Abstract
Individuals with autism have an atypical pattern of visual processing. Various studies have provided evidence that individuals with autism perceive the details of stimuli before the gestalt, the reverse of the typical pattern of visual processing. This study used the Rey Osterreith Complex Figure (ROCF) task and an objective scoring system to examine local/global processing approaches to its reproduction in 37 individuals diagnosed with high-functioning autism (HFA) compared to 49 age-, IQ-, and gender-matched typically developing controls (TD). The sample was divided into children (aged 8–14 years) and adolescents/adults (aged 15–47 years) to assess age effects. Results showed no difference in overall performance on the ROCF between HFA and TD children. TD participants displayed improved organizational and planning skills with age and a shift to global processing approaches, but there were no differences in erformance between children and adolescents/adults with HFA. There was no evidence of enhanced local processing in ither HFA group. These findings suggest that HFA individuals with average IQ scores do not have the clinically emonstrable evidence of the enhanced local processing thought to reflect increased local brain connectivity in more severely autistic individuals. The deficient global processing of the HFA adults reflects dependence of performance on impaired strategic problem-solving abilities, which has been demonstrated to result from under development of neural connectivity between visuo-spatial and frontal brain regions in HFA adults.
Keywords: autism, visual processing, visuo-spatial abilities, local processing, global processing, strategic planning, problem solving, neural connectivity
Introduction
Individuals with autism often manifest evidence of a distinctive atypical pattern of visual perception and visual information processing. In Kanner's original treatise, he described this feature more broadly as an “inability to experience wholes without full attention to the constituent parts” [Kanner, 1943]. In support, he described behavioral manifestations ranging from one child's extreme upset over the appearance of a crack in the plaster on the wall of a house passed on his daily walk, the distress of another child over the absence of a doll's hat from among the toys in Kanner's office, another child's incessant recitation of facts from the moment he entered Kanner's office until the moment of his departure, and the remarkable preoccupation of another individual with the inaugural ball gowns worn by each of the presidents' wives. This focus on minutiae of a perceptual or factual nature and the failure to attend to or appreciate the greater importance of the whole object, situation, or concept is currently captured under a number of signs and symptoms in the “Restricted, repetitive and stereotyped patterns of behavior, interests, and activities” category of the diagnostic criteria for Autistic Disorder, Asperger's Disorder, and Pervasive Developmental Disorder Not Otherwise Specified in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR) [APA, 2000].
Early research efforts directed at defining a cognitive basis for this increased awareness of details reported superior performance by children with autism and intellectual disability relative to mental age-matched peers without autism on Block Design subtests from the Wechsler Scales of Intelligence and on hidden figures tests such as the Children's Embedded Figures Test (CEFT) [Happé, 1996; Jolliffe & Baron-Cohen, 1997; Shah & Frith, 1983, 1993]. Superior performance on these tests bythese children with autism relative to matched typically developing (TD) controls was proposed to be the result of an inherent and atypical enhancement of detail-oriented processing, also referred to as a local processing bias [Frith, 1989; Happé, 1997, 1999]. For the CEFT, it is notable that the target shape embedded within the complex figure was shown to the subject and then removed from sight before the complex figure was presented; the subject was then asked to identify the previously shown target shape within the complex figure. The standard CEFT therefore imposed a working memory load in addition to the visual perceptual load.
Over time, more research studies in autism employing the CEFT and adult Embedded Figures Test (EFT) emerged [Brian & Bryson, 1996; Ozonoff, Pennington, & Rogers, 1991; Ropar & Mitchell, 2001; Schlooz et al., 2006]. Studies reporting superior performance on the embedded figures and Block Design tests often involved individuals with and without autism with IQ scores below 70 [Ropar & Mitchell, 2001; Shah & Frith, 1983]. Alternatively, studies reporting no differences in performance on these measures between those with and without autism involved average or superior IQ individuals [Brian & Bryson, 1996; Ozonoff et al., 1991; Schlooz et al., 2006]. It is important to highlight that an analysis of published studies using the preschool, child, and adult versions of the EFT indicates that reliable group differences on this task were evident in speed of response, not accuracy. Indeed, the majority of nonreplications of a local processing bias using the EFT with individuals with autism have been seen in studies measuring performance accuracy rather than reaction time [Burnette et al., 2005; Edgin & Pennington, 2005; Morgan, Maybery, & Durkin, 2003]. Group differences in reaction time vs. accuracy support a processing approach in autism in which individuals attend to the local level of a stimulus prior to the global level. In other words, they do not appear to have an inability to see global or contextual information, but rather a bias or predilection toward perceiving the local features or details [e.g., Plaisted, Swettenham, & Rees, 1999].
The EFT tasks with a time frame in seconds were followed by studies employing the finer grained Navon task with a millisecond time frame, which shifted task demands from a conceptual to a perceptual framework or from conscious or deliberate processes to automatic processes. The Navon task consists of a large letter filled with a quantity of a smaller letter, providing an opportunity for determining the innate predisposition to local or global processing (small letters are considered the local level of analysis, the large letter is considered the global level of analysis) on the subjects' perceptual processing [Navon, 1977].
The studies using the Navon task have, like the experience with the embedded figures tasks, reported inconsistent findings [Plaisted et al., 1999]. Research has explored how much of this variability was related to differences in autism severity, differences in stimulus features that influence perception, and how much was related to bona fide variability within the autism population that might be linked to meaningful intersubject behavioral differences [see Wang, Mottron, Peng, Berthiaume, & Dawson, 2007 for an overview]. While some studies have suggested that individuals with autism show a local processing bias, with fewer errors and faster speed when the target letter is located at the local level [e.g., Rinehart, Bradshaw, Moss, Brereton, & Tonge, 2000; Rinehart, Bradshaw, Moss, Brereton, & Tonge, 2001], others have found evidence of typical global precedence in autism [Ozonoff, Strayer, McMahon, & Filloux, 1994]. This research has also suggested that individuals with autism show a local bias when given a “divided attention” prompt (e.g., “Do you see an A?”), but demonstrate typical global precedence when given a “selective attention” prompt [e.g., “What is the large letter?”; Plaisted et al., 1999]. In other words, individuals with autism may have a bias to attend initially to the local level of the stimulus, but are able to process global information when prompted to attend to the global level. These findings support conclusions drawn from embedded figures testing that the natural local processing bias in autism can be over-ridden with deliberate conscious thought (or prompting/cueing) in some individuals with autism [Happé, 1999].
