Healthy young male carriers of the minor allele of IL1B -1473G/C have exaggerated lipid and inflammatory postprandial responses, which could help unravel these relationships during the postprandial state.
Abstract
Context:
IL1b (IL1B or IL1β), a key modulator of the immune response, exerts its functions mainly via IL6 regulation. Fatty meals cause transient hypertriglyceridemia and are considered to be proinflammatory, but the extent of these responses shows high interindividual susceptibility.
Objective:
We evaluated the influence of a genetic variant located in the promoter region of IL1B (-1473G/C) on fasting and postprandial lipids and IL6.
Design, Setting, and Participants:
A total of 477 people over age 65 yr were genotyped for IL1B -1473G/C, and we evaluated fasting lipids depending on genotype. Then, 88 healthy young men were also genotyped and were fed a saturated fatty acid-rich meal. Serial blood samples were drawn for 11 h after the meal, and lipid fractions and IL6 were assayed.
Main Outcome and Interventions:
Fasting lipids were studied in the aged persons. Fasting and postprandial measurements of lipids and IL6 were performed in the healthy young men.
Results:
In the aged persons, CC subjects (minor allele homozygotes) showed higher triglyceride (P = 0.002) and cholesterol (P = 0.011) levels. Healthy young male carriers of the minor C allele showed higher postprandial triglycerides (P = 0.037), and those carried into large triglyceride-rich lipoproteins (P = 0.004). In addition, they showed higher postprandial IL6 concentrations (P = 0.008).
Conclusions:
Our work shows that inflammatory genes may regulate fasting and postprandial lipids because the carriers of the minor allele of an IL gene variant have altered lipid metabolism. To reinforce these gene-phenotype findings, IL6 (the natural effector of IL1B) was increased in these persons.
In the modern concept of atherosclerosis, inflammation is an essential mechanism both in the origin and the development of the disease, involving both inflammatory cells (mainly mononuclear cells) and cytokines of several origins (1, 2). IL1B is an IL that is promoted in macrophage activation scenarios such as those of inflammatory/infectious states or chronic macrophage activation (3). Furthermore, IL1B has been implicated in atherosclerosis, controlling macrophage internalization to the subendothelial space where the cells transform into foam cells (2).
IL1B exerts its primary proinflammatory effects by stimulating the formation of its main effector, IL6, which drives the inflammation cascade (4). The biological significance of IL1B and IL6 in atherosclerosis is supported by experimental and epidemiological evidence. Both IL1B and IL6 gene transcripts are expressed in human atheroma (5). IL6, the final effector of IL1B, is directly correlated with a greater risk for cardiovascular disease and with a worse prognosis after coronary events (6). Furthermore, inhibiting IL1B reduces heart failure after acute myocardial infarct (7). Moreover, IL1 and IL6 also have an important impact on lipid metabolism. In fact, patients with chronic inflammatory diseases often display proatherogenic lipid profiles (3, 8).
The postprandial state after a fatty meal, especially when the meal is rich in saturated fatty acids, induces a “physiological” inflammatory response that includes IL6 (9, 10). Furthermore, it has been shown that pronounced postprandial hypertriglyceridemia may play a pivotal role in the control of atherogenesis (11). This postprandial state is subjected to external (mainly dietary) and internal regulation. Furthermore, genetic modulation has been identified as one of the key contributors to the extent of the postprandial lipemia (11, 12).
Some IL1B gene variants have been associated with increased cardiovascular risk (13), but to date there is no solid evidence that satisfactorily explains the underlying mechanisms. In this study, we explored the effects of a common variant of IL1B in the postprandial state after a saturated, rich fatty meal—a situation that promotes IL metabolism.
Subjects and Methods
Subjects and study design
Elderly cohort
Data for the present study come from preinterventional samples from 461 participants of a vitamin E intervention trial from Boston, Massachusetts (14). Briefly, the participants, mostly White/non-Hispanics (95%), were recruited from long-term care facilities, had life expectancy greater than 6 months, presented no neoplastic diseases at recruitment, and signed an informed consent.
Healthy young men cohort
The cohort consisted of 88 healthy men aged 18 to 33 yr from Cordoba, Spain, who signed an informed consent. A high-fat meal test based on saturated fatty acids was performed and was followed by 11 h of postmeal sample collection, as published elsewhere (15). IL6 plasma measurements were done at fasting and at 4 h. Sample size was calculated based on data obtained from a previous study (16): mean difference expected = 1.5, sd = 0.9, power = 80%, P ≤ 0.05, requiring at least six persons in each genotype group.
