Controlled studies demonstrate the absence of endogenous circadian rhythms of LH and FSH in early follicular phase women.
Abstract
Context:
Diurnal rhythms of LH and FSH have been reported in normal women, but it is unclear whether these reflect underlying circadian control from the suprachiasmatic nucleus and/or external influences.
Objective:
The aim of this study was to determine whether endogenous circadian rhythms of LH, FSH, and the glycoprotein free α-subunit (FAS) are present in reproductive-aged women.
Design and Setting:
Subjects were studied in the early follicular phase using a constant routine protocol in a Clinical Research Center at an academic medical center.
Subjects:
Subjects were healthy, normal-cycling women aged 23–29 yr (n = 11).
Main Outcome Measures:
Temperature data were collected, and blood samples were assayed for LH, FSH, FAS, and TSH.
Results:
Core body temperature and TSH were best fit by a sinusoid model, indicating that known circadian rhythms were present in this population. However, the patterns of FSH, LH, and FAS over 24 h were best fit by a linear model. Furthermore, there were no differences in LH and FAS interpulse intervals or pulse amplitudes between evening, night, and morning.
Conclusions:
Under conditions that control for sleep/wake, light/dark, activity, position, and nutritional cues, there is no circadian rhythm of LH, FSH, or FAS in women during the early follicular phase despite the presence of endogenous rhythms of TSH and core body temperature. These studies indicate that endogenous circadian control does not contribute to previously reported diurnal rhythms in reproductive-aged women and emphasizes the importance of environmental cues in controlling normal reproductive function.
Changes in the patterning of gonadotropin secretion provide powerful insights into physiological control mechanisms. Pulsatile gonadotropin secretion is widely used to reflect the underlying characteristics of pulsatile GnRH secretion. Diurnal patterns of hormone secretion provide further insights into the neuroendocrine control of reproductive hormones. Diurnal patterns are defined as changes in serum levels that occur over a 24-h period and reflect the combined influences of underlying circadian rhythms initiated in the suprachiasmatic nucleus and environmental signals. Endogenous self-sustaining circadian rhythms that persist in the absence of environmental stimuli are thought to represent a primary mechanism through which the body coordinates the timing of its activities with the outside environment. Light is a powerful synchronizer of circadian rhythms with the external environment. External factors such as the timing and quantity of nutritional intake, sleep, and activity may further modify biological patterns across a 24-h period (1, 2). Endogenous circadian rhythms have been clearly shown for cortisol, melatonin, and TSH as well as core body temperature, the classic marker of circadian rhythms across species (3, 4). Although there are known diurnal changes in reproductive hormones in a variety of physiological settings, the relative contributions of endogenous circadian control and/or exogenous modifiers have yet to be determined.
Control of reproductive function in rodents appears to have a strong circadian component. A neuronal signal confines the gonadotropin surge to the late afternoon of proestrus (5, 6), and importantly, ablation of the suprachiasmatic nucleus disrupts the estrous cycle (7, 8). Twenty-four-hour rhythms of gonadotropins have been reported in both men (9) and women (10–14). However, sleep is known to have profound effects on gonadotropin secretion during puberty and in specific phases of the menstrual cycle (15, 16). This was clearly demonstrated in our previous sleep-reversal studies in women in the early follicular phase (15). These studies also suggested the possibility of an additional circadian effect, but conditions were not controlled to address this question.
Application of a constant routine protocol of sleep/wake, light, temperature, position, and nutritional cues, which has been widely used to investigate circadian rhythms (17), failed to demonstrate changes in LH or FSH across a 24-h period in postmenopausal women despite the preservation of normal circadian changes in temperature, cortisol, and TSH (18). These studies suggest the absence of underlying circadian control of GnRH and/or pituitary responsiveness. However, aging is known to significantly dampen circadian rhythms of temperature, TSH, and cortisol (19–21), and thus we cannot exclude the possibility of an underlying circadian rhythm of the reproductive axis in younger women.
