Abstract
IL-33 is associated with atopic and autoimmune diseases and, as reported here, it interacts synergistically with Ag to markedly enhance production of inflammatory cytokines in rodent mast cells even in the absence of degranulation. Investigation of the underlying mechanisms revealed that synergy in signaling occurred at the level of TGFβ-activated kinase1 which was then transmitted downstream through JNK, p38 MAP kinase, and AP1. Stimulation of the Ca2+/calcineurin/NFAT pathway by Ag, which IL-33 did not, was critical for the synergy between Ag and IL-33. For example, selective stimulation of the NFAT pathway by thapsigargin also markedly enhanced responses to IL-33 in a calcineurin-dependent manner. As indicated by luciferase-reporter assays, IL-33 failed to stimulate the transcriptional activities of NFAT and AP1 but augmented the activation of these transcription factors by Ag or thapsigargin. Robust stimulation of NFκB transcriptional activity by IL-33 was also essential for synergy. These and pharmacologic data suggested that the enhanced production of cytokines resulted in part from amplification of the activation of AP1 and NFAT as well as co-operative interactions among transcription factors. IL-33 may retune mast cell responses to Ag towards enhanced cytokine production and thus determine the symptoms and severity of Ag-dependent allergic and autoimmune diseases.
Keywords: Mast Cells, Cytokine, IL-33, Ag, Signaling Mechanisms
Introduction
Mast cells participate in innate and adaptive immune responses as well as allergic and autoimmune diseases [1, 2]. Their best known role is mediating IgE-dependent allergic reactions through their ability to respond to allergens with release of inflammatory mediators via degranulation, production of inflammatory lipids, and synthesis of cytokines. These responses can be substantially enhanced by endogenous and exogenous factors such as Kit ligand (stem cell factor), adenosine, prostaglandin E2, and pathogenic TLR ligands [3, 4] and it is now apparent that the responses of mast cells to allergens are regulated by a variety of endogenous and exogenous ligands [1, 5, 6].
In cultured mast cells, Ag-induced aggregation of FcεRI through IgE results in the activation of tyrosine kinases such as Lyn, Fyn, and Syk. The ensuing tyrosine phosphorylation of adaptor proteins enables recruitment of other signaling molecules [2, 7, 8]. These molecules include PI3K, phospholipase (PL) Cγ1/2, and small GTPases including Ras and Rac. Further downstream, PLC-mediated increases in intracellular levels of free Ca2+ ([Ca2+]i) and activation of PKC provide necessary signals for degranulation [9, 10] whereas the activation of MAPK via GTPases is essential for engagement of transcription factors such as Elk, c-Jun, c-Fos, ATF2, and cMyc that promote cytokine gene transcription [8]. Additional cytokine-related transcription factors, NFAT and NF-κB, are activated through the Ca2+/calcineurin and PI3K/PKC-dependent pathways, respectively [4, 11]. The recruitment and extent of Ag-induced activation of transcription factors is enhanced on co-stimulation of mast cells with Kit ligand [12] or TLR ligands [4]. As a consequence, the production of cytokines is markedly augmented in a synergistic manner.
The recently described cytokine, IL-33, is also reported to stimulate cytokine production, but not degranulation or eicosanoid production in mast cells [13–16] and basophils [17–20], and may enhance FcεRI and G protein-coupled receptor mediated cytokine production in mast cells and basophils [14, 21] although the mechanism of this enhancement is unknown. IL-33 accumulates in the affected tissues of patients with Crohn’s disease, rheumatoid arthritis, atopic dermatitis, psoriasis, and anaphylactic reactions—afflictions thought to be associated with activation of mast cells [22–27]. In animal models, IL-33 is reported to exacerbate autoantibody-induced arthritis with enhanced autoantibody-mediated mast cell degranulation in synovial tissues [28] and to be essential for the late phase inflammatory reaction during passive cutaneous anaphylaxis [29]. IL-33 is a member of the Toll/interleukin-1 receptor (TIR) family of receptors [30] but operates through a unique ST2 receptor in conjunction with the IL-1 receptor accessory protein [31–33]. ST2, in common with IL-1/IL-18 receptors and TLR, contains a TIR domain which enables recruitment of the TIR domain-containing adaptor protein, MyD88, and in turn IL-1 receptor-associated kinase (IRAK) and TNF receptor-associated factor 6 (TRAF6) [34, 35]. The engagement of TRAF6 leads to the activation of two key transcription factors. These include the dimeric AP-1 through the JNK pathway as well as NF-κB through dissociation of the inhibitor of the NF-κB complex (IKK) from this complex. However, some aspects of IL-33-mediated signaling remain unresolved. p38 MAP kinase and ERK 1/2 are also activated in mast cells by IL-33 [14, 36] in a MyD88-dependent manner [15], but the downstream consequences are unclear. Also, it is not known if IL-33 operates through TGFβ-activated kinase1 (TAK1), which plays a pivotal role in the activation of the NF-κB, p38 MAP kinase, and JNK pathways in some types of cells when stimulated with agonists of other TIR family members including IL-1, IL-18, or TLR ligands [37–39]. Nevertheless, ST2-mediated signals appear to share the same general features as those mediated by IL-1 receptors and TLRs.
In investigating the mechanism of action of IL-33, and its similarities to that of TLR ligands, we found that as little as 10 pg/ml of IL-33 was capable of markedly amplifying production of inflammatory cytokines and chemokines when cells were stimulated with Ag along with IL-33. In contrast to Ag, IL-33 failed to induce a calcium signal for degranulation. Moreover, in the presence of low concentrations of both Ag and IL-33 mast cells exhibited no or minimal degranulation but produced inflammatory cytokines in amounts exceeding those achieved with optimal concentrations of either stimulant alone. The present studies were undertaken to examine the mechanisms underlying these phenomena with the expectation that the results would broaden our understanding how responses to Ag can be substantially retuned by other inflammatory factors.