Each study was approved by the Ethics Committee of the centers in which the study was carried out. Detailed information about the methodology of both studies has been published elsewhere (14, 15).
Biochemical measures
DNA amplification and genotyping
TaqMan assays, allele discrimination of PCR products, and collection of fluorescence data by the 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) were performed as previously described (15). Hardy-Weinberg equilibrium was tested by Fisher's exact test.
Lipoprotein separation and biochemical analysis
Large and small triglyceride (TG)-rich lipoproteins (TRL) were manually extracted after centrifugation in subdued light, and samples were stored at −70 C until analyzed. Cholesterol (CHOL), TG, apolipoprotein A1, apolipoprotein B, high-density lipoprotein-CHOL, and low-density lipoprotein-CHOL were measured in the hospital laboratory with standard methods and were performed as published (15, 16). Plasma IL6 concentration was determined by an ELISA kit (Quantikine IL6 ELISA kit; R&D Systems, Inc., Minneapolis, MN).
Statistical analysis
For the elderly cohort, association of the single nucleotide polymorphism (SNP) with TG concentration was made by univariate ANOVA, with age, sex, body mass index, and presence of diabetes mellitus as covariates. We excluded from the analysis those subjects that had their TG levels above the mean plus 3 sd values. Statistical methods employed for the analysis of the cohort of healthy men are comparable to those that we have published previously with regard to the gene-postprandial state interaction (15) and recently revised (12), using one-way ANOVA for area under the curve and repeated-measures ANOVA. We included the SNP as an independent factor and body mass index and age as covariates. A P value of less than 0.05 was considered significant. Data presented are mean ± se. We used SPSS 18.0 for Windows (SPSS Inc., Chicago, IL).
Results
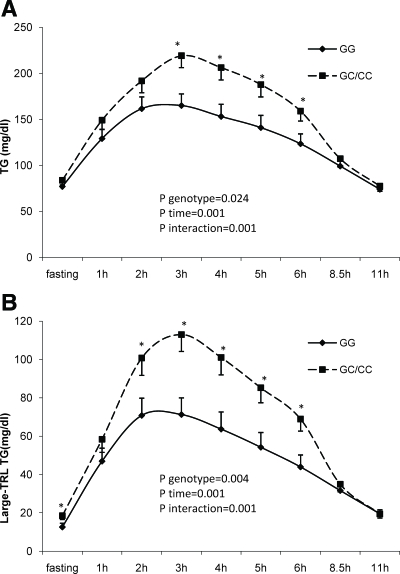
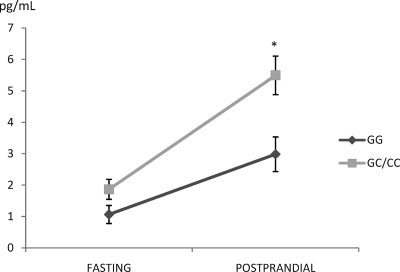
There was no departure from Hardy-Weinberg equilibrium (P > 0.40 in both samples). In the elderly cohort, we found a greater plasma concentration of TG and CHOL in homozygotes for the minor allele vs. the other two genotypes (for TG—CC 180.8 ± 12.1, CG 142.2 ± 4.9, and GG 140 ± 4.0 mg/dl, P = 0.002; for CHOL—CC 218.9 ± 7.9, CG 193.1 ± 3.3, and GG 197.1 ± 2.6 mg/dl, P = 0.011). In the healthy men cohort (baseline characteristics are shown in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), we found differences in the postprandial measurements (summarized in Figs. 1 and 2 and Supplemental Table 2). Carriers of the minor C allele of -1473G/C showed a higher area under the curve of total TG (P = 0.037) and large-TRL TG (P = 0.004) compared with homozygotes for the major G allele (Supplemental Table 2). In the repeated-measures ANOVA, differences were evident in the third to sixth hours for total TG and at h 0, 2, 3, 4, 5, and 6 for the large-TRL TG (Fig. 1). We found no differences in the other lipid parameters. IL6 values were dependent on the -1476G/C genotype (P = 0.005), with higher values in the GC/CC group. These differences were primarily due to the postprandial measurements (GC/CC 5.62 ± 0.62 vs. GG 2.96 ± 0.31 pg/ml; P = 0.008), and a similar trend was found after the overnight fasting (GC/CC 1.76 ± 0.41 vs. GG 0.99 ± 0.29 pg/ml; P = 0.083) (Fig. 2).
Fig. 1.
Evolution of TG (A) and large-TRL TG concentrations (B) depending on -1473G/C (rs1143623) genotype. Data are expressed as means ± se. *, P < 0.05.