The current study was, therefore, designed to determine whether endogenous circadian rhythms of LH and FSH are present in reproductive-aged women. Increases in menstrual cycle dysfunction, infertility, and spontaneous abortion have been documented in women who work rotating shifts (22–24). Thus, the information derived from an understanding of the factors that influence the central control of reproductive function is essential for determining optimal management strategies for the significant number of women with reproductive abnormalities in the setting of shift work or transmeridian travel.
Subjects and Methods
Subjects
Eleven women aged 23 to 33 yr with a body mass index of 30 kg/m2 or less were recruited for participation in this study. All subjects were nonsmokers in good health, as determined by physical exam, complete Beck's depression questionnaire, normal complete blood count, and urine toxicology screen. Patients had normal TSH levels and no history of thyroid disease. All women reported having normal menstrual cycles between 25 and 35 d in length and denied transmeridian travel within the previous 3 months. The subjects were not on any medications during the study, had not taken any gonadal steroids within the previous 6 months, and had no history of sleep disorders. The study was approved by the Institutional Review Board at the Massachusetts General Hospital, and a written informed consent was obtained from each subject before participation.
Experimental protocol
Subjects maintained a regular sleep-wake schedule for at least 4 wk and abstained from alcohol and caffeine for at least 7 d before their inpatient visit. Ovulatory cycles were confirmed for at least two cycles by urine ovulation tests, basal body temperature recordings, or luteal phase progesterone levels before the inpatient visit. During this time, a nutritional assessment was performed by nutritionists in the General Clinical Research Center (CRC) at the Massachusetts General Hospital. Resting energy expenditure was determined by indirect calorimetry (EMS/50; Life Energy Systems, Murray, UT) (25) for determination of basal energy expenditure using the Harris-Benedict equation.
Between d 1 and 7 after the onset of menses, each subject was studied during a 48-h inpatient admission at the CRC. Subjects began their inpatient visit at 1800 h and received a meal consisting of 40% of the calories to maintain body weight with no caffeine. Subjects then fasted until the start of their constant routine the following morning. Subjects went to bed at their usual sleep time. Nurses checked periodically during the night to confirm sleep. Subjects were awakened 8 h later and had a peripheral iv catheter placed within 1 h of wake time. The subjects then began a 32-h constant routine during which conditions of constant room lighting (50–100 lux), activity (bed rest), posture (recumbent at 45° angle), wakefulness, and nutritional intake were maintained (17). Subjects were instructed to remain awake during the entire 32-h period, and wakefulness was monitored by wrist activity monitors (Mini-Logger 2000; Mini Mitter Co., Bend, OR) and hired sitters. Isocaloric feeding was customized using the patient's basal energy expenditure to provide calories as 50% carbohydrate, 35% fat, and 15% protein and provided parenterally (n = 4) or in the form of hourly snacks (n = 7). Core body temperature, an established marker of circadian rhythms (3, 4), was recorded once per minute for the entire 32-h constant routine using an indwelling rectal probe.
During the first 5 h of the constant routine, the subjects received saline flushes every 30 min to acclimatize them to the frequent blood sampling. After the acclimatization period, frequent samples were drawn via the iv catheter at 10-min intervals for 27 h using a blood sparing technique previously described (26). Blood samples were assayed for LH, FSH, free α-subunit (FAS), and estradiol as markers of hypothalamic-pituitary-ovarian activity. TSH was assayed as a circadian marker and because FAS is secreted from both the gonadotrope and thyrotrope under the control of GnRH and TRH, respectively.