Results
IL-33 interacts synergistically with Ag to enhance cytokine production in a MyD88-dependent manner
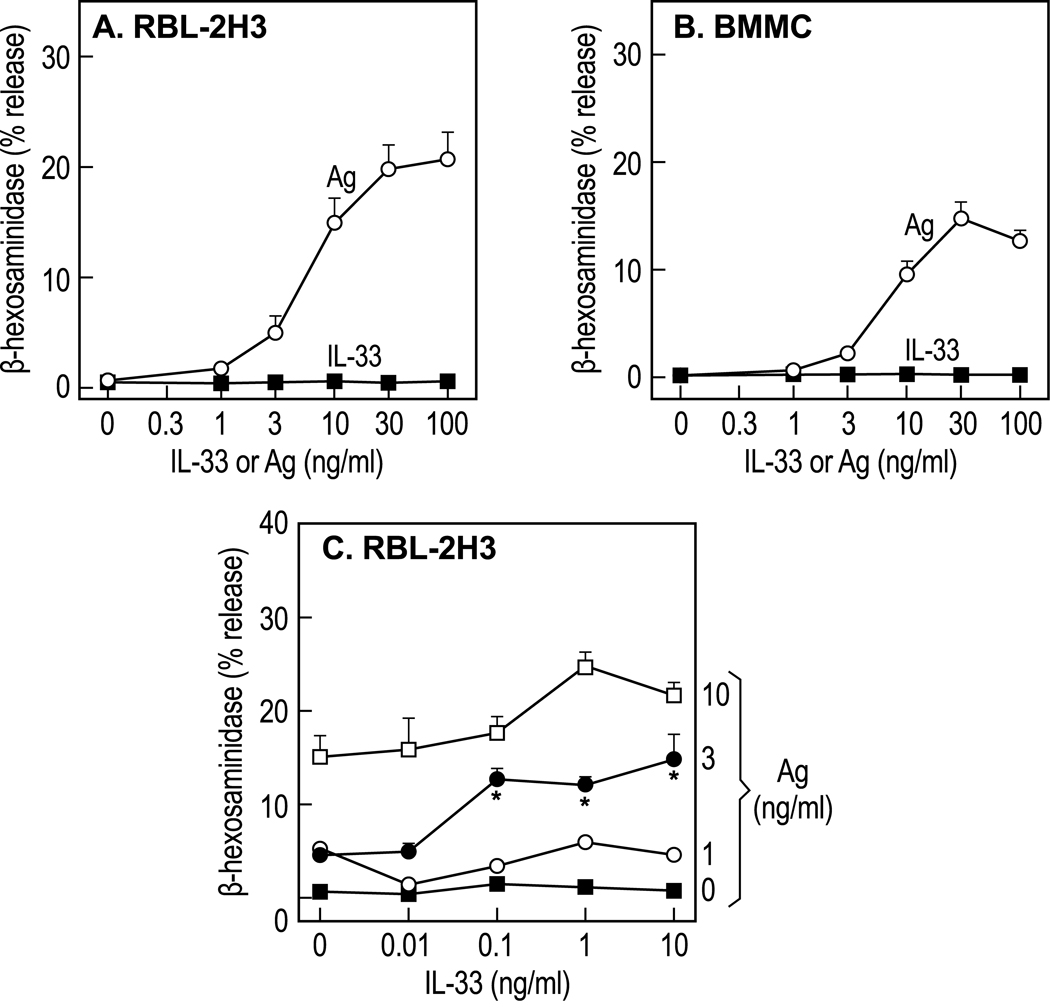
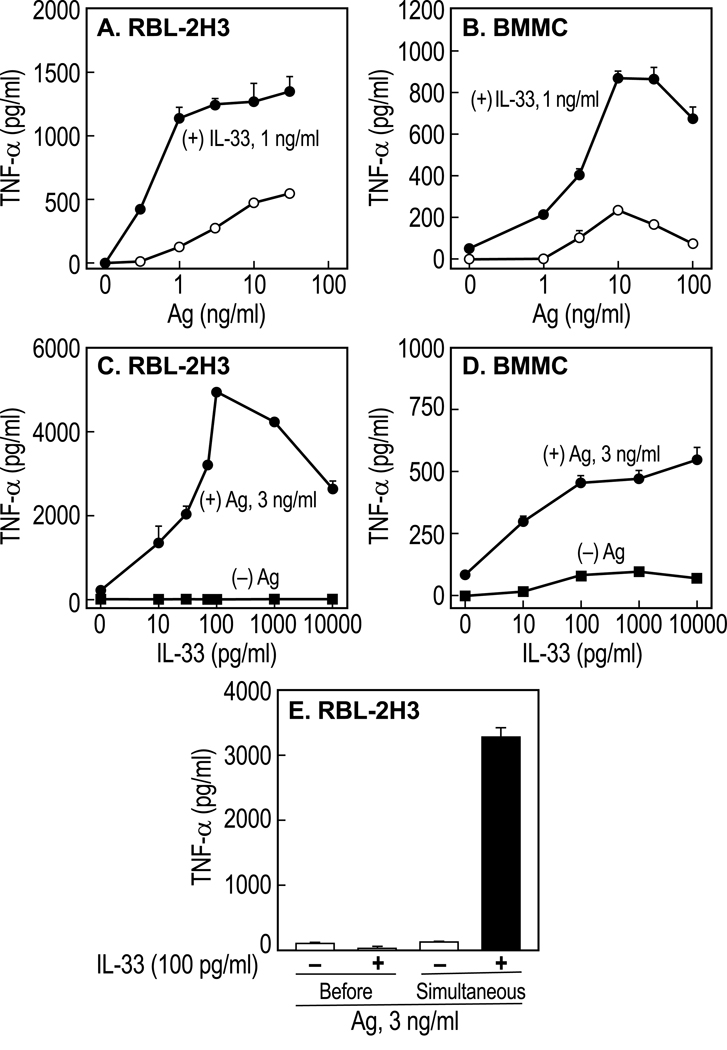
In contrast to Ag, IL-33 failed to stimulate degranulation in RBL-2H3 cells or BMMC (Fig. 1A and B) and enhanced degranulation to only a minimal or modest extent when added in combination with Ag (Fig. 1C). The most pronounced effect of IL-33 was its ability to synergistically augment production of cytokines by Ag in RBL-2H3 cells and BMMC. For example, production of TNFα was markedly enhanced when cells were simultaneously stimulated with various concentrations of Ag and 1 ng/ml IL-33 (Fig. 2A and B). Synergy was apparent over a wide range of concentrations of IL-33 and a fixed concentration of Ag (3 ng/ml) and was evident with as little as 10 pg/ml IL-33 (Fig. 2C and D). IL-33 alone did not stimulate production of TNFα in RBL-2H3 cells (Fig. 2C) and did so to a limited extent in BMMC (Fig. 2D). Therefore, IL-33 was capable of significantly amplifying production of TNFα at concentrations (i.e., 10 pg/ml) below those required for stimulation of TNFα production by IL-33 alone (i.e. 100 pg/ml in Fig. 2 D). Such synergy was not due to priming of cells by IL-33 as synergy was not observed when cells were incubated with IL-33 for 16 h before stimulation with Ag in the absence of IL-33 as compared to simultaneous addition of IL-33 and Ag (compare “Before” columns with “Simultaneous” columns in Fig. 2E).