Fig. 2.
Fasting and postprandial measures of IL6 depending on IL1B genotype. Data are expressed as means ± se. *, P < 0.05 GG vs. GC/CC.
Discussion
Our results show that healthy young male carriers of the minor C allele of IL1B SNP -1473G/C (rs1143623) have higher postprandial lipemia than homozygotes for the major allele. We also show how this IL1B variant affects inflammation by influencing IL6 concentrations postprandially. The postprandial state, especially after a meal rich in saturated fatty acids, induces inflammation primarily by monocyte/macrophage activation (9, 10, 16) and increased production of other proinflammatory, procoagulant species (1–3). An enlargement of postprandial inflammation, as proposed by the augmented increase in plasma IL6 seen in carriers of the -1473G/C minor allele, would theoretically confer a physiological disadvantage to these individuals by promoting atherosclerosis development via increased inflammation. Furthermore, carriers of the minor allele also exhibit an extended postprandial hypertriglyceridemia, which is also associated with accelerated atherosclerosis development. All of these results may be due to a combination of partial impairment of lipoprotein lipase to hydrolyze the TG contained in the TRL by either increased TRL particle formation or lower clearance, as previously proposed, and by an increase in adipocyte lipolysis and altered very low-density lipoprotein metabolism, which also has been reported for situations with increased IL6 (3, 17). Supporting our findings, we have reported previously other harmful effects of this polymorphism. Homozygotes for the minor allele had a higher risk for high blood pressure (P < 0.05) and a trend toward higher abdominal obesity and metabolic syndrome (P = 0.078) (17). Apart from the postprandial findings we report here, this SNP likely affects fasting lipids in general elderly populations (age, >65 yr) by provoking higher fasting CHOL and TG concentrations. To merge our two main findings, we hypothesize that young, normolipemic men only show the effects of the -1473 SNP in the postprandial state due to their “healthier, more resilient” background and younger age.
The experimental design does not allow us to identify the precise molecular mechanisms underlying our findings. However, previous knowledge demonstrates that the G allele of IL1B -1473G/C had decreased binding to nuclear extract of human monocytes (18) based on EMSA, suggesting weaker promoter activity (or increased activity for the C allele), which may shift gene transcription of C carriers in response to a proinflammatory stimulus, such as the postprandial state. Further computational analysis proposed that this SNP is functional with allele-specific participation of GATA-family transcription factors in regulating IL1B expression (18, 19). The increased transcriptional rate of IL1B may confer to this population additional biological disadvantages beyond lipid metabolism. In this sense, it has been reported recently that an artificial IL1B blockade with an artificial antagonist (anakinra) has favorable effects on several inflammatory diseases, such as gout, but also type II diabetes mellitus metabolic control, or, even, in multiple myeloma (3, 20).
In conclusion, we observed that carriers of the minor allele of a common SNP in IL1B (-1473G/C, rs1143623) have an exaggerated postprandial lipemia accompanied by an increased postprandial IL6 production, and that elderly homozygotes for the rare allele have increased levels of fasting TG. The combination of increased TG and IL6 levels allows us to hypothesize that these patients may have a higher inflammatory status and may overrespond to the proinflammatory stimulus that represents a fatty meal.
Acknowledgments
CIBER Fisiopatologia de la Obesidad y Nutricion is an initiative of Instituto de Salud Carlos III, government of Spain.
This work was supported by Proyectos de Investigación, Junta de Andalucia (PI-0252/2009, to J.D.-L.); Consejeria de Innovación, Proyectos de Investigación de Excelencia Junta de Andalucia (AGR 05/00922, to F.P.-J.; and P06-CTS-01425, to J.L.-M.); Ministerio de Educación y Ciencia (AGL-2006-01979/ALI, to J.L.-M.); National Institutes of Health (NIH) Grants R01 DK075030 and R01 HL054776 and U.S. Department of Agriculture (USDA) Grant 58-1950-9-001 (to J.M.O.); NIH Grant 5R01-AG013975 and USDA Grant 58-1950-7-707 (to S.N.M.); a grant for the preparation of study capsules from Hoffmann-La Roche Inc.; and a DSM Nutritional Products, Inc. scholarship. The study was also supported by Hebrew Rehabilitation Center for Aged/Harvard Research Nursing Home Grant PO1 AG004390.
Disclosure Summary: None of the authors declare any conflicts of interest regarding the present document.
For editorial see page 1279
- CHOL
- Cholesterol
- SNP
- single nucleotide polymorphism
- TG
- triglyceride(s)
- TRL
- TG-rich lipoprotein(s).