Assays
Serum LH, FSH, and estradiol were measured as previously described (27, 28). LH and FSH levels are expressed in international units per liter as equivalents of the Second International Pituitary Standard 80/552 for LH and the Second International Pituitary Reference preparation 78/549 for FSH. The intraassay coefficients of variation (CVs) for LH and FAS were 5.18 and 4.32%, respectively, with assay sensitivities of 0.3 and 0.7 IU/liter for LH and FSH, respectively. The interassay CV for the estradiol assay was 9.2%. FAS, measured by RIA as previously described (29), had an interassay CV of 5.0% and an assay sensitivity of 30 pg/ml. Serum TSH was assayed using an automated microparticle enzyme immunoassay (IMX System; Abbott Diagnostics, Chicago, IL) with a sensitivity of 0.02 μg/ml, as previously described (18). For quality controls ranging from 0.25 to 30.5 μIU/ml, the interassay CV of TSH was less than 10% for all three levels.
Data analysis
To objectively determine the presence of a circadian rhythm, the data were analyzed using three SAS-based models: a linear model, a cosinor model, and a model that combines linear and cosinor terms. Akaike Information Criteria was then used to determine which model best fit each data series: AIC = log(mean square error) + #terms*2/(number of data points − 1) (30). The Akaike Information Criteria uses both the fit of a particular model and the complexity of the model to generate a value that can be compared across models. The model with the lowest Akaike Information Criteria value is determined to be the model that best fits the data.
LH and FAS pulses were analyzed using a validated modification of the original Santen and Bardin algorithm (31, 32). Undetectable values were assigned the lowest measurable assay value and missing values (<1% of the total) were ignored. Mean amplitudes and interpulse intervals were calculated for each subject for evening, night, and morning. The amplitude for each pulse was calculated as the difference between the preceding nadir and the peak, whereas the interpulse interval was calculated as the interval between consecutive peaks. For assessment of pulse frequency and amplitude, data were indexed to the habitual time of onset of sleep for each subject and divided into evening (0–8 h), night (8–16 h), and morning (16–24 h). Repeated-measures ANOVA was used to compare pulse amplitudes and interpulse intervals between evening, night, and morning.
Baseline characteristics are expressed as mean ± sd, and all other results are expressed as mean ± sem. P < 0.05 was accepted as significant.