Other studies of the Navon task have highlighted interference effects in the explanation for the perceptual processing disturbance in autism. For example, a sample of adults with autism showed no differences in global vs. local letter recognition reaction time in a selective attention condition when the letters at the two levels had shared identity (e.g., a large A made of smaller As). In contrast, when the letters at the two levels were inconsistent, the individuals with autism had a local-toglobal interference effect in which they were faster at recognizing the letter in the local condition, and slower in the global condition due to distraction from the local level [Behrmann et al., 2006]. As a whole, the variability in results across studies using hierarchical letter tasks appear to relate to variations in autism severity across samples, child or adult age of samples, and specific task parameters, such as the density of the small elements [Behrmann et al., 2006].
In a third iteration of tasks investigating the local–global issue of altered visual perceptual processing in autism, studies began to focus on the manner in which individuals with autism completed the copy and reproduction from memory of the Rey Osterreith Complex Figure (ROCF) [Osterrieth, 1944]. The ROCF is a complex figure used in neuropsychology to assess visuo-spatial abilities, visual construction, visual planning and organization, and visual memory for complex visual
stimuli with inherent structure. Scoring methods have been developed to objectively analyze the strategies used to reproduce main or overall structural elements and incidental elements, and the approach to its reproduction in an organized or fragmented manner [e.g., Boston Qualitative Scoring System (BQSS); Stern et al., 1994].
Studies have investigated the reproduction of the ROCF in children, adolescents, and adults with autism. However, the studies either used a modified ROCF and found only a trend toward a local processing or piecemeal approach or failed to use an objective scoring method [Jolliffe & Baron-Cohen, 1997; Manjiviona & Prior, 1999; Prior & Hoffman, 1990; Ropar & Mitchell, 2001; Rumsey & Hamburger, 1990].
One study [Schlooz et al., 2006] has used an objective scoring method [Waber & Holmes, 1986] to examine the ROCF and CEFT issue in children with average intellectual ability scores aged 9–13 years old with: Pervasive Developmental Disorder Not Otherwise Specified (PDDNOS) (n512), Tourette Syndrome (n512), and typical development (n512). This study found no differences between groups in the performance of the CEFT, e.g., no local processing advantage for the average ability PDDNOS group compared to TD controls for identifying embedded figures, but did document a fragmented, detail-oriented reproduction of the ROCF with deficiency in global processing by the PDDNOS group. Hence, they confirmed that those adolescents with PDDNOS with average intellectual ability scores had a similar global processing deficit as those with Autistic Disorder with average or above average intellectual ability scores. They proposed that the “perceptual problems that lead to weak central coherence and cause part of the executive dysfunctions might be an integral part of the deficiencies in the cognitive functioning of all children with PDD” [Schlooz et al., 2006].
Studies of performance on the ROCF in the typical population from childhood to adulthood have documented that the performance of typical children is characterized by a detail-oriented approach. The emergence of the adult type approach involving planning and organization occurs between ages 11 and 13 years in typical children, at which time there is a shift to reproduction of structural and main elements first [Akshoomoff & Stiles, 1995a,b]. This age-related shift in approach coincides with the maturation of the frontal lobes that support the emergence of executive abilities in the second decade of life in TD individuals, which has been documented by neurophysiologic studies employing frontally dependent eye movement methods [Luna et al., 2001]. Oculomotor studies of high functioning adolescents and adults with autism have shown that the maturation of this frontal circuitry and emergence of these executive abilities was delayed in developing and failed to achieve adult levels [Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Luna et al., 2002; Minshew, Luna, & Sweeney, 1999].
The focus of this study was to investigate global and local processing approaches to problem solving using objectively scored ROCF designs in individuals with an Autism Diagnostic Interview-Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS) diagnosis of Autistic Disorder confirmed by expert clinical opinion across the age span from childhood to adulthood, compared to age-, IQ-, and gender-matched typical control groups. Based on prior neuropsychologic and fMRI studies and consistent with our altered connectivity model of autism [Just, Cherkassky, Keller, & Minshew, 2004, 2007; Kana, Keller, Cherkassky, Minshew, & Just, 2006; Kana, Keller, Minshew, & Just, 2007; Koshino et al., 2005, 2008; Minshew & Williams, 2007], we predicted that the adults with autism would perform poorly relative to adult controls on reproduction of the ROCF because of under development of frontal systems and related strategic abilities.
In this study we also proposed to correlate performance on the ROCF with performance on the BD subtest from the Wechsler Intelligence Scales as an index of local processing capacity, as was done in the study by Schlooz et al. [2006]. Because this autism subgroup did not have intellectual disability and we postulated that local processing superiority was an expression of enhanced local connectivity that is diminished or lost with the emergence, though under-development, of systems level connectivity, we did not predict superiority of Block Design subtest scores in the autism subgroups relative to the typical subgroups nor did we predict evidence of superior local processing on the ROCF e.g., superior recall of details.
Method
Participants
The participants were divided into two samples, one group of 49 children aged 8–14 years and a second group of 37 adolescents and adults aged 15–47 years. Inclusion criteria for the study included Full Scale and Verbal IQ scores of 77 or above and sufficient cooperation to complete neuropsychological testing. Additionally, participants were required to have Block Design subtest scores that were not lower than 1 SD below their Full Scale IQ score to avoid the confounding influence of poor visuospatial abilities on reproduction of the ROCF. Since low BD scores are uncommon in high-functioning autism (HFA), this did not prove to be an issue in recruitment.
There were no significant differences between the autism and control groups in age, gender (all male), Full Scale IQ score, Verbal IQ score, and socioeconomic status [Hollingshead, 1975]. Sample characteristics are presented in Table I.