Figure 1.
L-33 fails to stimulate mast cell degranulation but modestly enhances Ag-induced degranulation. RBL-2H3 cells (A and C) and (B) BMMC, sensitized with Ag-specific IgE, were stimulated for 15 min with the indicated concentrations of IL-33, Ag, or both simultaneously. The data show per cent release of cellular β-hexosaminidase into medium. Values are the mean and SEM of values from 3 cultures and are typical of two or more similar experiments. Statistically significant increases in degranulation are indicated thus *, p<0.05; **, p<0.001.
Figure 2.
Ag and IL-33 interact synergistically in the production of TNFα. Sensitized RBL-2H3 cells and BMMC were incubated for 3 h with the indicated concentrations of Ag, in the absence or presence of IL-33 (A and B), or the indicated concentrations of IL-33, in the absence or presence of Ag (C and D) before measurement of TNFα in the medium. For Panel E, cells were exposed to IL-33 for 16 h and then washed before stimulation with 3 ng/ml Ag (Before); alternatively cells were incubated for 16 h without IL-33, washed, and then stimulated simultaneously with IL-33 and Ag for 3 h (Simultaneous). Data depict mean and SEM of values from 3 cultures and are typical of two or more similar experiments. All differences were highly significant, p<0.001.
We next examined the effects of relatively low concentrations of IL-33 (70 pg/ml) and Ag (3 ng/ml) on production of a broad array of cytokines and chemokines in BMMC by the use of a multiplex assay system. These assays indicated that IL-33 alone induced production TNFα, IL-6, IL-13, MCP-1, MCP-3, MIP-1α either to the same extent or, in the case of IL-6 and IL-13, to a greater extent than Ag. However, both stimulants together interacted synergistically in stimulating production of (Fig. 3A–F) but they failed to stimulate production of IL-2 and RANTES when administered individually or in combination (data not shown). MyD88 was also examined, because of its known role in IL-33-mediated signaling events, to verify that MyD88 was also essential for the synergy between IL-33 and Ag (Fig. 3G–I). In BMMC derived from wild-type mice, the stimulated production of TNFα, IL-6, and IL-13 by optimal concentrations of IL-33 and Ag (10 ng/ml and 20 ng/ml respectively) was substantially enhanced on simultaneous addition of both stimulants. However, the responses to IL-33 and the synergy between IL-33 and Ag were no longer apparent in MyD88-deficient BMMC (Fig. 3G–I). The responses to Ag alone were not affected by the absence of MyD88.
Figure 3.
Ag and IL-33 interact synergistically in the production of several cytokines in wild type but not in MyD88-deficient BMMC. (A–F) BMMC were sensitized with Ag-specific IgE before stimulation or not (ns) with 3 ng/ml Ag or 70 pg/ml IL-33, individually or in combination, for 3 h. The indicated cytokines or chemokines were assayed by use of the Procarta assay kit as described in Materials and Methods. Data depict mean and SEM of values from 3 cultures and are typical of two similar experiments. (G–I) BMMC derived from wild type (WT) or MyD88−/− mice were sensitized with Ag-specific IgE before stimulation with 20 ng/ml Ag, 10 ng/ml IL-33, or both in combination. Note that these concentrations were higher than those used in panels A–F. Media were assayed for TNFα, IL-6, and IL-13 by ELISA 24 h later. Values are mean and SEM from 3 independent experiments except for panel I which shows mean values from 1 experiment. In all panels the differences between the sum of responses to individual stimulants and the response to the combination of stimulants were statistically significant at the p<0.05 (panels A to C) or p<0.01 (panels D to H) level.
The studies with BMMC indicated that synergistic production of cytokines was apparent at either low (3 ng/ml Ag and 70 pg/ml IL-33 in Fig. 3A–F) or high (20 ng/ml Ag and 10 ng/ml IL-33 in Fig. 3G–I) concentrations of Ag and IL-33. Subsequent experiments were conducted with the lower concentrations of stimulants because 70 pg/ml of IL-33 falls within the range concentrations normally found in synovial fluid from patients with rheumatoid arthritis [40] and less than optimal concentration of Ag (3 ng/ml) was the more likely situation in patients with atopic disease.