Results
Baseline characteristics
Subjects (n = 11) were 28.4 ± 4.7 yr old (mean ± sd) with a body mass index of 23.8 ± 3.6 kg/m2 (range, 18.6–29.8 kg/m2). The inpatient study was conducted on cycle d 4.1 ± 2.1 in relation to menses and −10.0 ± 2.0 d from ovulation. Of the 11 women that participated, five were Caucasian, three were African-American, two were Asian, and one was Hispanic. The range of bedtimes was 2200–2400 h, with an average of 2300 h.
Circadian study
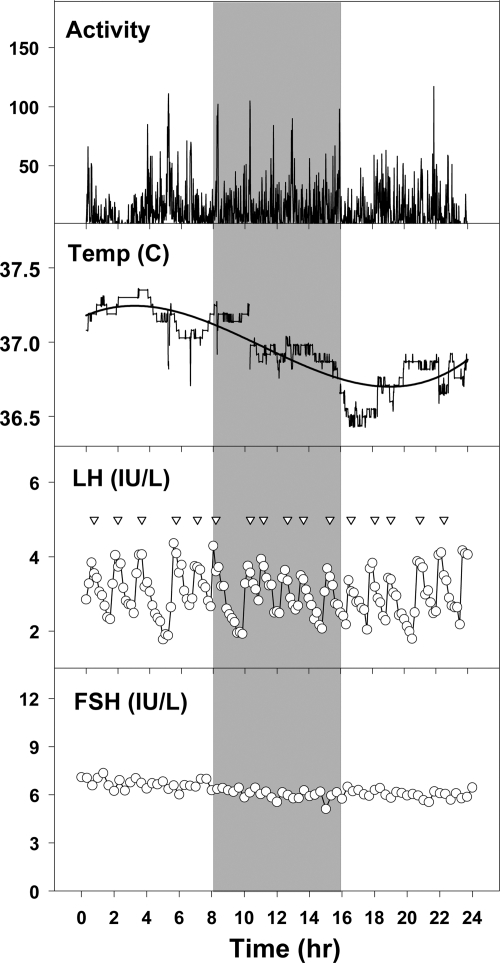
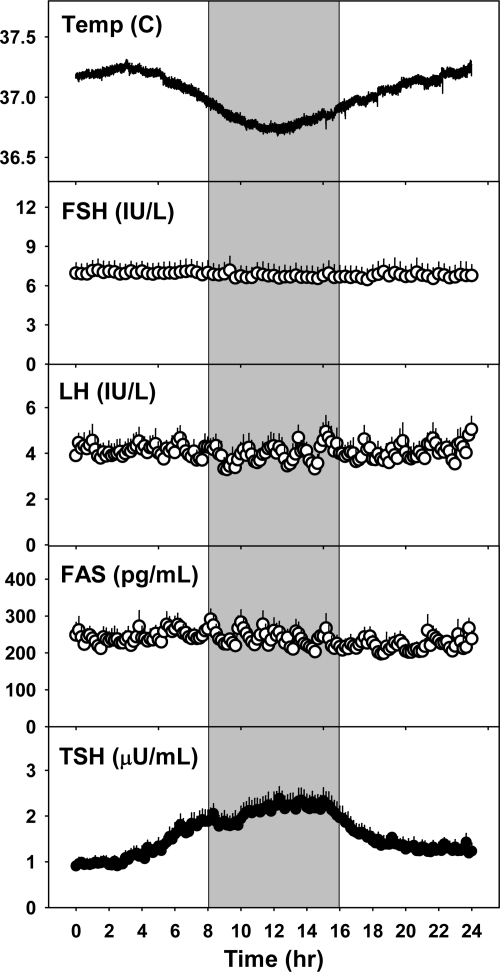
Actigraphy data indicated that all subjects remained awake for the duration of the constant routine protocol, confirming sitter reports (Fig. 1). Circadian rhythms were present for core body temperature and TSH (Fig. 2). The temperature curves for each of the subjects were best fit by a sinusoidal term with a nighttime nadir. The mean amplitude of the rhythm was 0.24 ± 0.02 C. The time of the nadir was 11.7 h (3.7 h after habitual bedtime), which corresponds to approximately 0300 h. TSH rhythms for each subject demonstrated a nighttime rise and were also best fit by a sinusoidal term. The mean amplitude of the TSH rhythm was 0.63 ± 0.1 mIU/liter. The time of the maximum was 12.3 h (4.3 h after habitual bedtime), which corresponds to approximately 0330 h.
Fig. 1.
Activity counts, temperature, LH, and FSH levels over 24 h in a subject studied during a 32-h constant routine. The gray shaded area represents the subject's habitual sleep time, during which she was kept awake. Note the circadian rhythm of temperature, but the lack of evidence of a circadian rhythm of LH or FSH. Inverted triangles mark statistically identified LH pulses.
Fig. 2.
Mean + sem of temperature, FSH, LH, FAS, and TSH levels (n = 11). The gray shaded area represents subjects' habitual sleep time, during which they were kept awake for the constant routine. Temperature and TSH are best fit with a sinusoidal model, whereas FSH, LH, and FAS are best fit with a linear model.
In contrast, FSH and LH were best fit by a linear model with no evidence of a sinusoidal component (Figs. 1 and 2). Consistent with this finding, mean estradiol did not change significantly between evening, night, and morning, with an overall mean of 49.7 ± 3.0 pg/ml (182 ± 11 pmol/liter). Despite the presence of a sinusoidal rhythm for TSH, FAS was also best fit by a linear model alone (Fig. 2), indicating that the 24-h levels of FAS are more reflective of control by GnRH than TRH.