Table I. Sample Characteristics.
| Children | Adolescents/adults | |||||||
|---|---|---|---|---|---|---|---|---|
| Autism | Control | Autism | Control | |||||
| Variable | M | SD | M | SD | M | SD | M | SD |
| n | 23 | 26 | 14 | 23 | ||||
| Age | 11.00 | 1.86 | 11.23 | 1.68 | 24.14 | 8.72 | 22.91 | 7.42 |
| Full scale IQ | 103.87 | 12.55 | 106.23 | 8.96 | 96.14 | 15.07 | 99.30 | 7.71 |
| Verbal IQ | 106.83 | 14.74 | 105.81 | 8.26 | 99.86 | 17.38 | 99.35 | 7.30 |
| Performance IQ | 100.30 | 12.83 | 92.86 | 12.45 | 105.77 | 9.80 | 99.87 | 9.10 |
| Socioeconomic Status | 3.05 | 1.43 | 3.43 | 0.95 | 4.08 | 1.56 | 3.83 | 1.10 |
Autism group
The participants with autism (n537) were recruited by the University of Pittsburgh NICHD Collaborative Program of Excellence in Autism (CPEA) Subject Core as consecutive cases meeting the inclusion and exclusion criteria for the study. They were recruited through announcements at local and national parent meetings and autism group newsletters. The diagnosis of autism was established through two structured research diagnostic instruments, the Autism Diagnostic Interview (ADI) [LeCouteur et al., 1989; Lord, Rutter, & LeCouteur, 1994] and the Autism Diagnostic Observation Schedule (ADOS) [Lord et al., 1989, 2000], and confirmed by expert clinical evaluation in accordance with accepted clinical descriptions of high functioning individuals with autism [Filipek et al., 1999; Minshew, 1996]. Subjects who met ADI and ADOS criteria for autism but showed no history of delayed or disordered language development were considered to have Asperger's disorder and were excluded from the study. This decision was made to preserve as much uniformity in the autism group as possible.
Control participants
Medically healthy individuals (n549) were recruited from community volunteers through newspaper announcements in areas with the same socioeconomic status as the families of origin of the participants with autism. All subjects in the control group were selected based on an absence of a history of birth or developmental abnormalities; brain injury; poor school attendance; current or past history of psychiatric or significant neurological disorder; family history of autism, and family history in first degree relatives of developmental cognitive disorder, learning disability, and mood or anxiety disorder.
Procedure
All tests were administered by trained technicians under the supervision of a licensed psychologist. All procedures were prospectively reviewed and approved by the Institutional Review Board (IRB) at the University of Pittsburgh. Participants received the age-appropriate version of the Wechsler Intelligence Scales.
Rey Complex Figure Test
[Osterrieth, 1944]: Participants were presented with a blank sheet of paper displaying the Rey Figure on the top half and blank space on the bottom half (Fig. 1). Subjects were instructed to copy the picture as carefully and accurately as possible, immediately after which they were to reproduce it from memory. After a delay of 20–30 min filled with other standardized activities, the subjects were again asked to reproduce the design from memory.
Figure 1.
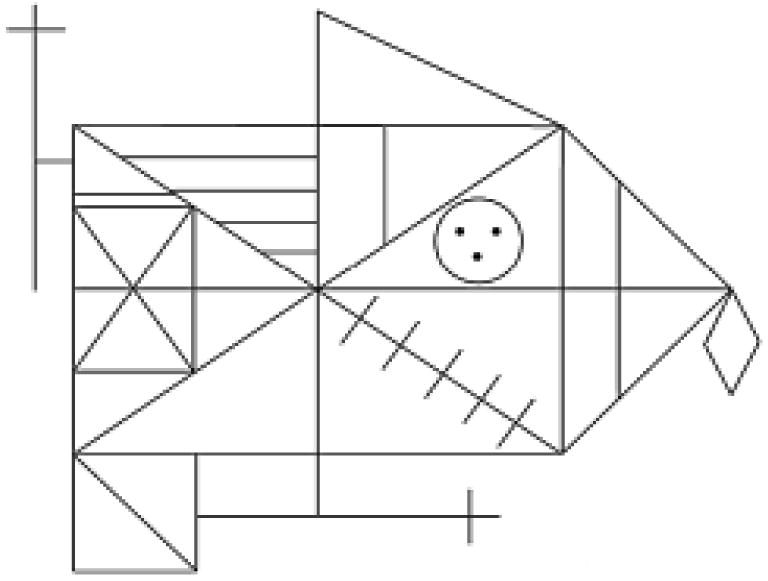
The Rey-Osterrieth Complex Figure [Stern et al., 1994].
Each participant's copy and reproductions of the ROCF were scored using the Boston Qualitative Scoring System [Stern et al., 1994]. This scoring system divides the Rey design into Configural Elements, Clusters, and Details. Configural Elements are considered to be the most “global” parts of the design, the Details as the most “local” elements, and the Clusters in between. A Configural
Element is a large global element such as the base rectangle of the design. A Cluster is a distinguishable component of the total figure, such as the small rectangle containing two intersecting diagonal lines that appears in the left portion of the large rectangle. A Detail is a smaller, less distinguishable component of the figure, such as the vertical line positioned in the triangle adjacent to the right side of the rectangle. These Configural Elements, Clusters, and Details are scored for Presence, Accuracy, Placement, and Fragmentation. These scores contribute to Summary Scores, including Presence and Accuracy for each of the three conditions, and the Organization of the reproduction. The complex design is also scored for characteristics of how the reproduction is approached, resulting in an overall Planning Score. All of the Rey figures were scored blind to group status by a single trained staff member (EK) trained to reliability in the use of the scoring system.
Data analysis
The study's hypotheses were tested in a series of analyses to examine general performance on the ROCF as well as the organizational approach to production of the design with regard to local vs. global processing.
First, the Presence and Accuracy summary scores were analyzed using a mixed ANOVA with Condition as a within-subjects factor (Copy vs. Immediate Recall vs. Delayed Recall) and Diagnostic Group (Autismvs. Control) and Age (Child vs. Adolescents/Adults) as between-subjects factors. Presence and Accuracy scores were on a 7-point scale (0–6). This initial set of analyses looked at basic production of the complex figure and whether its components were present in the participants' designs and accurately drawn (e.g., four sides of rectangle roughly parallel, diagonal line drawn between upper left and lower right quadrants within 3mm of corners).
To evaluate our hypotheses on local vs. global approaches to completing the ROCF, a second phase of analysis used the same mixed ANOVA design on the Planning Scores across the three conditions and the Organization Summary Score. The Planning Score focused on whether the figure was produced with consideration of the gestalt. For example, a higher Planning Score would be achieved by drawing global elements like the configural rectangle in one piece (rather than quadrants), and drawing these global features prior to drawing smaller elements like clusters or details. The Planning Score also accounted for whether the figure was drawn within the boundaries of the page, and completed in a logical and systematic manner. Planning was scored on a 5-point scale ranging from significantly poor (0) to good (4) planning. The Organization Summary Score focused on this local vs. global approach to the drawing, and also accounted for fragmentation of the design (e.g., overdrawn lines, lines drawn with more than one pen stroke, beginning a second element before completing the first). This summary score focused on approach to the design for only the copy condition, allowing for an analysis of approach to the design without any effect of memory load. For both the Planning scores and Organization Summary Score, higher scores represent a more strategic (planned), globally oriented approach to completing the design.
