Closed-loop insulin delivery in individuals with type 1 diabetes improves nighttime glucose control, but the meal response needs to be improved.
Abstract
Context:
Initial studies of closed-loop proportional integral derivative control in individuals with type 1 diabetes showed good overnight performance, but with breakfast meal being the hardest to control and requiring supplemental carbohydrate to prevent hypoglycemia.
Objective:
The aim of this study was to assess the ability of insulin feedback to improve the breakfast-meal profile.
Design and Setting:
We performed a single center study with closed-loop control over approximately 30 h at an inpatient clinical research facility.
Patients:
Eight adult subjects with previously diagnosed type 1 diabetes participated.
Intervention:
Subjects received closed-loop insulin delivery with supplemental carbohydrate as needed.
Main Outcome Measures:
Outcome measures were plasma insulin concentration, model-predicted plasma insulin concentration, 2-h postprandial and 3- to 4-h glucose rate-of-change following breakfast after 1 d of closed-loop control, and the need for supplemental carbohydrate in response to nadir hypoglycemia.
Results:
Plasma insulin levels during closed loop were well correlated with model predictions (R = 0.86). Fasting glucose after 1 d of closed loop was not different from nighttime target (118 ± 9 vs. 110 mg/dl; P = 0.38). Two-hour postbreakfast glucose was 132 ± 16 mg/dl with stable values 3–4 h after the meal (0.03792 ± 0.0884 mg/dl · min, not different from 0; P = 0.68) and at target (97 ± 6 mg/dl, not different from 90; P = 0.28). Three subjects required supplemental carbohydrates after breakfast on d 2 of closed loop.
Conclusions/Interpretation:
Insulin feedback can be implemented using a model estimate of concentration. Proportional integral derivative control with insulin feedback can achieve a desired breakfast response but still requires supplemental carbohydrate to be delivered in some instances. Studies assessing more optimal control configurations and safeguards need to be conducted.
Advancing closed-loop insulin delivery into a product that can be made widely available to patients with diabetes continues to be a challenge. Medtronic Diabetes has approached the development of such a system by combining sc glucose sensing and sc insulin delivery with an algorithm that emulates the multiphase response of the β-cell to glucose (1). The multiphase response was shown to be well characterized by a classical proportional integral derivative (PID) control model (2, 3), and the combination of external insulin pump, sc sensor, and PID algorithm was described as an external physiological insulin delivery (ePID) system (4). The ePID system produced good overnight control in early clinical studies, but the peak postprandial glucose level after breakfast was higher than desired, and low nadir values after breakfast frequently required supplemental carbohydrates to correct (5). This was widely attributed to an inability to have compensated for the pharmacokinetic (PK) delay associated with sc insulin delivery. In a subsequent study conducted in pediatric subjects, an insulin bolus equal to 25–50% of the normal meal bolus given 15 min in advance of the meal was shown to reduce the postprandial peak (6). In that study (6), glucose concentrations typically returned to target within 4 h, but the levels were often observed to be still falling. This suggested that if the subjects had not consumed lunch at 1200 h, they would have been at risk for hypoglycemia.
Subsequent to the pediatric study (6), the PID algorithm was modified to include feedback of a model-predicted insulin profile (7). Insulin feedback (IFB) allows the PID algorithm to better emulate the β-cell physiology, which is widely believed to reduce insulin secretion as plasma insulin levels increase (8). The theoretical effect of such a mechanism is to make it appear as if the insulin PK profile is faster (see Fig. 46 of Ref. 7). Model simulations showed that the IFB mechanism could have improved the meal response observed in the original adult study [see Refs. 9–11 for model description/identification and Fig. 48 in Ref. 7 for predicted improvement]. This observation motivated the present study in which we assess whether the improvement could be achieved in a clinical study.