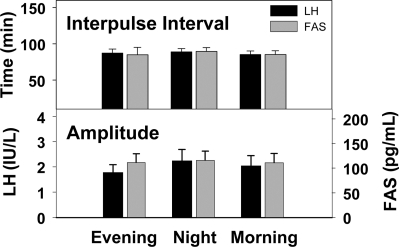
Pulsatile secretion of GnRH was equally represented by LH and FAS in these women studied in the early follicular phase. Interpulse intervals did not vary over the 24-h constant routine, nor was there a difference in pulse amplitudes between evening, night, and morning for LH and FAS (Fig. 3).
Fig. 3.
Interpulse intervals (upper panel) and pulse amplitudes (lower panel) of LH and FAS did not differ between evening, night, and morning during the constant routine studies.
Discussion
Although endogenous circadian rhythms contribute to the 24-h variability in melatonin, cortisol, TSH, and to a lesser extent GH, prolactin, and PTH (3), there has yet to be established a true circadian rhythm of gonadotropins in women or men. Using a study design that controlled environmental cues and prohibited sleep (17), we have now demonstrated the absence of circadian rhythms in LH, FSH, and FAS in young women during the early follicular phase of the menstrual cycle. The presence of prominent circadian rhythms of core body temperature and TSH, both well-established endogenous rhythms (4, 33), validated the protocol and reinforced the absence of endogenous 24-h rhythms of gonadotropins in these subjects.
Diurnal changes in gonadotropin levels have been well documented in puberty and in adult men and women. In puberty, the nocturnal augmentation of gonadotropin levels has been shown to be due to sleep rather than time of day (34, 35). In adult men, a nighttime rise in LH (9) and an early morning rise in testosterone (9, 36) and inhibin B (37) have been noted under normal sleep-wake conditions, but sleep and circadian components have not been dissected. In adult women, diurnal rhythms of LH and FSH have been widely documented during the early follicular phase with conflicting results in other phases of the menstrual cycle (10–14). Unlike the sleep-related stimulation of gonadotropin secretion in puberty, sleep specifically inhibits gonadotropin secretion in early follicular phase women (15, 16). Our previous sleep-reversal study demonstrating the profound inhibitory effect of sleep on pulsatile GnRH/LH secretion also included a 24-h period of wake, during which mean LH was higher in the evening compared with day or night (15). Although this study suggested the possibility of an underlying circadian rhythm, it was not controlled for other environmental influences such as nutritional intake and meal timing. In addition, subjects were not consistently protected from exogenous cues such as changes in light or knowledge of time of day.
We have previously demonstrated the absence of day-night differences in LH, FSH, and FAS, as well as LH and FAS pulse frequency and amplitude under a strict 24-h constant routine protocol in postmenopausal women, although blood drawing constraints did not allow for blood sampling across the full 24-h period (18). Some circadian rhythms are known to dampen with age (19–21), but the present studies indicate that the absence of circadian rhythms of gonadotropins and GnRH is characteristic of the reproductive axis in young as well as older women. The current study focused on women in the early follicular phase for two reasons. The first is that previous diurnal studies demonstrated that 24-h gonadotropin rhythms are most consistently seen in this phase of the cycle. In addition, the relatively low estrogen environment is most comparable to that of postmenopausal women in whom we had previously documented the lack of apparent circadian rhythms of gonadotropins. The current study, which carefully controlled exogenous cues, indicates that endogenous circadian rhythms do not contribute to diurnal gonadotropin secretion in women in the early follicular phase. Taken together, these studies suggest that, in the absence of underlying circadian control, both sleep and other environmental factors contribute to the diurnal variability in gonadotropin levels in women.