Finally, given the common use of the Block Design (BD) subtest as a index of local processing in autism, we examined correlations between the BD subtest scaled score and the Presence and Accuracy Summary Scores, the Planning score, and the Organization Summary Score. In addition, we conducted several analyses to parallel those reported by Schlooz et al. [2006] and further examined the relationship between the ROCF and BD. By calculating difference scores between Configural Element Presence Scores (representing global design components) and Detail Presence Scores (representing local, detailed design components), we analyzed the relationship between BD scores and the local vs. global components of the ROCF.
Results
Presence and Accuracy
Results for the Presence and Accuracy Summary Scores are presented in Table II. Although there was a strong effect of condition, such that Presence and Accuracy Scores decreased in the recall conditions, there was no significant interaction between condition and diagnostic group or age. Therefore, only between subjects effects will be discussed.
Table II. Mean and Standard Deviations for Presence and Accuracy, Planning, and Organization Scores.
| Children | Adolescents/adults | |||||||
|---|---|---|---|---|---|---|---|---|
| Autism (n = 23) | Control (n = 26) | Autism (n = 14) | Control (n = 23) | |||||
| Variable | M | SD | M | SD | M | SD | M | SD |
| Copy Presence and Accuracy | 15.09 | 3.59 | 16.73 | 1.89 | 17.39 | 1.69 | 18.09 | 1.54 |
| Immediate Presence and Accuracy | 9.74 | 5.20 | 11.35 | 3.67 | 12.29 | 3.65 | 13.00 | 2.52 |
| Delay Presence and Accuracy | 8.96 | 4.67 | 10.69 | 3.87 | 11.50 | 3.18 | 13.13 | 2.94 |
| Copy Planning | 1.80 | 1.20 | 1.72 | 1.02 | 1.77 | 1.09 | 2.04 | 1.19 |
| Immediate Planning | 1.85 | 1.27 | 1.68 | 0.90 | 1.54 | 0.88 | 2.22 | 0.95 |
| Delay Planning | 1.85 | 1.35 | 1.60 | 1.00 | 1.46 | 0.97 | 2.48 | 0.95 |
| Organization | 3.70 | 1.79 | 3.23 | 1.61 | 3.57 | 1.95 | 4.65 | 2.04 |
| Block Design Subtest Scaled Score | 12.52 | 3.94 | 11.92 | 3.26 | 10.36 | 3.25 | 10.96 | 2.14 |
| Configural Element Presence | ||||||||
| Copy Condition | 3.83 | 0.58 | 4.00 | 0.00 | 4.00 | 0.00 | 4.00 | 0.00 |
| Immediate Recall Condition | 2.96 | 1.36 | 3.23 | 1.07 | 3.50 | 0.86 | 3.70 | 0.64 |
| Delayed Recall Condition | 2.87 | 1.22 | 3.83 | 1.02 | 3.71 | 0.73 | 3.78 | 0.67 |
| Detail Presence | ||||||||
| Copy Condition | 3.30 | 0.97 | 3.54 | 0.71 | 3.86 | 0.36 | 3.74 | 0.54 |
| Immediate Recall Condition | 1.48 | 1.20 | 1.69 | 0.88 | 2.00 | 1.04 | 1.61 | 0.72 |
| Delayed Recall Condition | 1.22 | 0.90 | 1.54 | 1.03 | 1.79 | 0.98 | 1.65 | 0.71 |
Note: Planning Scores are presented for 20 children with autism, 25 control children, 13 adolescents/adults with autism, and 23 adolescent/adult controls.
There was a main effect of diagnostic group for the Presence and Accuracy Summary Scores, F(1,82)54.25, P50.04. The individuals with autism had fewer items present and accurately drawn in their figures when compared to the TD individuals. There was also a main effect of age, F(1,82)510.72, P50.002, with the children across both diagnostic groups having fewer items present and accurately drawn when compared to the adolescents/adults across both diagnostic groups. There was, however, no significant interaction between diagnostic group and age, F(1,82)50.24, P50.63. These results suggest a developmental effect across age for both groups in the presence and accuracy of the elements drawn in the ROCF.
Planning and Organization
Results for the Planning Scores are also presented in Table II. Planning scores were unable to be derived for five participants (three children with autism, one control child, and one adolescent/adult with autism) whose Rey reproductions were insufficient to score on the components that contribute to the planning variable. There was no effect of condition for these Planning Scores and therefore all subsequent analyses were conducted on Planning Scores averaged across the three conditions. There was also no main effect of diagnostic group or of age but, as predicted, there was an interaction effect of diagnostic group and age, F(1,77)54.38, P50.04. Follow-up analyses using the collapsed Planning Score demonstrated that while there were no significant differences in Planning Scores between the children and adolescents/adults with autism, F(1,31)50.42, P50.52, the TD adolescents/adults had significantly higher planning scores than the TD children, F(1,46)58.49, P50.006. (See Fig. 2 for a depiction of these Planning Scores by age.)
Figure 2.
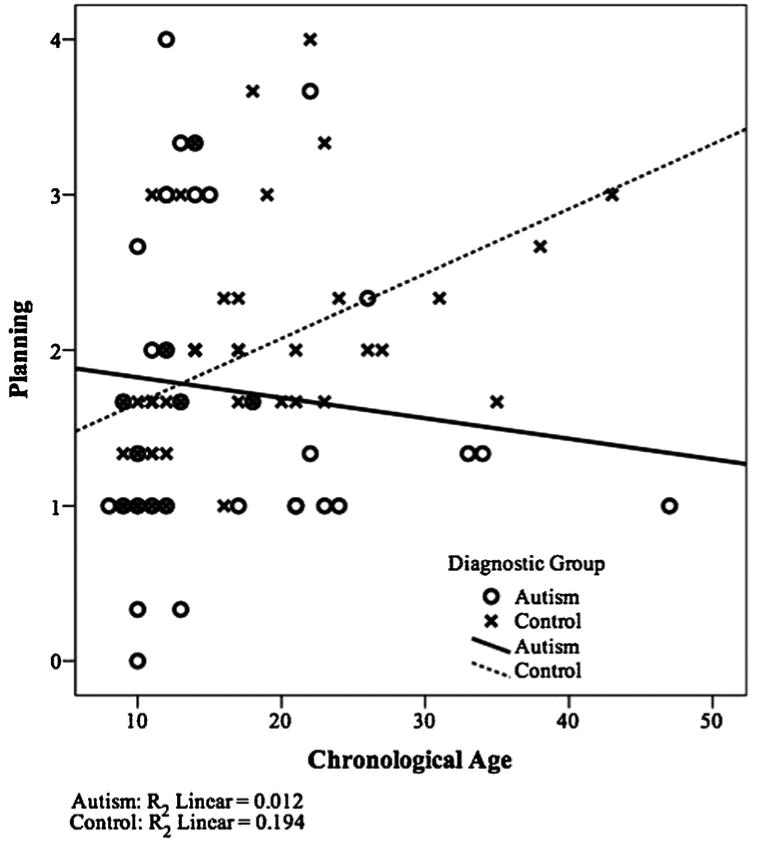
Scatterplot of correlation (by diagnostic group) of planning scores and chronological age.