Synergistic interactions among signaling pathways
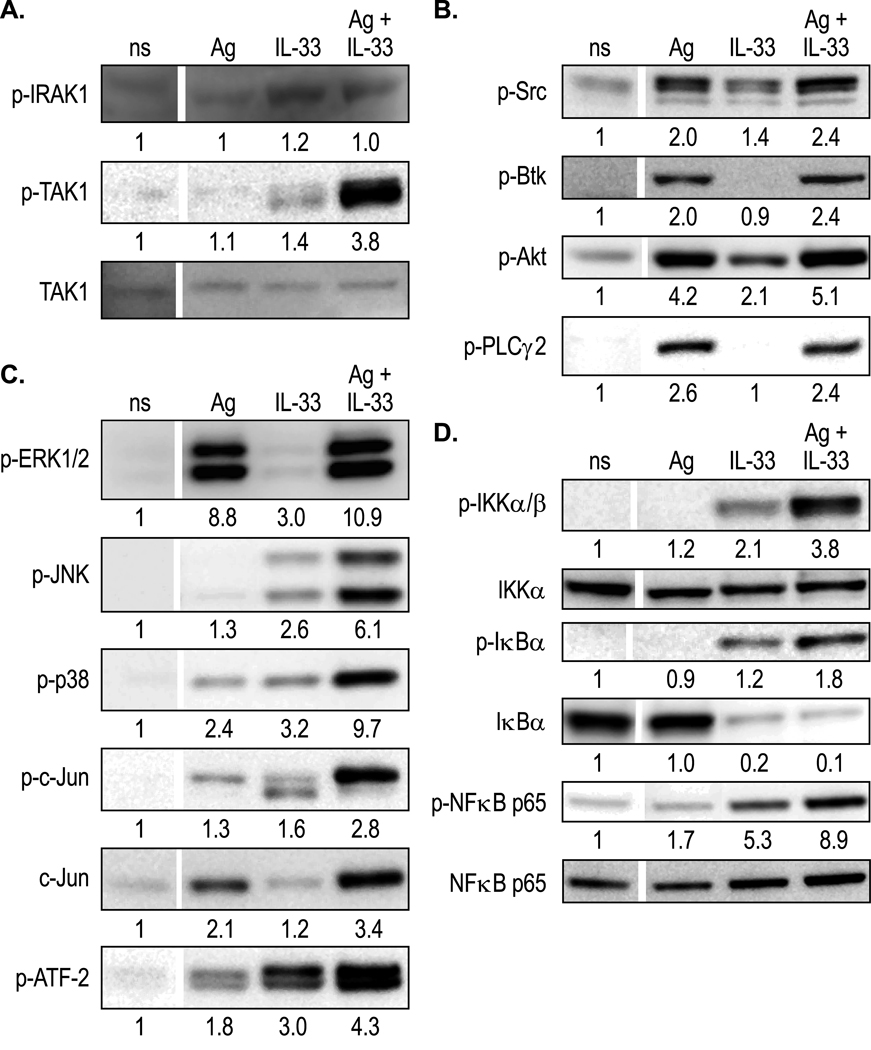
Others have reported that ST2 receptors are expressed in mouse BMMC [15, 16, 41] and we have verified by immunoblotting that both RBL-2H3 cells and BMMC express ST2 receptors (data not shown). Examination of early ST2-mediated signaling events in RBL-2H3 cells indicated that both IRAK1 and TAK1 were phosphorylated following stimulation with 70 pg/ml IL-33 but this was not so with 3 ng/ml Ag (Fig. 4A). Although the extent of IRAK1 phosphorylation under these conditions was modest, substantial phosphorylation was apparent at higher concentrations of IL-33 (data not shown). Of note though, the phosphorylation of IRAK1 was not enhanced by the combination of stimulants whereas the phosphorylation of TAK1 was enhanced several fold on co-stimulation (Fig. 4A). Although 3 ng/ml Ag did not induce detectable phosphorylation of TAK1 such phosphorylation was detectable at higher concentrations of Ag (e.g. 50 ng/ml as shown in supplemental Figure S1). Collectively, the data in Figures 3, 4, and S1 are consistent with the notion that IL-33 operates via ST2 through MyD88, the IRAKs, and TAK1 and that TAK1 is a possible point of convergence of signals from ST2 and FcεRI.
Figure 4.
Pattern of phosphorylation of signaling proteins in RBL-2H3 cells in response to Ag and IL-33 individually or in combination. RBL-2H3 cells sensitized with Ag-specific IgE were stimulated or not (ns) with 3 ng/ml Ag, 70 pg/ml IL-33, or both for 30 min. (A and B) Western blots were prepared from cell lysates for detection of phosphorylated rat IRAK1 (Thr 209), TAK1 (Thr 187), pan Src (Tyr 416), Btk (Ser 180), Akt (Ser 473), and PLCγ2 (Tyr 1217) as well as TAK1 protein. (C and D) Western blots were prepared from cell lysates to detect downstream phosphorylations which included phosphorylated rat ERK1/2 (Thr 202/Tyr 204), JNK (Thr 183/Tyr 185), p38 MAP kinase (Thr 180/Tyr 182), c-Jun (Ser 63), ATF-2 (Thr 69/71), and c-Jun protein. Blots were also probed for phosphorylated IKKα/β (Ser 176/180), IκBα (Ser 32), and NF-κB (Ser 536) and their protein counterparts. The blots shown were typical of results from three or more experiments and the numeric values indicate average density of bands from these experiments after correction for their protein counterparts. For this and the following figure, images were cropped to allow direct visual comparison of bands from non-stimulated and stimulated cells from the same gel.
As expected for FcεRI-mediated signaling events, Ag stimulation resulted in phosphorylation of Src kinase, Bruton’s tyrosine kinase (Btk), protein kinase B (Akt), and PLCγ2 (Fig. 4B). IL-33 also stimulated, although to a lower extent than Ag, phosphorylation of Src kinase and Akt, which is an indicator of PI3K activation, but not Btk or PLCγ2. None of these phosphorylation events were significantly enhanced on co-stimulation of cells.
Several downstream signaling events were also enhanced on co-stimulation of cells with low concentrations of IL-33 and Ag (Fig. 4C and D). Low concentrations of Ag (3 ng/ml) stimulated activating phosphorylations of ERK and p38 MAP kinase (Fig. 4C) and minimally stimulated phosphorylation of JNK (detectable after longer exposure than shown in Fig. 4C). IL-33 (70 pg/ml) induced modest phosphorylation of all three kinases. However, phosphorylation of JNK and p38 MAP kinase, but not ERK, was markedly augmented on stimulation with both Ag and IL-33. Phosphorylation of the downstream targets of JNK and p38 MAP kinase, the transcriptions factor c-Jun and ATF2, as well as the increase in levels of c-Jun that normally occurs after Ag stimulation [42] were either synergistically or additively enhanced by co-stimulation with IL-33 and Ag (Fig. 4C). Some enhancement of phosphorylation was observed throughout the NF-κB pathway to include IKKα/β, IκBα, and NF-κB even though Ag barely stimulated this pathway at low concentrations (Fig. 4D). The levels of IKKα and NF-κB protein remained unchanged but degradation of IκBα by the polyubiquitination pathway [43, 44] was apparent following stimulation by IL-33 alone or in combination with Ag (Fig. 4D).