There is ample evidence from both animal and human studies that pulsatile LH secretion directly reflects underlying GnRH secretion. There is also evidence that, in euthyroid women, the pulsatile component of FAS secretion is under the direct control of GnRH, whereas baseline levels of FAS are supported by TRH (38). In the current study, we used both LH and FAS as markers of pulsatile GnRH secretion because previous studies have shown that FAS may be a preferable marker in settings of high frequency and/or low estrogen (31, 39). The absence of changes in the amplitude or frequency of pulsatile secretion of both LH and FAS over the 24-h constant routine supports the absence of circadian control of hypothalamic GnRH secretion in early follicular phase women.
The most compelling evidence for the circadian control of reproduction in rodents is the precisely timed proestrous gonadotropin surge, which is dependent on a high estrogen environment (5, 40). Although we cannot exclude the possibility that reproductive circadian rhythms in women might emerge in a different hormonal milieu, most (10, 14, 41), but not all (13, 42), diurnal studies in the high estrogen environment of the late follicular phase do not support this hypothesis. Importantly, whereas Clock mutants have disrupted estrous cyclicity and increased pregnancy loss (43), the effect is relatively subtle (44), suggesting that even in rodents there is considerable redundancy in the control of reproduction, and endogenous circadian controls may be less important than previously thought. It is tempting to speculate that in the absence of circadian control, the reproductive axis in women may be more sensitive to disruption by exogenous influences such as the sleep disturbance associated with shift work or factors such as exercise or nutritional imbalance that can lead to hypothalamic amenorrhea (45).
A potential limitation of this study is that although light exposure remained constant throughout, these studies were conducted at 50–150 lux, which has been shown to suppress, but not abolish, circadian rhythms of melatonin. The intensity of light exposure in the current study did not abolish circadian rhythms of core body temperature or TSH, and thus we do not expect that this would have been the case for the gonadotropins. The characteristics of the TSH rhythm in the current study closely mimic those in previous studies with a similar time of peak and amplitude (33). Although an additional potential limitation of this study is the relatively small number of subjects studied, post hoc analysis indicates that the study was adequately powered to detect a difference in pulse frequency and amplitude of less than 10% and less than 25%, respectively.
In conclusion, the results from these studies indicate the absence of circadian rhythms of gonadotropins and FAS in the early follicular phase, despite the presence of robust circadian rhythms of temperature and TSH. These studies resolve interests from our previous work and are the first to be conducted under a strict 24-h constant routine protocol that permits investigation of underlying circadian rhythms of the reproductive axis in women. These studies further suggest that menstrual cycle disturbances and reproductive dysfunction reported in women working night or rotating shifts (22–24) do not result from alterations of circadian rhythms, but more likely result from the impact of sleep disruption on GnRH pulse frequency and FSH secretion and/or effects of stress or other changes in environmental cues on the reproductive axis.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant R01-AG-13241, M01 RR 01066, and NIH P01-AG009975, NIH K02-HD045459, and NIH R01-HD40291 (to E.B.K.).
This study was initiated before 1997 and was therefore not registered with www.clinicaltrials.gov.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- Coefficients of variation
- FAS
- free α-subunit.