Results for the Organization Summary Scores are presented in Table II. These analyses yielded no main effects of diagnostic group, F(1,82)50.57, P50.45, or age, F(1,82)52.54, P50.12, but did show a trend toward an interaction between diagnostic group and age, F(1,82)53.60, P50.06. Given a priori predictions about organizational approach to completing the ROCF, followup analyses were conducted. Findings showed that while organizational scores (only calculated for the copy condition) were consistent between children and adults with autism, F(1,35)50.039, P50.84, there was significant improvement in these scores between TD children and typical adults, F(1,47)57.44, P50.009. These result parallel those described above for the Planning score.
Finally, results indicated that there was no effect of diagnostic group on the Block Design (BD) subtest scores, F(1,82)50.00, P50.99, and no interaction effect of group by age, F(1,82)50.71, P50.40. These findings, e.g., the failure to demonstrate a Block Design subtest performance superiority in the autism group, are consistent with the absence of a local processing bias on the BD subtest in the individuals with autism. The exclusion criteria precluded the possibility of subjects with autism who had below average block design scores, although in reality this did not limit the sample. Given the absence of group differences on this subtest, subsequent correlations were conducted on all individuals in the sample. There were no significant correlations found between BD and the Presence and Accuracy Summary Scores, the Planning Scores, or the Organization Summary Score. We did also examine the correlations between the BD subtest scores and the Detail Presence scores to explore whether the two measures capture similar constructs of detai lfocused processing. These correlations were positively correlated for the individuals with autism (rcopy50.25, rimmediate recall50.29, rdelayed recall50.21), while they were unrelated in the control group (rcopy50.04, rimmediate recall5_0.07, rdelayed recall5_0.05), suggesting that the presence of details in the ROCF designs likely represent locally focused processing that is often observed in BD performance in individuals with autism. As originally presented by Schlooz et al. [2006], we also conducted correlations between BD scores and difference scores between Configural Element and Detail Presence scores in order to capture evidence of a detail-oriented style. We found negative correlations in the direction of those reported by Schlooz et al. [2006], but these relationships were not statistically significant in any of the three conditions (all P's40.3).
However, given that heterogeneity of performance is the rule in groups of individuals with autism, we examined whether the pattern of these correlations was statistically different between diagnostic groups. We used Fisher r-to-z transformations to compare the relationship between BD scores and the difference scores between Configural Element Presence Scores (representing global design components) and Detail Presence Scores (representing local, detailed design components). These analyses indicated that the correlation between BD scores and the difference scores were statically significant between diagnostic groups in the immediate condition (rautism5_0.37, rcontrol50.17, Z5_2.47, P50.007), and showed a trend in the delayed condition (rautism5_0.18, rcontrol50.13, Z5_1.42, P50.08). These findings suggest that for the autism group, better BD performance (i.e., more locally focused processing) is related to a smaller difference in the presence of Configural Elements vs. Details in the complex design. This might mean that the individuals with autism view configural elements and details as equivalent “pieces” of the design, and do not differentiate the local vs. global nature of the components. In contrast, better BD performance in the control group was related to a larger difference in Configural Element presence vs. Detail presence, suggesting that the individuals in the control group perceive and produce these elements differently and the gestalt nature of Configural Elements is still maintained in the ROCF. These findings could indicate that while performance on the BD subtest did not differ between the individuals with autism and typical controls at an outcome level, the approach differs between groups (i.e., locally oriented processing may drive better scores for the individuals with autism, but not for typical controls).
Discussion
In Kanner's original descriptions [1943, 1971; Kanner, Rodriguez, & Ashenden, 1972], the predisposition of individuals with autism for perceiving details and missing the significance of the whole entity was as characteristic of this syndrome as the disturbances in social contact, and continues to be reflected in current diagnostic criteria under the symptom category of restricted and repetitive behavior and interests [DSM-IVTR, APA, 2004]. Initial research led to a range of evidence supporting a local processing bias, and to a number of cognitive theories, the most prominent of which was weak central coherence [Frith, 1989]. The utilization of complex visual tasks as stimuli led to the introduction of a distinction between local/global processing at the perceptual level and local/global processing at the conceptual level. Only few studies have been completed in autism using the ROCF thus far, with most reporting a detail-oriented approach though often only qualitative assessments have been used.
This study found no evidence of a local processing superiority in either the children or the adults with autism. That is, there was no evidence of an increased number or increased accuracy of reproduction of details of the Rey Osterreith Figure, or of elevated Block Design scores in this group of individuals with autism. When individual variability in processing approaches was considered by performing the within-group analysis used in the prior study by Schlooz and colleagues [2006], there were negative correlations in the individuals with autism between Block Design scores and Configural Elements Present Score minus Details Present Score suggesting a local processing bias, but these correlations were not significant. However, in one of our prior studies, a comparably defined group of adolescent and adult subjects with and without autism were tested with the Navon task, which did demonstrate a local processing bias in the individuals with autism [Behrmann et al., 2006]. The Navon task is a finer grained and more sensitive assessment of local–global processing, and thus is capable of detecting subtle differences not apparent with clinical tests, such as Block Design and the ROCF. Hence, this subgroup of individuals with autism without intellectual disability do not have clinically apparent evidence of local processing bias but do exhibit this propensity on more fine grained measures supporting the presence of this alteration in visual processing even in the mildest expression of autism. The findings of this study are consistent with recent studies of the absence of clinically apparent evidence of local processing bias in individuals with autism without intellectual disability, or when the properties of the Navon stimulus favored global processing [reviewed in: Behrmann et al., 2006; Schlooz et al., 2006].