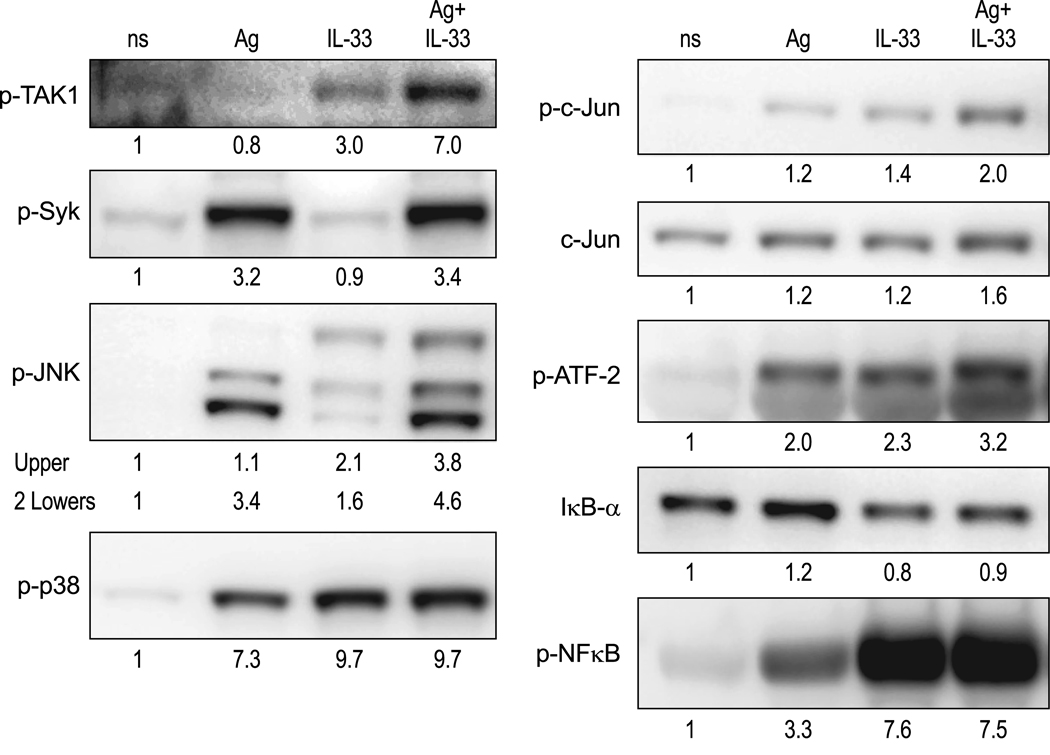
As in RBL-2H3 cells, the phosphorylation of TAK1 in BMMC was enhanced by simultaneous stimulation with 3 ng/ml Ag and 70 pg/ml IL-33 (Fig. 5). In contrast to Ag, IL-33 neither stimulated phosphorylation of Syk, which lies upstream of PLCγ, nor enhanced Syk phosphorylation by Ag (Fig. 5). The signaling pathways that lead to degranulation were not investigated in further detail because of the relatively modest effects of IL-33 on degranulation. With respect to pathways that regulate cytokine production, the phosphorylations of JNK, c-Jun, and ATF-2 were enhanced on costimulation with IL-33 and Ag but to a lesser extent than that observed in RBL-2H3 cells. There was no remarkable synergy in the phosphorylation of NFκB but these and other experiments suggested that NFκB was already optimally phosphorylated by IL-33. As described in a later section, the activation of NFκB was further examined by luciferase-reporter assays.
Figure 5.
Pattern of phosphorylation of signaling proteins in BMMC following stimulation with Ag and IL-33 individually in combination. Experiments were performed with sensitized BMMC as described for RBL-2H3 cells in Figure 4 except that cells were stimulated for 10 min. The blots shown were typical of results from two or more experiments and as for Figure 4 the numeric values indicate average corrected density of bands from these experiments.
Role of the Ca2+/calcineurin/NFAT pathway in cytokine production
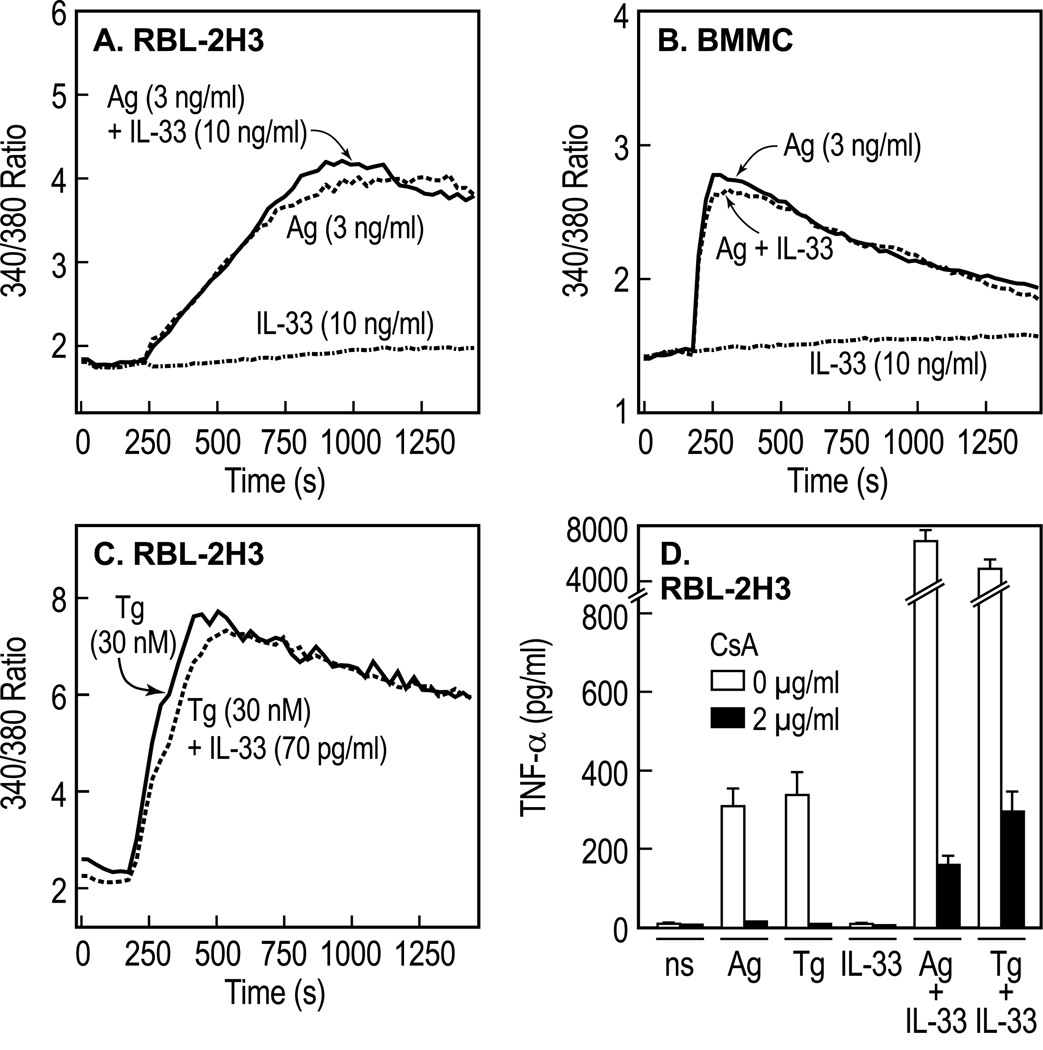
Even at high concentrations, IL-33 (10 ng/ml) failed to elicit an increase in [Ca2+]i or alter the Ca2+-response to Ag in RBL-2H3 cells or BMMC (Fig. 6A and B). The lack of a calcium response was consistent with the failure of IL-33 to induce phosphorylation of Syk (Fig. 5) and PLCγ (Fig. 4B) or degranulation (Fig. 1). An increase in [Ca2+]i is a necessary signal not only for degranulation [10] but also for the activation of the calcineurin/NFAT pathway and cytokine production in Ag-stimulated mast cells [4]. Therefore, experiments were performed to determine whether or not activation of the Ca2+/calcineurin/NFAT pathway acts in synergy with IL-33-activated Ca2+-independent signaling pathways to enhance production of cytokines. Thapsigargin was tested as a potential selective activator of the calcineurin/NFAT pathway because of its unique ability to increase [Ca2+]I by blocking uptake into intracellular Ca2+-stores [10]. A concentration of 30 nM thapsigargin was chosen as this causes little or no stimulation of phospholipid hydrolysis by PLC or PLD, phosphorylation of Akt and MAP kinases, or degranulation (reference [45] and our unpublished data). At this concentration, thapsigargin