Subjects and Methods
Nine subjects previously diagnosed with type 1 diabetes mellitus, managing their diabetes with continuous sc insulin infusion [although not continuous glucose monitoring (CGM) per se], and having glycosylated hemoglobin of less than 9% were recruited [four females, five males; median (range) age, 44 (22–60) yr; body mass index, 24.4 (19.7–28.4) kg/m2; glycosylated hemoglobin, 7.1 (6.5–8.5)%]. After an initial screening visit, subjects were monitored for 3 d using a CGM (Medtronic Guardian-RealTime; Medtronic Minimed, Northridge, CA). During this period, patients were asked to perform blood glucose checks at least six times daily and to maintain a record of carbohydrates consumed. After at least 1 wk, subjects were admitted (∼1800 h) to the City of Hope Medical Center and studied for approximately 30 h using the PID algorithm modified for IFB. Consistent blood draws could not be obtained in the last subject (subject 9, male), and no closed-loop data could be obtained. Open-loop vs. closed-loop comparisons were subsequently performed by excluding this subject's open-loop data. The first four subjects were also studied using a self-tuning version of the algorithm, described as adaptive proportional derivative (7), but the results were deemed unacceptable, and the approach was discontinued pending further refinement. The present manuscript only reports data on eight subjects who completed both the open-loop CGM procedure and the closed-loop PID-with-IFB procedure.
Closed loop was performed as follows. On the day of admission, two sc glucose sensors (identical to those used in the Guardian REAL-Time System; Medtronic MiniMed) were inserted in the abdominal area and connected to radio frequency transmitters communicating with a laptop computer on a 1-min interval. At the time of admission, the patient's insulin pump was replaced with a Medtronic 715 Paradigm Pump capable of receiving radio frequency bolus commands on the same 1-min interval. That night, open-loop pump adjustments were made to normalize blood glucose to between 90 and 120 mg/dl using self-monitored blood glucose values (patient meter) at 2100, 2400, and 0300 h. At approximately 0600 h, an iv catheter was inserted for collection of venous blood samples. Sensors were calibrated at approximately 0630 h using the blood glucose values obtained overnight. Pump basal rates were then set to zero, and the closed-loop insulin delivery initiated.
During closed-loop insulin delivery, blood samples were collected at 10-min intervals from 0600 to 0840 h, then at 20-min intervals until 2200 h, 60-min intervals until 0600 h, 20-min intervals through 0720 h, 10-min intervals until 0840 h, and finally every 20 min until the end of closed-loop control (1200 h on d 2 of closed loop). Samples were centrifuged, and approximately 25–50 μl of plasma was used to assess glucose (YSI, Inc., Yellow Springs, OH). Meals were served at 0700, 1200, and 1800 h, with a 15-g snack given at 2100 h. Subjects were free to choose meal items from the full cafeteria menu. A manual 2-U meal bolus was delivered at the start of each meal. If plasma glucose fell below 50 mg/dl, 15 g of supplemental carbohydrate (juice) was provided, and more frequent blood samples were obtained to assess glucose concentration.
Insulin delivery algorithm
The PID insulin delivery algorithm with IFB can be reduced to the following equations:
 |
In this formulation, “n,” “n − 1,” and “n − 2” denote the most recent time value, the value 1 min back in time, and the value 2 min back in time, respectively. P(n), I(n), and D(n) denote the proportional, integral, and derivative terms of the PID algorithm; SG(n) denotes sensor glucose; and dSGdt(n) denotes the rate of change of SG. A real-time estimate of insulin concentration (Îp(n)); normalized to insulin clearance) was obtained from the closed-loop insulin delivery [ID(n)] profile.
