2).
Table 2.
Correlation coefficients between the GIR needed to maintain constant glucose levels and the Rd (both assessed during the high dose insulin infusion) vs. the bone turnover markers and OPG levels (also as assessed during the high dose insulin infusion) in the study subjects
| GIR | Rd | |
|---|---|---|
| PINP | 0.450.111 | 0.450.105 |
| Osteocalcin | 0.010.981 | 0.040.894 |
| Undercarboxylated osteocalcin | 0.330.250 | 0.290.308 |
| CTX | 0.560.036 | 0.550.041 |
| OPG | −0.300.316 | −0.280.362 |
Shown are the r and P values (in superscript).
Fig. 4.
Plot showing the correlation between the Rd, a measure of insulin sensitivity, and CTX.
Because the relationship between CTX and measures of insulin sensitivity noted above could be confounded by gender or BMI, we also assessed the impact of adjusting for these variables. Thus, adjusting for effects of gender had little impact on the correlation coefficients between CTX and either GIR or Rd (adjusted R = 0.56, P = 0.045, and adjusted R = 0.55, P = 0.053 for GIR and Rd, respectively). Adjusting for BMI did reduce the correlation coefficients (adjusted R = 0.49, P = 0.092, and adjusted R = 0.47, P = 0.102 for GIR and Rd, respectively) to the point at which these were no longer statistically significant, although the correlation coefficients themselves were only slightly reduced.
Discussion
Using a study design that allowed us to isolate possible effects of insulin on bone metabolism independent of changes in glucose, GH, or glucagon, we demonstrate that changes in insulin levels that span the physiological range (pre- or postprandially) fail to alter levels of bone formation or bone resorption markers or circulating OPG levels. In addition, plasma undercarboxylated osteocalcin levels were unaffected by the changes in insulin levels. These data thus indicate that in humans the variations in insulin levels that occur over the course of the day do not impact bone metabolism. We acknowledge that although isolated changes in insulin levels (i.e. in the absence of changes in glucose levels or in other hormones) would not occur physiologically, our goal was to test for any possible direct effects of insulin, independent of these other biochemical or hormonal changes, on bone metabolism.
Our findings are consistent with a previous study by Clowes et al. (7), who used a pharmacologic hyperinsulinemic clamp and found that, at least over 2 h, a euglycemic, hyperinsulinemic clamp failed to alter serum levels of CTX, PINP, or osteocalcin. Our study used a somewhat different design, achieving insulin levels spanning the physiological range (rather than pharmacological insulin levels), and maintaining three different insulin levels for at least 2 h each (3 h for the low dose and 2 h each for the intermediate and high doses). In addition, we also measured circulating levels of undercarboxylated osteocalcin and OPG at each insulin infusion dose, thereby providing the most comprehensive test of the hypotheses generated from the mouse studies.
Interestingly, Clowes et al. (7) did find, using a hypoglycemic, hyperinsulinemic clamp, that hypoglycemia itself resulted in a reduction in serum CTX, PINP, and osteocalcin levels, with these changes becoming evident between 75 and 120 min after the induction of hypoglycemia. In a subsequent study (8), these investigators found that an oral glucose load also reduced all three bone turnover markers, although these reductions occurred much sooner (starting within 20 min of the glucose load) than the changes observed after hypoglycemia. Of note, iv infusion of somatostatin abolished the effect of oral glucose on bone turnover markers, suggesting a role for as-yet-unknown somatostatin-inhibitable factor(s) in mediating the effects of glucose on bone turnover. This finding underscores the importance of the somatostatin infusion used in the present study to isolate possible effects of insulin on bone, independent of other hormonal factors.
Although the skeleton is the major source for the bone turnover markers we assessed (PINP, osteocalcin, CTX), there are multiple sources of circulating OPG, including the vasculature (9). Thus, we recognize that there may well have been changes in local OPG production in the bone microenvironment that were not reflected in peripheral blood OPG measurements. However, mitigating this concern is the fact that we also failed to observe any changes in peripheral blood CTX levels, which should have been responsive to changes in OPG production within bone.
Whereas our data indicate that, in humans, physiological variations in insulin levels do not impact bone turnover over the time course of hours, they do not exclude the possibility that insulin may regulate bone metabolism over a longer time frame. Along these lines, we did find that measures of insulin sensitivity (GIR and Rd) were positively correlated with CTX levels and tended to be inversely associated with OPG levels and positively with PINP and undercarboxylated osteocalcin levels. These data are consistent with the findings from the mouse studies and suggest that, as predicted by the mouse models (3, 4), increased insulin action (i.e. sensitivity) is associated with increased bone resorption and perhaps with decreased OPG and increased PINP and undercarboxylated osteocalcin levels. In agreement with our findings, Yaturu et al. (10) previously found that serum OPG levels were elevated in patients with type 2 diabetes and correlated positively with a measure of insulin resistance, the homeostatic model assessment insulin resistance index.