induced a substantial increase in [Ca2+]i that was unaffected by the presence of IL-33 (Fig. 6C). Also, 30 nM thapsigargin stimulated production of TNFα to the same extent as 3 ng/ml Ag and this production was enhanced by more than 20-fold upon costimulation with 70 pg/ml IL-33 (Fig. 6D). The production of TNFα in response to Ag or thapsigargin, individually or in combination with IL-33, was inhibited by more than 95% by the calcineurin inhibitor, cyclosporin A (Fig. 6D). Therefore, both thapsigargin and Ag interact synergistically with IL-33 in a calcineurin-dependent manner. The presumed downstream activation of NFAT via Ca2+/calcineurin [46] was verified by luciferase reporter assays as demonstrated in the next section.
Figure 6.
IL-33 fails to mobilize Ca2+ but mobilization of Ca2+ upon stimulation with Ag or thapsigargin markedly potentiates TNFα production by IL-33 in a calcineurin-dependent manner. (A–C) RBL-2H3 cells or BMMC, sensitized with Ag-specific IgE, were stimulated with the indicated concentrations of Ag, IL-33, thapsigargin (Tg) or combinations thereof for measurement of changes in [Ca2+]i. (D) Sensitized cells were stimulated with 3 ng/ml Ag, 70 pg/ml IL-33, 30 nM thapsigargin, or the indicated combinations in the absence or presence of 2 µg/ml cyclosporin A (CsA). Cells were stimulated for the indicated times (A–C) or 90 min (D). Values are mean and SEM from 3 separate cultures and are representative or two or more experiments.
Enhancement of transcriptional activities by IL-33
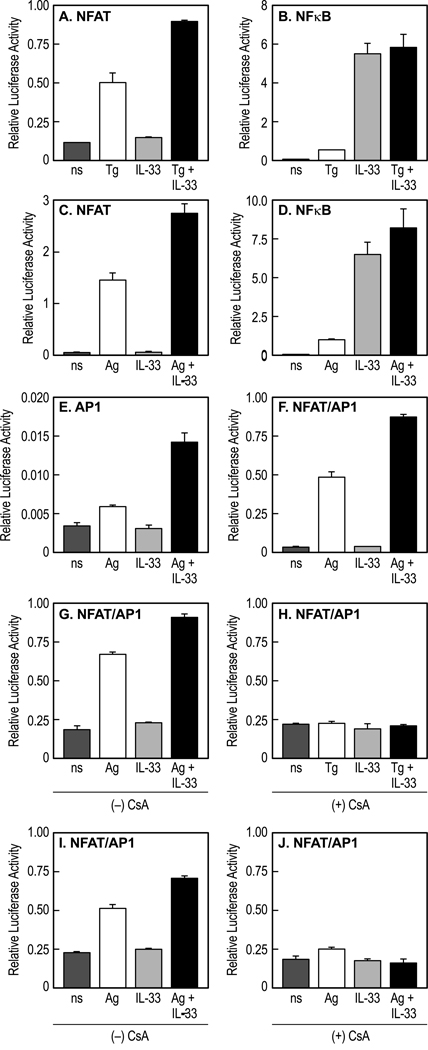
Luciferase reporter assays were used to investigate interactions of low concentrations of IL-33 (70 pg/ml), thapsigargin (30 nM), and Ag (3 ng/ml) at the level of gene transcription (Fig. 7). The action of thapsigargin was selective in that it stimulated NFAT activity (Fig. 7A) but minimally so NF-κB (Fig. 7B) and it failed to stimulate AP-1 (data not shown). In contrast, Ag stimulated all three transcription factors (Fig. 7C–E) as well as NFAT and AP1 in combination (Fig. 7F). IL-33 stimulated NFκB activity (Fig. 7B) but failed to stimulate NFAT transcriptional activity (Fig. 7A and C), as would be expected from the inability of IL-33 to generate a calcium signal. IL-33 also failed to stimulate AP-1 and NFAT/AP-1 activities (Fig. 7E and F). Nevertheless, IL-33 enhanced the activation of NFAT by thapsigargin (Fig. 7A) and Ag (Fig. 7C) and of AP1 and NFAT/AP1 by Ag (Fig. 7E and F). Additional studies with the calcineurin inhibitor, cyclosporin A, reaffirmed that IL-33 potentiated the coactivation of NFAT/AP1 by Ag or thapsigargin and, moreover, this activation was completely blocked by cyclosporin A whatever the mode of stimulation (Fig. 7G–J).
Figure 7.