Control parameters were set as follows: gain (KP) was set in proportion to the subject's daily insulin requirement (IDIR; units per kilogram per day; KP = IDIR/135). During the daytime (0600 to 2200 h), the integration time (TI) was set to 450 min and the derivative time (TD) to 90 min; during the nighttime, TI was set to 150 min and TD was set to 60 min. Insulin PK model parameters K1 and K2 were set to 1.966308 and 0.966584, respectively. K0 and γ need only to be set in combination because multiplying K0 by a constant and dividing γ by the same constant has no effect. K0 and γ are provided here as 1 − K1+K2 and 0.5, respectively, with Îp(n) shown throughout the manuscript without normalization to clearance (scaled to have units of μU/ml). Target was set to 90 mg/dl during the day and 110 mg/dl during the night. On initialization, the I-component and predicted initial insulin levels Îp(n - 1) and Îp(n - 2) were set to the subject's overnight basal rate, allowing bumpless transfer between open and closed-loop insulin delivery for subjects at target. A maximum rate for the integral component (IMAX) was set to three times the 0600 h open-loop basal rate when glucose was above 60 mg/dl, and to KP · [target − GRESET] if glucose was below this level (GRESET = 60 mg/dl). ID(n) was converted to a discrete series of 0.1 U insulin boluses by integrating the insulin delivery rate expressed in boluses/minute and applying a 0.1 U threshold.
Sensor calibration
Sensor current (nA) was filtered using a finite impulse response filter with 3 cycles/h cutoff frequency (see Ref. 12 for discussion of cutoff frequency). The rate of change of current [dIdt(n); nA/min] was estimated from the slope of current vs. time over 15 min (12). SG(n) was calculated using a calibration factor (CF; mg/dl per nA) and offset (OS; nA) estimated from a linear regression of plasma glucose and sensor current [SG(n) = CF · (IFiltered(n) − OS)]. The rate of change of SG was obtained from the rate of change of sensor current [dSGdt(n) = CF · dIdt(n)].
Biochemical measurements
Once the blood sample was obtained and centrifuged, plasma glucose concentration was determined with a YSI 2300 STAT Plus Glucose and Lactate Analyzer (YSI, Inc., Yellow Springs, OH). Remaining plasma was stored at −30 C for subsequent measurement of plasma insulin, free fatty acid (FFA), and cortisol. Insulin was measured using an ELISA (Mercodia, Uppsala, Sweden); plasma cortisol was measured using ELISA (IBL International, Hamburg, Germany); and plasma FFA was measured by enzymatic colorimetric method using reagents from Wako Diagnostics (Richmond, VA).
Statistical analysis
Data were analyzed using tools available in GraphPad Prism (GraphPad Software, Inc., La Jolla, CA), Microsoft Excel (Microsoft Corporation, Redmond, WA), and MATLAB with the Statistics Toolbox (The MathWorks, Inc., Natick, MA). The ability of the insulin model to predict plasma insulin kinetics was assessed by correlating the real-time prediction with measured concentrations. Closed-loop control was assessed by measuring peak and nadir glucose concentration during meals. Stability was assessed by monitoring the need for supplemental carbohydrate and by calculating the rate of glucose change in the 3- to 4-h period after breakfast (linear regression slope of SG vs. time). Nighttime open- and closed-loop glucose control was compared using open-loop profiles during the nights when the subjects did not report having consumed meals while under open-loop control (data are presented as mean and variability about the mean). Sensor performance was quantified as mean absolute relative difference between the calibrated sensor and blood glucose measurements. Data are presented as median (range) or mean ± sem unless otherwise noted. The study was approved by the City of Hope Institutional Review Board, and all patients gave written informed consent.