References
- 1. Duffy JF, Czeisler CA. 2009. Effect of light on human circadian physiology. Sleep Med Clin 4:165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mistlberger RE, Skene DJ. 2005. Nonphotic entrainment in humans? J Biol Rhythms 20:339–352 [DOI] [PubMed] [Google Scholar]
- 3. Czeisler CA, Klerman EB. 1999. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res 54:97–130; discussion 130–132 [PubMed] [Google Scholar]
- 4. Hofstra WA, de Weerd AW. 2008. How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav 13:438–444 [DOI] [PubMed] [Google Scholar]
- 5. Legan SJ, Coon GA, Karsch FJ. 1975. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology 96:50–56 [DOI] [PubMed] [Google Scholar]
- 6. Norman RL, Blake CA, Sawyer CH. 1973. Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93:965–970 [DOI] [PubMed] [Google Scholar]
- 7. Stetson MH, Watson-Whitmyre M. 1976. Nucleus suprachiasmaticus: the biological clock in the hamster? Science 191:197–199 [DOI] [PubMed] [Google Scholar]
- 8. Brown-Grant K, Raisman G. 1977. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci 198:279–296 [DOI] [PubMed] [Google Scholar]
- 9. Spratt DI, O'Dea LS, Schoenfeld D, Butler J, Rao PN, Crowley WF., Jr 1988. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol 254:E658–E666 [DOI] [PubMed] [Google Scholar]
- 10. Filicori M, Santoro N, Merriam GR, Crowley WF., Jr 1986. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 62:1136–1144 [DOI] [PubMed] [Google Scholar]
- 11. Hall JE, Schoenfeld DA, Martin KA, Crowley WF., Jr 1992. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab 74:600–607 [DOI] [PubMed] [Google Scholar]
- 12. Mortola JF, Laughlin GA, Yen SS. 1992. A circadian rhythm of serum follicle-stimulating hormone in women. J Clin Endocrinol Metab 75:861–864 [DOI] [PubMed] [Google Scholar]
- 13. Rossmanith WG, Lauritzen C. 1991. The luteinizing hormone pulsatile secretion: diurnal excursions in normally cycling and postmenopausal women. Gynecol Endocrinol 5:249–265 [DOI] [PubMed] [Google Scholar]
- 14. Soules MR, Steiner RA, Cohen NL, Bremner WJ, Clifton DK. 1985. Nocturnal slowing of pulsatile luteinizing hormone secretion in women during the follicular phase of the menstrual cycle. J Clin Endocrinol Metab 61:43–49 [DOI] [PubMed] [Google Scholar]
- 15. Hall JE, Sullivan JP, Richardson GS. 2005. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab 90:2050–2055 [DOI] [PubMed] [Google Scholar]
- 16. Kapen S, Boyar R, Hellman L, Weitzman ED. 1976. The relationship of luteinizing hormone secretion to sleep in women during the early follicular phase: effects of sleep reversal and a prolonged three-hour sleep-wake schedule. J Clin Endocrinol Metab 42:1031–1040 [DOI] [PubMed] [Google Scholar]
- 17. Duffy JF, Dijk DJ. 2002. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 17:4–13 [DOI] [PubMed] [Google Scholar]
- 18. Lavoie HB, Marsh EE, Hall JE. 2006. Absence of apparent circadian rhythms of gonadotropins and free α-subunit in postmenopausal women: evidence for distinct regulation relative to other hormonal rhythms. J Biol Rhythms 21:58–67 [DOI] [PubMed] [Google Scholar]
- 19. van Coevorden A, Mockel J, Laurent E, Kerkhofs M, L'Hermite-Balériaux M, Decoster C, Nève P, Van Cauter E. 1991. Neuroendocrine rhythms and sleep in aging men. Am J Physiol 260:E651–E661 [DOI] [PubMed] [Google Scholar]
- 20. Van Cauter E, Plat L, Leproult R, Copinschi G. 1998. Alterations of circadian rhythmicity and sleep in aging: endocrine consequences. Horm Res 49:147–152 [DOI] [PubMed] [Google Scholar]
- 21. Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sánchez R, Ríos CD, Ronda JM. 1992. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet 340:933–936 [DOI] [PubMed] [Google Scholar]
- 22. Zhu JL, Hjollund NH, Andersen AM, Olsen J. 2004. Shift work, job stress, and late fetal loss: the National Birth Cohort in Denmark. J Occup Environ Med 46:1144–1149 [DOI] [PubMed] [Google Scholar]
- 23. Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. 1996. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. J Occup Environ Med 38:352–358 [DOI] [PubMed] [Google Scholar]