Stimulation of NFAT, NF-κB, and AP-1 transcriptional activity by thapsigargin, Ag, and IL-33 and the effects of cyclosporin A. Sensitized RBL-2H3 cells were stimulated or not (ns) with 30 nM thapsigargin (Tg), 3 ng/ml Ag, 70 pg/ml IL-33, or combinations thereof (A–F). In an additional set of experiments run in parallel, cyclosporin A (CsA, 2µg/ml) was added or not 10 min before addition of stimulants (G–J). Transcriptional activities were measured 90 min later by the dual luciferase reporter assay. Values are the mean and SEM from three to five separate experiments and are expressed as the ratio of firefly/Renilla luminescence activities. Statistical analyses indicated that stimulation of NFAT, NFκB, AP1, and NFAT/AP1 by Ag and that of NFAT and NFAT/AP1 by thapsigargin were highly significant (p<0.001) as was the stimulation of NFκB by IL-33. The enhancement of NFAT, AP1, and NFAT/AP1 activities by IL-33 in Ag- or thapsigargin- stimulated cells was also highly significant (p<0.001) except in the presence of cyclosporin A.
These results demonstrated that NFAT and AP1 interacted co-operatively and that activation of both these factors was further enhanced by IL-33 even though IL-33 itself activates neither transcription factor at low concentrations. These co-operative interactions likely occur at the level of gene transcription because regulatory signaling events upstream of NFAT, namely phosphorylation of PLCγ and calcium signal, are neither activated nor enhanced by IL-33. In contrast to NFAT and AP1, synergy was not observed in the activation of NFκB. The relatively robust activation of NFκB by IL-33 was not further enhanced by either thapsigargin or Ag (Fig. 7B and D).
Pharmacologic evaluation of the roles of signaling pathways in cytokine production
The possibility that TAK1 (Fig. 4A) participates in the synergistic interaction of Ag and IL-33 was examined by knock-down of TAK1 and by use of the irreversible fungal TAK1 inhibitor, 5Z-7-oxozeaenol [47] in RBL-2H3 cells. The enhanced production of TNFα was indeed much reduced in cells transfected with siRNA against TAK1 (Fig. 8A) or when cells were exposed to 100 nM 5Z-7-oxozeaenol (Fig. 8B). 5Z-7-Oxozeaenol also blocked Ag-induced TNFα production (Fig. 8B) to indicate possible involvement of TAK1 in the responses to Ag as well as IL-33 and was equally effective in suppressing production of TNFα and IL-6 although some decrease in degranulation was noted with high concentrations of 5Z-7-oxozeaenol (Supplemental Figure S2). In this series of experiments, expression of TAK1 was reduced by 62–69% in siRNA-transfected cells and the phosphorylation of TAK1(Thr 187) by >60% in 100 nM 5Z-7-oxozeaenol-treated cells (data not shown). However, we were unable to obtain satisfactory blots with available antibodies against phosphorylated TAK1(Thr 184) leaving the question unresolved as to why suppression of TAK1 activity by 5Z-7-oxozeaenol was more effective than knockdown with siRNA in suppressing TNFα production in Ag-stimulated cells (Fig. 8A and B).
Figure 8.
Suppression of TNFα production by knock-down of TAK1 and pharmacologic inhibitors of various signaling proteins. RBL-2H3 cells (A and B) and BMMC (C–E), sensitized with Ag-specific IgE, were stimulated with Ag and IL-33, individually or in combination, for 3 h for measurement of TNFα by ELISA. (A) Cells were transfected with scrambled control siRNA or anti-TAK1 siRNA 48 h before stimulation with 3 ng/ml Ag or 70 pg/ml IL-33. (B–E) Cells were exposed to the inhibitors for 20 min before addition of stimulants. Cells were stimulated with 3 ng/ml Ag and 70 pg/ml IL-33 (B) or at the concentrations indicated (C–E). Data are mean and SEM of values from 3 separate cultures and are representative of two separate experiments. Differences from controls are indicated thus *, p<0.05; **, p<0.001; ***, not detectable.
Key for inhibitors in panels C–E: Ox, 100 nM 5Z-7-oxozeaenol; Bay, 10 µM Bay 11–7082; 10 µM SB 202190; 20 µM SP 600125; CsA, 2 µg/ml cyclosporin A.
Studies with 5Z-7-oxozeaenol and other pharmacological inhibitors (identified in parentheses) of IκBα (Bay 11–7082, 10 µM), JNK (SB 202190, 10 µM), p38 MAP kinase (SP 600125, 20 µM), and calcineurin (cyclosporin A, 2 µg/ml) suggested that all of these signaling proteins as well as TAK1 regulated production of TNFα to some degree in BMMC (Fig. 8C–D). However, the extent of regulation varied according to mode of stimulation. In Ag-stimulated BMMC, production of TNFα was partially suppressed by inhibitors of TAK1, IκBα, and JNK and totally blocked by inhibitors of p38 MAP kinase and calcineurin (Fig. 8C). In IL-33-stimulated BMMC, TNFα production was totally blocked by inhibition of TAK1, IκBα, and JNK and partially repressed by inhibition of p38 MAP kinase, and was unaffected by inhibition of calcineurin (Fig. 8D). These results suggested that p38 MAP kinase and calcinerin were the preeminent regulators in Ag-stimulated cells and TAK1, IκBα, and, JNK were predominant in IL-33-stimulated BMMC. In co-stimulated BMMC the results were a hybrid of those observed with the individual stimulants. Even though the inhibitors may have unintended and unknown consequences, it would appear that all five signaling molecules play substantial roles in regulating TNFα production on costimulation with Ag and IL-33 (Fig. 8E).
Discussion
We find that low, clinically relevant concentrations of IL-33 substantially amplify Ag-induced production of chemokines and inflammatory cytokines in mast cells. In addition, Ag in combination with IL-33 elicits greater production of cytokines than is achieved with optimally effective concentrations of either stimulant alone (Figs. 2 and 3G–I). However, IL-33 failed to induce a calcium signal (Fig. 6) and degranulation (Fig. 1A). Although the synergy in cytokine production was apparent over a wide range of concentrations of IL-33 and Ag, the underlying mechanisms were investigated at low concentrations of stimulants to reflect as far as possible conditions that might occur in allergic/inflammatory diseases.