Results
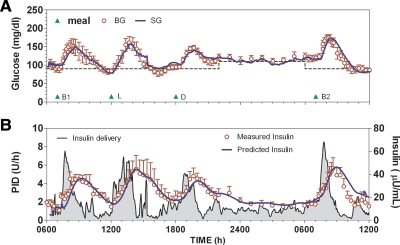
Closed-loop control (Fig. 1) was initiated at approximately 0630 h with a glucose concentration of 106 ± 10 mg/dl. Subjects consumed 44.5 (30–100) g during breakfast on day 1 (B1), followed by 62.5 (37–95) g for lunch (L), 59.5 (35–103) g for dinner (D), and 45 (27–99) g for breakfast on d 2 (B2). Carbohydrate accounted for 30 (26–67), 41 (27–49), 34 (20–48), and 33.5 (21–54)% of calories consumed during meals (B1, L, D, and B2, respectively). Two-hour postprandial glucose level values were 138 ± 24, 158 ± 17, 138 ± 9, and 132 ± 16 mg/dl (B1, L, D, and B2, respectively). No hypoglycemia was observed overnight, and fasting glucose was at nighttime target the following morning (118 ± 9 mg/dl, not different from 110 mg/dl; P = 0.38). Sensors tracked plasma glucose with a mean absolute relative difference of 11.9 (7.3–20.6)%. Calibrations were performed every 4.2 (0.1–23.4) h. Insulin was rapidly delivered during each meal (Fig. 1B, shaded region left axis) with the plasma insulin profile well predicted by the model [median correlation, 0.86 (0.71–0.90)]. Glucose concentration during the 3- to 4-h period after B2 was stable (rate of change of glucose, −0.03792 ± 0.0884 mg/dl · min, not different from zero; P = 0.68) and at daytime target (97 ± 6 mg/dl, not different from 90; P = 0.28). Supplemental carbohydrates were given on eight occasions during the 30-h closed-loop period, with six subjects receiving supplemental carbohydrates on at least one occasion. Three subjects received supplemental carbohydrate during B2.
Fig. 1.
A, Average glucose profile obtained using the ePID system modified to include IFB. B, Insulin delivery during closed loop (left axis) with measured and model-predicted plasma insulin concentration (right axis). Model-predicted and measured insulin are shown in μU/ml (1 μU/ml = 6.00 pmol/liter); glucose concentration is shown in mg/dl (1 mg/dl = 0.055 mmol/liter). BG, Blood glucose; SG, sensor glucose; B1 and B2, breakfast on d 1 and 2, respectively; L, lunch; D, dinner.
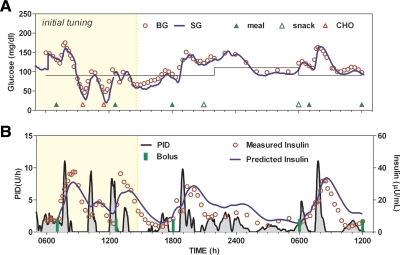
Closed-loop gain (KP) was adjusted in one subject during the study (Fig. 2). Open-loop adjustment of insulin delivery the evening before starting closed-loop control had not achieved the morning target, and closed-loop control was initiated at a value of approximately 150 mg/dl. The initial closed-loop response resulted in a glucose value of less than 50 mg/dl, which was treated with supplemental carbohydrate at 0930 h. Blood glucose increased with the accompanying closed-loop insulin response again generating a need for supplemental carbohydrate at 1130 h. After the second instance, KP was reduced by 20% with no change in any other control parameter. The dinner response that day peaked at approximately 160 mg/dl, and the controller achieved target at approximately 0200 h. No nighttime hypoglycemia was observed, and fasting concentration the following morning was near target. Thereafter, B2 peaked at 163 mg/dl, with a 2-h value of 121 mg/dl and a 3- to 4-h rate of change of 0.0017 ± 0.0535 mg/dl · min.
Fig. 2.
Subject in which closed-loop control was initiated at gain proportional the subject's daily insulin requirement (shaded area) but subsequently decreased (not shaded). A, Glucose. B, Insulin delivery (left axis) and measured and predicted insulin concentration (right axis). BG, Blood glucose; SG, sensor glucose; CHO, supplemental carbohydrate.