- 24. Labyak S, Lava S, Turek F, Zee P. 2002. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int 23:703–714 [DOI] [PubMed] [Google Scholar]
- 25. Segal KR. 1987. Comparison of indirect calorimetric measurements of resting energy expenditure with a ventilated hood, face mask, and mouthpiece. Am J Clin Nutr 45:1420–1423 [DOI] [PubMed] [Google Scholar]
- 26. Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. 1994. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab 79:858–864 [DOI] [PubMed] [Google Scholar]
- 27. Welt CK, Adams JM, Sluss PM, Hall JE. 1999. Inhibin A and inhibin B responses to gonadotropin withdrawal depends on stage of follicle development. J Clin Endocrinol Metab 84:2163–2169 [DOI] [PubMed] [Google Scholar]
- 28. Welt CK, Falorni A, Taylor AE, Martin KA, Hall JE. 2005. Selective theca cell dysfunction in autoimmune oophoritis results in multifollicular development, decreased estradiol, and elevated inhibin B levels. J Clin Endocrinol Metab 90:3069–3076 [DOI] [PubMed] [Google Scholar]
- 29. Whitcomb RW, O'Dea LS, Finkelstein JS, Heavern DM, Crowley WF., Jr 1990. Utility of free α-subunit as an alternative neuroendocrine marker of gonadotropin-releasing hormone (GnRH) stimulation of the gonadotroph in the human: evidence from normal and GnRH-deficient men. J Clin Endocrinol Metab 70:1654–1661 [DOI] [PubMed] [Google Scholar]
- 30. Priestley MB. 2001. Spectral analysis and time series. London: Academic Press; 367–370 [Google Scholar]
- 31. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. 1999. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab 84:1028–1036 [DOI] [PubMed] [Google Scholar]
- 32. Santen RJ, Bardin CW. 1973. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allan JS, Czeisler CA. 1994. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. J Clin Endocrinol Metab 79:508–512 [DOI] [PubMed] [Google Scholar]
- 34. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. 1972. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287:582–586 [DOI] [PubMed] [Google Scholar]
- 35. Boyar RM, Rosenfeld RS, Kapen S, Finkelstein JW, Roffwarg HP, Weitzman ED, Hellman L. 1974. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest 54:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Resko JA, Eik-nes KB. 1966. Diurnal testosterone levels in peripheral plasma of human male subjects. J Clin Endocrinol Metab 26:573–576 [DOI] [PubMed] [Google Scholar]
- 37. Carlsen E, Olsson C, Petersen JH, Andersson AM, Skakkebaek NE. 1999. Diurnal rhythm in serum levels of inhibin B in normal men: relation to testicular steroids and gonadotropins. J Clin Endocrinol Metab 84:1664–1669 [DOI] [PubMed] [Google Scholar]
- 38. Hall JE, Whitcomb RW, Rivier JE, Vale WW, Crowley WF., Jr 1990. Differential regulation of luteinizing hormone, follicle-stimulating hormone, and free α-subunit secretion from the gonadotrope by gonadotropin-releasing hormone (GnRH): evidence from the use of two GnRH antagonists. J Clin Endocrinol Metab 70:328–335 [DOI] [PubMed] [Google Scholar]
- 39. Hall JE, Lavoie HB, Marsh EE, Martin KA. 2000. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab 85:1794–1800 [DOI] [PubMed] [Google Scholar]
- 40. Christian CA, Mobley JL, Moenter SM. 2005. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoff JD, Quigley ME, Yen SS. 1983. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab 57:792–796 [DOI] [PubMed] [Google Scholar]
- 42. Kerdelhué B, Brown S, Lenoir V, Queenan JT, Jr, Jones GS, Scholler R, Jones HW., Jr 2002. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology 75:158–163 [DOI] [PubMed] [Google Scholar]
- 43. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. 2004. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14:1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kennaway DJ, Boden MJ, Voultsios A. 2004. Reproductive performance in female Clock Delta19 mutant mice. Reprod Fertil Dev 16:801–810 [DOI] [PubMed] [Google Scholar]
- 45. Gordon CM. 2010. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med 363:365–371 [DOI] [PubMed] [Google Scholar]