Both the children with and without autism used a detail-oriented or local processing approach to reproducing the ROCF. Likewise, neither the children with autism nor the TD children exhibited strategic approaches to reproducing the ROCF. As a result, there were no significant differences between the autism and control child groups for any of the variables examined.
However, there were consistent differences between the adolescent/adult groups with and without autism, reflecting the acquisition of strategic and problemsolving skills in the TD adolescents/adults but not the adolescent/adult autism group. Thus, the adult autism group demonstrated poorer performance than controls on the Planning scores across the three conditions, and the Organization score (which evaluates the approach to the design without any memory load). These findings define a developmental pattern in which strategy formation and planning abilities have not yet been acquired in the children regardless of diagnosis, thereby producing the absence of a difference between the groups of children using this procedure. The global processing deficit that emerged in these adults with autism reflects the failure of the autism group to make expected developmental gains in acquiring strategic problem solving and planning skills.
The lack of use of a global approach by the TD children is consistent with normative studies of the ROCF, which have documented that the use of global approaches does not begin to emerge in TD children until 11–13 years of age [Akshoomoff & Stiles, 1995a,b]. The failure to document a deficit in planning and strategy use in the children with autism without intellectual disability is also consistent with prior reports of failure to document executive function deficits in children with HFA [Baron- Cohen et al., 1999; Edgin & Pennington, 2005; Hill, 2004; Q1 Hill & Russell, 2002; Russell & Hill, 2001]. This pattern of delayed development and failure to achieve adult norms for performance has also been seen in postural control, suggesting it may be a general feature of the disturbance Q2 in development of connectivity in integrative circuitry in autism [Minshew, Meyer, & Goldstein, 2002].
Although delayed development, particularly of social and language skills, is an integral aspect of the early presentation of autism, developmental delays associated with delay and underdevelopment of frontal systems in autism in the second decade of life is less widely appreciated, though well documented. Sweeney, Luna and colleagues [Luna et al., 2001, 2002, 2007; Minshew et al., 1999; Sweeney et al., 1996] have demonstrated the same age-related performance pattern with the oculomotor delayed response task in children and adults with and without autism, showing that deficits did not emerge in this frontally dependent task until the second decade of life in those with autism. During the second and third decade, this phenomenon is also apparent in the increasing divergence between Vineland Adaptive Behavior Scale scores and IQ scores in high functioning adults with autism [Howlin, Mawhood, & Rutter, 2000].
A clinical decline in function is relatively common in high-functioning children when they enter middle or high school. This decline has often been attributed to the greater challenges posed by the social and academic environment; however, this and other studies demonstrate that the failure of higher order frontally mediated skills to emerge is likely a major factor. The emergence of deficits in the second decade of life in adults with HFA is a reminder that brain development is not static after five years of age, nor is the impact of autism on brain functions.
We hypothesized that there would not be notable superiority of Block Design scores for this autism group with FS and VIQscores above 77. This hypothesis was based on prior observations of average but not superior performance on visuo-spatial tasks in individuals with autism without intellectual disability [Minshew, Goldstein, & Siegel, 1997; Williams, Goldstein, & Minshew, 2006] and also on a developmental neurobiologically based model for autism in which brain connectivity, if it develops, is characterized by abnormal increased local connectivity and reduced distributed connectivity. As predicted, we found an absence of differences on the BD scores between the autism and control groups, and also no significant correlations between BD scores and the ROCF scores. We did find, however, that for the autism group, better BD performance (i.e., more locally focused processing) is related to a smaller difference in Configural Elements vs. Details in the complex design. These findings support literature suggesting that the gestalt nature of Configural Elements is still maintained in the ROCF by the individuals in the control group, but that the individuals with autism do not perceive this local vs. global difference [Akshoomoff & Stiles, 1995b; Schlooz et al., 2006].
There is evidence that disturbances in local–global processing have neurobiological correlates in autism. Functional imaging data have demonstrated that individuals with autism activate different neural regions than typical controls when completing visuo-spatial tasks. When performing the EFT, control subjects, but not subjects with autism, activated prefrontal cortical areas. In contrast, participants with autism showed greater activation of the ventro-occipito-temporal regions [Ring et al., 1999]. This study suggests over reliance of task performance on basic visual and spatial abilities secondary to enhanced local connectivity and reduced ability to bring frontal circuitry on-line and associated planning abilities in high functioning individuals with autism. Under-connectivity of posterior regions with frontal cortex has been repeatedly demonstrated in higher functioning individuals with autism [Cherkassky, Kana, Keller, & Just, 2006; Just et al., 2004, 2007; Kana et al., 2006, 2007; Koshino et al., 2005, 2008; Villalobos, Mizuno, Dahl, Kemmotsu, & Muller, 2005]. Ring et al. [1999] documented no difference in Accuracy performance on the EFT between the groups despite the discrepant areas of activation. That is, there was neither a deficit nor an enhanced ability to perform the EFT.
Hence, we would propose that these subjects with autism had neither the degree of enhancement of local connections to produce superior performance, nor was the task sufficiently challenging of planning abilities to demonstrate deficits resulting from reduced frontal abilities and distributed connections. These findings, along with others [Baron-Cohen et al., 2006; de Jonge, Kemner, & van Engeland, 2006], emphasize that the cognitive and neural approach used to complete these local–global tasks differentiates individuals with autism without intellectual disability from TD controls, even when overall performance does not. Our findings support this argument, given the between group differences in performance on the ROCF and the Navon task. However, our results also confirm that the ROCF task should not be considered interchangeable with the EFT or BD as measures of local–global processing.
This study adds to the evidence of a typical altered pattern of visual processing in autism that can be discerned across the autism spectrum, if sufficiently sensitive behavioral methods are used. Although a local processing bias has been most conspicuous in children with autism with intellectual disability and on simpler visuo-spatial tasks, this and other studies have demonstrated that this distinctive processing pattern is present across the spectrum. The use of a more challenging visuospatial task in higher functioning individuals with autism has led to evidence of impairments in planning and strategy use needed for such tasks, demonstrating again that the alteration in processing consists of two elements— enhanced local connectivity and impaired distributed connectivity. In lower functioning individuals with autism, it is their enhanced local connectivity and elementary visuo-spatial skills that dominate task performance resulting in superior performance on visuo-spatial tasks relative to TD controls. In more able individuals with autism, the local processing bias can be detected with the Navon task but task performance is now dominated by their deficient strategy and planning related to under-development of distributed connections with frontal lobe. The absence of superior performance on BD or of increased recall of details on the ROCF suggests that the process of developing even some distributed connections has involved a trade-off with local connectivity.