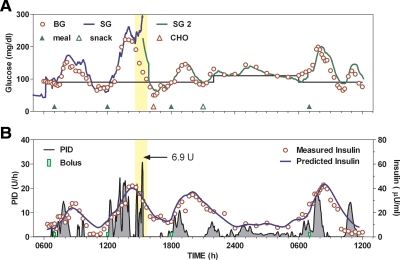
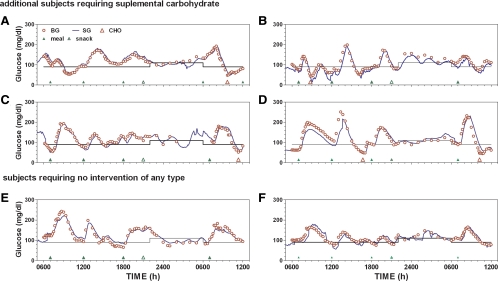
One occurrence of supplemental carbohydrate was deemed to have been related to sensor error. In this instance (Fig. 3, red open triangle), B1 peaked at 145 mg/dl with a nadir value of 65 mg/dl and a slightly increasing 3- to 4-h rate of change (0.0921 mg/dl · min). During lunch, glucose peaked at approximately 200 mg/dl, but approximately 2 h after lunch SG increased unexpectedly, and control was changed to a second sensor (SG2; Fig. 3, green line). The second sensor was recalibrated within the first hour of use. During the transition from sensor 1 to sensor 2, approximately 6.9 U of insulin was delivered (yellow shaded region), and supplemental carbohydrate was required at approximately 1500 h. No hypoglycemia was observed with sensor 2. Five additional instances of hypoglycemia requiring supplemental carbohydrate were observed (Fig. 4, A–D). Of these, two instances occurred after B1, three occurred after B2, and one during L. Two subjects did not require any interventions with closed-loop insulin delivery (Fig. 4, E and F).
Fig. 3.
Subject in which closed-loop control was initiated with one sensor but where the sensor was subsequently replaced (yellow shaded region). A, Glucose; and B, insulin delivery (left axis); measured and predicted insulin concentration (right axis). BG, Blood glucose; SG, sensor glucose; SG 2, replacement sensor; CHO, supplemental carbohydrate.
Fig. 4.
Glucose profiles obtained in four subjects requiring supplemental carbohydrate on at least one occasion (A–D) together with profiles obtained in two subjects with no interventions (E and F). BG, Blood glucose; SG, sensor glucose; CHO, supplemental carbohydrate.
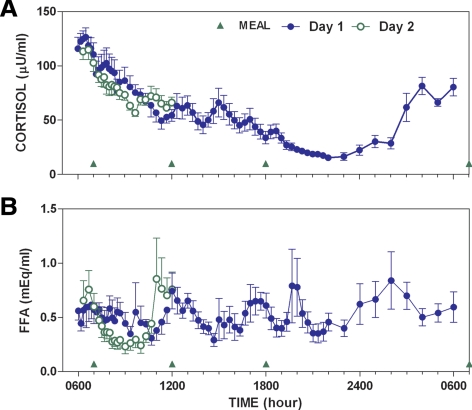
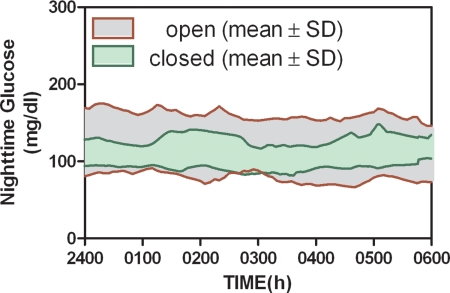
Plasma cortisol showed a marked circadian rhythm with peak values corresponding to approximately 0600 h and a nadir value at approximately 2400 h (Fig. 5A). Cortisol levels were similar for B1 (Fig. 5, blue symbols) and B2 (Fig. 5, green symbols). FFA concentration (Fig. 4B) tended to increase between 1000 and 0300. FFA was also more suppressed during B2 than B1 (compare green open and blue-closed symbols in Fig. 5), with the response on d 2 abruptly increasing at approximately 1100 h, just before lunch. Variability about the mean glucose obtained during the nighttime was reduced with PID (Fig. 6; variability expressed as mean ± sd). This analysis, which only included open-loop profiles during the nights in which the subjects did not report consuming carbohydrates, also showed that lower mean values were obtained with closed-loop control (101 ± 3 vs. 120 ± 6 mg/dl; P < 0.05).