Although hyperinsulinemia has generally been associated with increased bone mass and decreased markers of bone resorption (reviewed in Ref. 11), this association may, in fact, be driven by the associated insulin resistance. Thus, in 55 male patients with ischemic heart disease and 40 healthy men, Abrahamsen et al. (12) found that insulin sensitivity (estimated as the Rd divided by the area under the insulin curve during an iv glucose tolerance test) was inversely related to femur and whole-body bone density by dual-energy x-ray absorptiometry. Moreover, data from the Tromso study indicates that nonvertebral fracture risk declines with increasing insulin resistance (13). These findings are consistent with our observation that the measures of insulin sensitivity in our study, GIR and Rd, were positively associated with CTX levels.
We do recognize several limitations of our study. First, we used archived samples that had been stored for a number of years at −20 C. Whereas only previously unthawed samples were used in this study, it is possible that prolonged storage at this temperature may have affected some of the bone turnover markers, although for all the assays in this study, the values obtained were well within the ranges specified by the kit manufacturers, arguing against significant degradation being a problem. Second, although we did note associations between serum CTX levels and measures of insulin sensitivity, the correlation coefficients were attenuated after adjustment for BMI. In addition, some of the other associations we noted between the bone turnover markers and measures of insulin sensitivity failed to reach statistical significance, likely due to our relatively small sample size. Thus, we recognize that studies assessing these relationships in larger cohorts of patients are needed. Third, it is possible that there were very transient changes in one or more of the bone turnover markers that occurred early after changes in the insulin infusion rates that were no longer evident 2 h later, when we performed our measurements. If this did occur, however, the physiological relevance of these very transient changes would be open to question.
These limitations notwithstanding, our findings do indicate that acute changes in insulin levels, as would occur during meals and over the course of a day, do not regulate bone turnover, undercarboxylated osteocalcin, or OPG levels in humans. Nonetheless, the associations we noted between specific measures of insulin sensitivity and CTX levels, in the context of increasing evidence that increased insulin sensitivity is associated with reduced bone mass (12), increased fracture risk (13), and reduced OPG levels (10), do suggest that at least part of the predictions from the mouse models may be relevant to humans. Further studies examining the effects of altering insulin sensitivity bone resorption, OPG production, and bone formation (including undercarboxylated osteocalcin levels) are warranted to better define the possible role of insulin in regulating bone metabolism in humans.
Acknowledgments
This work was supported by National Institutes of Health Grants AG004875, DK29953, and UL1-RR24150 (Center for Translational Science Activities), U.S. Public Health Service.
Disclosure Summary: None of the authors have a conflict to disclose.
Footnotes
- BMI
- Body mass index
- CTX
- C-terminal telopeptide of type I collagen
- CV
- coefficient of variation
- GIR
- glucose infusion rate
- OPG
- osteoprotegerin
- PINP
- amino terminal propeptide of type I collagen
- Rd
- glucose disappearance rate.
References
- 1. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. 2010. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. 2010. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. 2004. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes 53:2042–2050 [DOI] [PubMed] [Google Scholar]
- 6. Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R. 2010. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab 95:3502–3506 [DOI] [PubMed] [Google Scholar]
- 7. Clowes JA, Robinson RT, Heller SR, Eastell R, Blumsohn A. 2002. Acute changes of bone turnover and PTH induced by insulin and glucose: euglycemic and hypoglycemic hyperinsulinemic clamp studies. J Clin Endocrinol Metab 87:3324–3329 [DOI] [PubMed] [Google Scholar]
- 8. Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. 2003. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab 88:4867–4873 [DOI] [PubMed] [Google Scholar]
- 9. Hofbauer LC, Schoppet M. 2004. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292:490–495 [DOI] [PubMed] [Google Scholar]
- 10. Yaturu S, Rains J, Jain SK. 2008. Relationship of elevated osteoprotegerin with insulin resistance, CRP, and TNF-α levels in men with type 2 diabetes. Cytokine 44:168–171 [DOI] [PubMed] [Google Scholar]
- 11. Reid IR. 2010. Fat and bone. Arch Biochem Biophys 503:20–27 [DOI] [PubMed] [Google Scholar]
- 12. Abrahamsen B, Rohold A, Henriksen JE, Beck-Nielsen H. 2000. Correlations between insulin sensitivity and bone mineral density in non-diabetic men. Diabet Med 17:124–129 [DOI] [PubMed] [Google Scholar]
- 13. Ahmed LA, Schirmer H, Berntsen GK, Fonnebø V, Joakimsen RM. 2006. Features of metabolic syndrome and the risk of non-vertebral fractures: the Tromso study. Osteoporos Int 17:426–432 [DOI] [PubMed] [Google Scholar]