This neurological model based on developmental disturbances in connectivity make it possible to integrate weak central coherence and the various local processing theories within a complex information processing model. This information processing model has the advantage of having a neurologic substrate, as well as the ability to predict and accommodate impairments in sensory, motor, memory, and postural control domains not readily explained by weak central coherence, even in combination with theory of mind and executive function deficits [Ozonoff & McEvoy, 1994], or other local processing based theories [Mottron, Burack, Iarocci, Belleville, & Enns, 2003; Mottron, Burack, Stauder, & Robaey, 1999; Plaisted, 2001].
Several limitations of this study should be noted. Smaller sample sizes were evident after participants were divided by both diagnostic group and age. Future research with larger samples would allow for larger cells when analyzing by subgroups including the opportunity to look at more incremental development across the teenage and adult years. Although the inclusion–exclusion criteria requiring potential participants to have a Block Design subtest score no lower than one SD of their FSIQ to eliminate a visuo-spatial deficit rather than poor planning or strategy use as the basis for poor performance on the ROCF, this criteria also may have restricted the range of scores and limited the power to detect differences between the groups. In addition, the focus of the study on high functioning individuals with autism, and the exclusion of individuals with Asperger's Disorder and Pervasive Developmental Disorder Not Otherwise Specified, limits the generalizability of the present findings to individuals with these other autism spectrum disorders. Future studies should be expanded to explore these constructs in individuals with autism spectrum disorder.
Acknowledgments
We thank all participants and their families for contributing their time and effort to complete this study for the benefit of all those affected by autism spectrum disorders. We also thank the CPEA Subject Core staff for their effort and diligence in recruitment and testing. In addition, this manuscript is dedicated to the memory of my son Jonathan (N. J. M.) who died during its original preparation.
Contributor Information
Emily S. Kuschner, Department of Psychiatry, University of Rochester Medical Center, Rochester, New York
Kimberly E. Bodner, Department of Psychological Sciences, University of Missouri, Columbia, Missouri
Nancy J. Minshew, Autism Center of Excellence, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, Departments of Psychiatry and Neurology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
References
- 1.Akshoomoff NA, Stiles J. Developmental trends in visuospatial analysis and planning: I. Copying a complex figure. Neuropsychology. 1995a;9:364–377. [Google Scholar]
- 2.Akshoomoff NA, Stiles J. Developmental trends in visuospatial analysis and planning: II. Copying a complex figure. Neuropsychology. 1995b;9:378–389. [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition., text rev. Washington, DC: Author; 2000. [Google Scholar]
- 4.Baron-Cohen S, Ring H, Chitnis X, Wheelwright S, Gregory L, et al. fMRI of parents of children with Asperger syndrome: a pilot study. Brain and Cognition. 2006;61:122–130. doi: 10.1016/j.bandc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 6.Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44:110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Bennetto L, Pennington BF. Executive functioning in normal and abnormal development. In: Segalowitz SJ, Rapin I, editors. Handbook of neuropsychology. Vol. 8. Amsterdam: Elsevier Science B.V.; 2003. pp. 785–802. [Google Scholar]
- 8.Brian JA, Bryson SE. Disembedding performance and recognition memory in autism/PDD. Journal of Child Psychology and Psychiatry. 1996;37:865–872. doi: 10.1111/j.1469-7610.1996.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 9.Burnette CP, Mundy PC, Meyer JA, Sutton SK, Vaughan AE, Charak D. Weak central coherence and its relations to theory of mind and anxiety in autism. Journal of Autism and Developmental Disorders. 2005;35:63–73. doi: 10.1007/s10803-004-1035-5. [DOI] [PubMed] [Google Scholar]
- 10.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 11.Edgin JO, Pennington BF. Spatial cognition in autism spectrum disorders: superior, impaired, or just intact? Journal of Autism and Developmental Disorders. 2005;35:729–745. doi: 10.1007/s10803-005-0020-y. [DOI] [PubMed] [Google Scholar]
- 12.Filipek PA, Accardo PJ, Baranek GT, Cook EH, et al. The screening and diagnosis of autistic spectrum disorders. Journal of Autism and Developmental Disorders. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 13.Frith U. Explaining the enigma. Oxford: Blackwell; 1989. [Google Scholar]; Happé FGE. Studying weak central coherence at low levels: children with autism do not succumb to visual illusions. A research note. Journal of Child Psychology and Psychiatry. 1996;37:873–877. doi: 10.1111/j.1469-7610.1996.tb01483.x. [DOI] [PubMed] [Google Scholar]
- 14.Happé FGE. Central coherence and theory of mind in autism: reading homographs in context. British Journal of Developmental Psychology. 1997;15:1–12. [Google Scholar]
- 15.Happé FGE. Autism: cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3:216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- 16.Hollingshead AB. Four factor index of social status. Yale University, Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- 17.Howlin P, Mawhood L, Rutter M. Autism and developmental receptive language disorder—a follow-up comparison in early adult life. II: social, behavioural, and psychiatric outcomes. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41:561–578. doi: 10.1111/1469-7610.00643. [DOI] [PubMed] [Google Scholar]
- 18.Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the embedded figures test? Journal of Child Psychology and Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 19.de Jonge MV, Kemner C, van Engeland H. Superior disembedding performance of high-fucntioning individuals with autism spectrum disorers and their parents: the need for subtle measures. Journal of Autism and Developmental Disorders. 2006;36:677–683. doi: 10.1007/s10803-006-0113-2. [DOI] [PubMed] [Google Scholar]
- 20.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 21.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biological Psychology. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–240. [PubMed] [Google Scholar]
- 25.Kanner L. Follow-up study of 11 autistic children originally reported in 1943. Journal of Autism and Childhood Schizophrenia. 1971;1:119–145. doi: 10.1007/BF01537953. [DOI] [PubMed] [Google Scholar]
- 26.Kanner L, Rodriguez A, Ashenden B. How far can autistic children go in matters of social adaptation? Journal of Autism and Childhood Schizophrenia. 1972;2:9–33. doi: 10.1007/BF01537624. [DOI] [PubMed] [Google Scholar]