Fig. 5.
Plasma cortisol (A) and FFA levels (B) during closed-loop control.
Fig. 6.
Comparison of open and closed-loop control during the nighttime.
Discussion
The present study assessed the ability of an insulin PK model (13) to predict the plasma insulin profile obtained with sc insulin delivery and whether feedback of the profile would allow acceptable closed-loop meal responses to be obtained in humans. The insulin model predicted measured plasma insulin concentration with a high correlation (Fig. 1B), and feedback of the predicted profile resulted in an average peak meal response of less than 180 mg/dl with a stable target value obtained within 3 h (Fig. 1A). Nighttime results support the hypothesis that better control can be achieved under closed loop than open loop (Fig. 5); however, a better method to establish tuning parameters and protect against sensor error will need to be developed if the need for supplemental carbohydrates is to be eliminated.
It is unclear whether the need for supplemental carbohydrate can be eliminated by changes in the PID algorithm parameters or whether the algorithm needs further modification. A 20% decrease in algorithm gain (KP) improved the performance for one subject (Fig. 2), and it is possible that other subjects in which supplemental carbohydrates were used would have also benefited from similar adjustments. That a 20% decrease in gain results in stable control suggests that the gain may still be too close to the critical value for which the system becomes unstable. Virtually all closed-loop systems go unstable as the overall system gain is increased; however, the overall gain is determined by the product of the controller gain and insulin sensitivity (14). It may be possible to have the PID algorithm automatically adapt KP to changes in SI [adaptive or self-tuning control (7)], but this has not been proven.
Control gain (KP) is only one of the PID parameters needing to be optimized. Additional parameters include TI and TD, which are used to define the relative magnitudes of the integral and derivative components; γ, K1, and K2, which are used to configure the IFB; and IMAX and GRESET, which are used to limit the underlying basal rate and define the glucose value at which the pump is suspended. The IFB parameters K1 and K2 can be obtained by identifying time constants in the insulin PK response (15). Parameters defining the pump shutoff (GRESET = 60 mg/dl in this study) and the maximum basal rate (IMAX = three times the subject's fasting basal rate) are relatively straightforward to set. Few subjects require basal rates to increase by more than a factor of 3 (Steil, G. M., unpublished observation), and most clinicians agree that insulin should not be delivered below 60 mg/dl. Still, deriving optimal values for TI, TD, and γ presents a substantial challenge, analogous to the open-loop problem with setting an insulin sensitivity factor, insulin to carbohydrate ratio, and, for pump therapy, the insulin-on-board half-time.
It may be possible to obtain an optimal control configuration by identifying a metabolic model using readily available open- or closed-loop data (9–11). This will require that changes in model parameters be well characterized. Changes in model parameters, particularly insulin sensitivity, have previously been linked to circadian changes in cortisol (16). In this study, cortisol showed a marked circadian pattern, but there with little variability between B1 and B2 (Fig. 4). FFA levels are thought to affect insulin sensitivity and/or reduce the ability of insulin to suppress endogenous glucose production (17–20). Evidence for a change in model/subject parameters can be inferred from studies showing that high-fat meals elevate glucose levels well after the meal can reasonably be expected to have been absorbed (21, 22). In the present study, FFA levels were markedly different during B1 and B2 (Fig. 4B).