References
- 1. Libby P, Okamoto Y, Rocha VZ, Folco E. 2010. Inflammation in atherosclerosis: transition from theory to practice. Circ J 74:213–220 [DOI] [PubMed] [Google Scholar]
- 2. Woollard KJ, Geissmann F. 2010. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 7:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinarello CA. 2010. IL-1: discoveries, controversies and future directions. Eur J Immunol 40:599–606 [DOI] [PubMed] [Google Scholar]
- 4. Woods A, Brull DJ, Humphries SE, Montgomery HE. 2000. Genetics of inflammation and risk of coronary artery disease: the central role of interleukin-6. Eur Heart J 21:1574–1583 [DOI] [PubMed] [Google Scholar]
- 5. Rus HG, Vlaicu R, Niculescu F. 1996. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 127:263–271 [DOI] [PubMed] [Google Scholar]
- 6. Blake GJ, Ridker PM. 2002. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med 252:283–294 [DOI] [PubMed] [Google Scholar]
- 7. Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. 2010. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 105:1371.e1–1377.e1 [DOI] [PubMed] [Google Scholar]
- 8. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45:1169–1196 [DOI] [PubMed] [Google Scholar]
- 9. Margioris AN. 2009. Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care 12:129–137 [DOI] [PubMed] [Google Scholar]
- 10. Fuentes F, López-Miranda J, Pérez-Martínez P, Jiménez Y, Marín C, Gómez P, Fernández JM, Caballero J, Delgado-Lista J, Pérez-Jiménez F. 2008. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with α-linolenic acid on postprandial endothelial function in healthy men. Br J Nutr 100:159–165 [DOI] [PubMed] [Google Scholar]
- 11. López-Miranda J, Pérez-Martínez P, Marín C, Moreno JA, Gómez P, Pérez-Jiménez F. 2006. Postprandial lipoprotein metabolism, genes and risk of cardiovascular disease. Curr Opin Lipidol 17:132–138 [DOI] [PubMed] [Google Scholar]
- 12. Perez-Martinez P, Delgado-Lista J, Perez-Jimenez F, Lopez-Miranda J. 2010. Update on genetics of postprandial lipemia. Atheroscler Suppl 11:39–43 [DOI] [PubMed] [Google Scholar]
- 13. Iacoviello L, Di Castelnuovo A, Gattone M, Pezzini A, Assanelli D, Lorenzet R, Del Zotto E, Colombo M, Napoleone E, Amore C, D'Orazio A, Padovani A, de Gaetano G, Giannuzzi P, Donati MB. 2005. Polymorphisms of the interleukin-1β gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler Thromb Vasc Biol 25:222–227 [DOI] [PubMed] [Google Scholar]
- 14. Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. 2004. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 292:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delgado-Lista J, Perez-Martinez P, Perez-Jimenez F, Garcia-Rios A, Fuentes F, Marin C, Gómez-Luna P, Camargo A, Parnell LD, Ordovas JM, Lopez-Miranda J. 2010. ABCA1 gene variants regulate postprandial lipid metabolism in healthy men. Arterioscler Thromb Vasc Biol 30:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiménez-Gómez Y, López-Miranda J, Blanco-Colio LM, Marín C, Pérez-Martínez P, Ruano J, Paniagua JA, Rodríguez F, Egido J, Pérez-Jiménez F. 2009. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis 204:e70–e76 [DOI] [PubMed] [Google Scholar]
- 17. Shen J, Arnett DK, Peacock JM, Parnell LD, Kraja A, Hixson JE, Tsai MY, Lai CQ, Kabagambe EK, Straka RJ, Ordovas JM. 2007. Interleukin1β genetic polymorphisms interact with polyunsaturated fatty acids to modulate risk of the metabolic syndrome. J Nutr 137:1846–1851 [DOI] [PubMed] [Google Scholar]
- 18. Lee KA, Ki CS, Kim HJ, Sohn KM, Kim JW, Kang WK, Rhee JC, Song SY, Sohn TS. 2004. Novel interleukin 1β polymorphism increased the risk of gastric cancer in a Korean population. J Gastroenterol 39:429–433 [DOI] [PubMed] [Google Scholar]
- 19. Inagaki T, Tachibana M, Magoori K, Kudo H, Tanaka T, Okamura M, Naito M, Kodama T, Shinkai Y, Sakai J. 2009. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes Cells 14:991–1001 [DOI] [PubMed] [Google Scholar]
- 20. Dinarello CA, Donath MY, Mandrup-Poulsen T. 2010. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17:314–321 [DOI] [PubMed] [Google Scholar]