- 27.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. FMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cerebral Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeCouteur A, Rutter M, Lord C, Rios P, Robertson S, et al. Autism diagnostic interview: a standardized investigator- based instrument. Journal of Autismand Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- 30.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, et al. The Autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 31.Lord C, Rutter M, Good S, Heemsbergen J, Jordan H, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 32.Lord C, Rutter M, LeCouteur AL. Autism diagnostic interview revised. A Revised Version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 33.Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychology. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, et al. Neocortical system abnormalities in autism. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 35.Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 36.Manjiviona J, Prior M. Neuropsychological profiles of children with Asperger syndrome and autism. Autism. 1999;3:327–356. [Google Scholar]; Minshew NJ. Autism. In: Berg BO, editor. Principles of child neurology. New York: McGraw-Hill; 1996. pp. 1713–1730. [Google Scholar]
- 37.Minshew NJ, Goldstein G. The pattern of intact and impaired memory functions in autism. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:1095–1101. doi: 10.1111/1469-7610.00808. [DOI] [PubMed] [Google Scholar]
- 38.Minshew N, Goldstein G, Siegel D. Neuropsychological functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3:303–316. [PubMed] [Google Scholar]
- 39.Minshew N, Luna B, Sweeney J. Oculomotor evidence for neocortical systems but not cerebellar dysfunctions in autism. Neurology. 1999;52:917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minshew NJ, Meyer J, Goldstein G. Abstract reasoning in autism: a dissociation between concept formation and concept identification. Neuropsychology. 2002;16:327–334. doi: 10.1037//0894-4105.16.3.327. [DOI] [PubMed] [Google Scholar]
- 41.Minshew NJB, Williams DL. The new neurobiology of autism: coretex, connectivity, and neuronal organization. Archives of Neurology. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan B, Maybery M, Durkin K. Weak central coherence, poor joint attention, and low verbal ability: independent deficits in early autism. Developmental Psychology. 2003;39:646–656. doi: 10.1037/0012-1649.39.4.646. [DOI] [PubMed] [Google Scholar]
- 43.Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. Journal of Child Psychology and Psychiatry. 2003;44:904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- 44.Mottron L, Burack JA, Stauder JEA, Robaey P. Perceptual processing among high-functioning persons with autism. Journal of Child Psychology and Psychiatry. 1999;40:203–211. [PubMed] [Google Scholar]
- 45.Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulated system. Journal of Child Psychology and Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- 46.Navon D. Forest before trees: the precedence of global features in visual perception. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- 47.Osterrieth P. Le test de copie d'une figure complexe. Archieves de Psychologie. 1944;30:206–356. [Google Scholar]
- 48.Ozonoff S. Reliability and validity of theWisconsin Card Sorting Test in studies of autism. Neuropsychology. 1995;9:491–500. [Google Scholar]
- 49.Ozonoff S, McEvoy RE. A longitudinal study of executive function and theory of mind development in autism. Development and Psychopathology. 1994;6:415–431. [Google Scholar]
- 50.Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. Journal of Child Psychology and Psychiatry. 1991;32:1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 51.Ozonoff S, Strayer DL, McMahon WM, Filloux F. Executive function abilities in autism and Tourette syndrome: an information processing approach. Journal of Child Psychology and Psychiatry. 1994;35:1015–1032. doi: 10.1111/j.1469-7610.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 52.Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Journal of Child Psychology and Psychiatry and Allied Discipline. 1999;40:733–742. [PubMed] [Google Scholar]
- 53.Plaisted KC. Reduced generalization: an alternative to weak central coherence. In: Burack JA, Charman A, Yirmiya N, Zelazo PR, editors. Development and autism: perspectives from theory and research. New Jersey: Lawrence Erlbaum Associates; 2001. pp. 149–169. [Google Scholar]
- 54.Prior M, Hoffman W. Brief report: neuropsychological testing of autistic children through an exploration with frontal lobe tests. Journal of Autism and Developmental Disorders. 1990;20:581–590. doi: 10.1007/BF02216063. [DOI] [PubMed] [Google Scholar]
- 55.Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Atypical interference of local detail on global processing in high-functioning autism and Asperger's disorder. Journal of Child Psychology and Psychiatry. 2000;41:769–778. [PubMed] [Google Scholar]
- 56.Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. A deficit in shifting attention present in high-fuctioning autism but not Asperger's disorder. Autism. 2001;5:67–80. doi: 10.1177/1362361301005001007. [DOI] [PubMed] [Google Scholar]
- 57.Ring H, Baron-Cohen S, Wheelwright S, Williams S, Brammer M, et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- 58.Ropar D, Mitchell P. Susceptibility to illusions and performance on visuospatial tasks in individuals with autism. Journal of Child Psychology and Psychiatry. 2001;42:539–549. [PubMed] [Google Scholar]
- 59.Rumsey JM, Hamburger SD. Neuropsychological divergence of high-level autism and severe dyslexia. Journal of Autism and Developmental Disorders. 1990;20:155–168. doi: 10.1007/BF02284715. [DOI] [PubMed] [Google Scholar]
- 60.Schlooz WAJM, Hulstijn W, Van den Broek PJA, Van der Pijll ACAM, Gabreels F, et al. Fragmented visuospatial processing in children with pervasive developmental disorder. Journal of Autism and DevelopmentalDisorders. 2006;36:1025–1037. doi: 10.1007/s10803-006-0140-z. [DOI] [PubMed] [Google Scholar]
- 61.Shah A, Frith U. An islet of ability in autism: a research note. Journal of Child Psychology and Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 62.Shah A, Frith U. Why do autistic individuals show super performance on the block design task? Journal of Child Psychology and Psychiatry and Allied Disciplines. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 63.Stern R, Javorsky D, Singer E, Harris N, Somerville J, et al. The Boston Qualitative Scoring System for the Rey- Osterrieth Complex Figure. Odessa, FL: Psychological Assessment Resources, Inc.; 1994. [Google Scholar]
- 64.Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- 65.Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. NeuroImage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waber DP, Holmes JM. Assessing children's memory productions of the Rey-Osterrieth Complex Figure. Journal of Clinical Experimental Neuropsychology. 1986;8:563–580. doi: 10.1080/01688638608405176. [DOI] [PubMed] [Google Scholar]
- 67.Wang L, Mottron L, Peng D, Berthiaume C, Dawson M. Local bias and local-to-global interference without global deficit: a robust finding in autism under various conditions of attention, exposure time, and visual angle. Cognitive Neuropsychology. 2007;24:550–574. doi: 10.1080/13546800701417096. [DOI] [PubMed] [Google Scholar]
- 68.Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing. Child Neuropsychology. 2006;12:279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]