The closed-loop configuration used in this study produced better results than those obtained in previous clinical studies (5, 6). Peak breakfast glucose levels on d 2 were reported as 231 ± 12 mg/dl in Ref. 5, reduced to 204 ± 17 mg/dl with the addition of a premeal insulin bolus in Ref. 6, and to 175 ± 8 mg/dl in this study with the use of IFB. Generally, the model used to predict insulin concentration worked well, producing correlation with the measured values of between 0.71 and 0.90, despite using population-based PK parameters (K1 and K2 in Eq. 1). A similar model-based approach to real-time estimation of insulin concentration has been implemented by El-Khatib et al. (23) [model predictive control has been clinically evaluated by several groups (24–26)]. Generally, the results by El-Khatib et al. (23) were positive; however, the group observed substantially slower kinetics in a subset of patients compared with others. We have previously observed slow kinetics in subjects known to have insulin antibodies (Steil, G. M., N. Kurtz, and G. Voskanyan, unpublished observations), but we have not otherwise observed any substantial mismatch between model estimates and measured values (see results reported in Refs. 5 and 6). Still, further studies will be required to fully quantify the intrasubject variability of the PK profile and to ensure that the PID control algorithm is robust to any mismatch. Finally, the present study emphasizes that the control algorithm will need to be more robust to sensor errors. Generally, glucose sensor signals tend to decrease over time as sensors lose sensitivity, but it is possible for the sensors to fail high, as shown in Fig. 3. The present PID algorithm was not equipped with safeguards to detect this type of error because the study was performed under staff supervision. It is possible, however, that a metabolic model could serve in a similar supervisory role (27).
Although the study highlights aspects of the ePID system needing to be refined, the overall results support the hypothesis that closed-loop control can be achieved with the existing technology. Better nighttime control was already achieved in this study (Fig. 6), although the comparison is not ideal in that the open-loop control was done as an outpatient procedure. Still, it is noteworthy that no closed-loop interventions were performed at night in the present study, and that the open-loop data were prescreened to include profiles where the subjects did not report any meal consumption. Also, the open-loop data reported can be expected to be better than that normally obtained by the subjects because they had the benefit of the CGM (subjects were not blinded to the CGM readings). Also noteworthy is that two of the eight subjects underwent the entire 30-h closed-loop duration with no interventions of any kind (Fig. 4, E and F). Thus, we conclude that the addition of IFB to the previously studied PID closed-loop system results in a substantial improvement over prior iterations, although further research is still needed to optimize control tuning and to develop safety mechanisms to protect against sensor error.
Acknowledgments
The authors thank Mikhail Loutseiko and Kenny Long from Medtronic and the staff at City of Hope Medical Center for their valuable assistance during the study; Raymond Cartaya (Medtronic) for his assistance in writing and editing a first version of this manuscript; as well as the subject participants.
This study was funded in part by National Institutes of Health Grant R01-DK-0064567 (to G.M.S.).
Disclosure Summary: C.C.P., N.K., G.V., and A.R. are employees and shareholders of Medtronic MiniMed. G.M.S. is a former employee of Medtronic MiniMed. S.P. and F.R.K. have nothing to declare.
Footnotes
- B1
- Breakfast on d 1
- B2
- breakfast on d 2
- CF
- calibration factor
- CGM
- continuous glucose monitoring
- D
- dinner
- ePID
- external physiological insulin delivery
- FFA
- free fatty acid(s)
- IFB
- insulin feedback
- L
- lunch
- PID
- proportional integral derivative
- PK
- pharmacokinetic
- SG
- sensor glucose.
References
- 1. Elahi D. 1996. In praise of the hyperglycemic clamp. A method for assessment of B-cell sensitivity and insulin resistance. Diabetes Care 19:278–286 [DOI] [PubMed] [Google Scholar]
- 2. Steil GM, Panteleon AE, Rebrin K. 2004. Closed-loop insulin delivery—the path to physiological glucose control. Adv Drug Deliv Rev 56:125–144 [DOI] [PubMed] [Google Scholar]
- 3. Steil GM, Rebrin K, Janowski R, Darwin C, Saad MF. 2003. Modeling β-cell insulin secretion—implications for closed-loop glucose homeostasis. Diabetes Technol Ther 5:953–964 [DOI] [PubMed] [Google Scholar]
- 4. Steil GM, Rebrin K. September 2004. Medtronic MiniMed Inc., ed. Closed-loop method for controlled insulin infusion. Patent 5954643
- 5. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. 2006. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 6. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. 2008. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 31:934–939 [DOI] [PubMed] [Google Scholar]
- 7. Kanderian S, Steil GM. July 2007. Medtronic MiniMed Inc., ed. Apparatus and method for controlling insulin infusion with state variable feedback. Patent 7,806,886
- 8. Argoud GM, Schade DS, Eaton RP. 1987. Insulin suppresses its own secretion in vivo. Diabetes 36:959–962 [DOI] [PubMed] [Google Scholar]
- 9. Steil GM, Clark B, Kanderian S, Rebrin K. 2005. Modeling insulin action for development of a closed-loop artificial pancreas. Diabetes Technol Ther 7:94–108 [DOI] [PubMed] [Google Scholar]
- 10. Stocker DN, Kanderian S, Cortina GJ, Nitzan T, Plummer J, Steil GM, Mastrototaro JJ. 2009. Virtual patient software system for educating and treating individuals with diabetes. U.S. Patent application 20060272652
- 11. Kanderian SS, Weinzimer S, Voskanyan G, Steil GM. 2009. Identification of intraday metabolic profiles during closed-loop glucose control in individuals with type 1 diabetes. J Diabetes Sci Technol 3:1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rebrin K, Sheppard NF, Jr, Steil GM. 2010. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol 4:1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sherwin RS, Kramer KJ, Tobin JD, Insel PA, Liljenquist JE, Berman M, Andres R. 1974. A model of the kinetics of insulin in man. J Clin Invest 53:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panteleon AE, Loutseiko M, Steil GM, Rebrin K. 2006. Evaluation of the effect of gain on the meal response of an automated closed-loop insulin delivery system. Diabetes 55:1995–2000 [DOI] [PubMed] [Google Scholar]
- 15. Steil GM, Rebrin K. 2005. Closed loop insulin delivery—what lies between where we are and where we are going? Expert Opin Drug Deliv 2:353–362 [DOI] [PubMed] [Google Scholar]
- 16. Trümper BG, Reschke K, Molling J. 1995. Circadian variation of insulin requirement in insulin dependent diabetes mellitus the relationship between circadian change in insulin demand and diurnal patterns of growth hormone, cortisol and glucagon during euglycemia. Horm Metab Res 27:141–147 [DOI] [PubMed] [Google Scholar]
- 17. Rebrin K, Steil GM, Getty L, Bergman RN. 1995. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 44:1038–1045 [DOI] [PubMed] [Google Scholar]
- 18. Rebrin K, Steil GM, Mittelman SD, Bergman RN. 1996. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest 98:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mittelman SD, Fu YY, Rebrin K, Steil G, Bergman RN. 1997. Indirect effect of insulin to suppress endogenous glucose production is dominant, even with hyperglucagonemia. J Clin Invest 100:3121–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mittelman SD, Bergman RN. 2000. Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab 279:E630–E637 [DOI] [PubMed] [Google Scholar]
- 21. Ahern JA, Gatcomb PM, Held NA, Petit WA, Jr, Tamborlane WV. 1993. Exaggerated hyperglycemia after a pizza meal in well-controlled diabetes. Diabetes Care 16:578–580 [DOI] [PubMed] [Google Scholar]
- 22. Jones SM, Quarry JL, Caldwell-McMillan M, Mauger DT, Gabbay RA. 2005. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther 7:233–240 [DOI] [PubMed] [Google Scholar]
- 23. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. 2010. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2:27ra27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. 2009. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol 3:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Dalla Man C, Place J, Facchinetti A, Guerra S, Magni L, De Nicolao G, Cobelli C, Renard E, Maran A. 2009. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol 3:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. 2010. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 375:743–751 [DOI] [PubMed] [Google Scholar]
- 27. Steil GM, Kanderian S, Cantwell MT, Hoss U. July 2008. Medtronic MiniMed, editor. Model predictive method for controlling and supervising insulin infusion. U.S. Patent application 0183060