At low concentrations, IL-33 by itself stimulated phosphorylation of TAK1, JNK, p38 MAP kinase, c-Jun, ATF2, and robustly so components of the NF-κB pathway (Figs. 4 and 5) as would be expected of ST2-mediated responses. IL-33 also induced phosphorylation of Src kinase and Akt in common with Ag (Fig. 4B). In contrast to Ag, IL-33 failed to induce phosphorylation of LAT (data not shown), Syk (Fig. 5), Btk, and PLCγ (Fig. 4B) which is consistent with the lack of a calcium signal and degranulation. When IL-33 was used in combination with Ag, synergy in signaling was evident at the level of TAK1 (Fig. 4A). The enhanced signaling was then transmitted downstream through the JNK, p38 MAP kinase, c-Jun and, ATF-2 in RBL-2H3 cells (Fig. 4B) although this was less evident in BMMC (Fig. 5). The involvement of TAK1 and these downstream molecules in cytokine production was verified by the inhibitory effects of pharmacological inhibitors (Fig. 8). A pathway that is independent of the canonical IRAK/TRAF6/TAK1 signaling pathway has been detected in IL-33-stimulated basophils [18] but our pharmacological studies suggest that this is not the case in mast cells. In addition, TAK1 is critical for the activation of cells through IL-1R and TLRs [39, 48] but this has not been formally established in the case of ST2. Our studies with anti-TAK1 siRNA and 5Z-7-oxozeaenol (Fig. 8) suggest that TAK1 is indispensible for IL-33 activation of mast cells and for the synergy between IL-33 and Ag. Also, the studies with MyD88-deficient cells (Fig. 3G–I) and visualized phosphorylated proteins (Figs. 4 and 5) suggest that the MyD88/IRAK/ TAK1 pathway is engaged in IL-33-stimulated mast cells as depicted in Figure 9.
Figure 9.
Proposed mechanisms for amplification of cytokine gene transcription on co-stimulation of mast cells with low concentrations of Ag (or thapsigargin) and IL-33. Phosphorylation of TAK1 by IL-33 via the ST2/IL-1 receptor accessory protein (IL-1RAcP) complex, MyD88, the IRAKs, and TRAF6 is amplified by Ag via FcεRI by an as yet undetermined mechanism. This results in enhanced phosphorylation of p38 MAP kinase and JNK and, in turn, phosphorylation of ATF-2 and c-Jun (Fig. 4). Enhancement of phosphorylation events throughout the NFκB pathway were also observed (Fig. 4) but this did not result in detectable augmentation of NFκB transcriptional activity as determined by luciferase assays (Fig. 7). Synergy was not apparent in the phosphorylation of PLCγ (Fig. 4B) and calcium mobilization, whether initiated by Ag or thapsigargin (Tg) (Fig. 6A–C), but amplification of NFAT activation was noted (Fig. 7). Studies with cyclosporin A, an inhibitor of calcineurin that dephosphorylates and hence activates NFAT, indicate that the Ca2+/calcineurin/NFAT pathway is necessary for TNFα-gene transcription (Fig. 6D) and the activation of NFAT/AP1 (Fig. 7G–J) to suggest essential co-operative interactions between NFAT and AP1. In total, the data suggest that Ag and IL-33 together recruit a more effective combination of transcription factors and an essential role for NFAT.
Nothing is known about the role of TAK1 in FcεRI-activated mast cells although our data suggest engagement of TAK1 by FcεRI. Low concentrations of Ag failed to stimulate detectable phosphorylation of TAK1 (Fig. 3A) yet the same concentration of Ag substantially enhanced phosphorylation of TAK1 by IL-33 (Fig. 3A). Also high concentrations of Ag alone can stimulate TAK1 phosphorylation (Supplemental Fig. 1) indicating that strength of the Ag stimulus may be an important determinant. FcεRI-mediated activation of NF-κB is reported to be dependent on PI3K, phosphoinositide-dependent kinase, PKB, and PKC upstream [11] and the Bcl10 and Malt1 downstream [49]. However, Bcl10 and Malt1 regulate activation of TAK1 and the NF-κB pathway in T cell receptor signaling [50] and TAK1 is essential for engagement of the p38 MAP kinase pathway but not for activation of NF-κB during B cell receptor signaling [48]. Therefore, TAK1 appears to be engaged in T cell and B cell receptor signaling although the downstream consequences may differ. Further studies are necessary to define the signaling links between FcεRI and TAK1, if any, as well as the pathways activated through TAK1.
Synergy also appears to be highly dependent on co-operative interactions among transcription factors particularly between NFAT and AP1. An essential role for NFAT in the activation of AP1 (Fig. 7G–I) and production of TNFα (Fig. 8) is indicated by the inhibitory actions of cyclosporin A. IL-33 activates neither of these transcription factors but it does enhance their activation by mechanisms that need to be clarified. For example, the NFAT pathway [4] is selectively activated by low concentrations of thapsigargin but this activation is augmented by IL-33 (Fig. 7). IL-33 also enhanced the Ag-induced activation of NFAT, AP-1, or NFAT/AP-1 in combination (Fig. 7). As a result production of TNFα is substantially enhanced whether cells are stimulated by thapsigargin or Ag in the presence of IL-33 (Fig. 6D). The relatively robust stimulation of NF-κB transcriptional activity by IL-33, however, was not further augmented by Ag. Examination of the genomic sequences by the rVISTA [51] and Mulan [52] programs indicated highly conserved putative binding sites for NFAT, AP-1, and NF-κB in the promoter regions of TNFα and IL-13 genes and for AP-1 and NF-κB in the IL-6 gene. The AP-1- and NFAT- binding sites are located in close proximity at several sites in the TNFα gene and synergistic cooperative interaction between AP1 and NFAT is well documented [53]. In addition, NFAT and NF-κB show considerable functional overlap in co-ordinating induction of many cytokines [54]. Therefore, it seems possible that the combination of Ag and IL-33 brings into play a much more effective combination of transcription factors for production of least a subset of cytokines than do the individual stimulants.
The studies with pharmacologic inhibitors, in general, support the notion that the hybridization of the different signaling mechanisms for Ag and IL-33 results in robust augmentation of cytokine production (Fig. 8C–D). The studies indicate that the production of TNFα by Ag is absolutely dependent p38 MAP kinase and the calcineurin/NFAT pathways and only partially dependent on the NFκB, JNK, and TAK1 pathways whereas the effects of IL-33 are absolutely dependent on the latter pathways. In contrast, the enhanced production of TNFα by the combination of Ag and IL-33 becomes almost totally dependent on all pathways.
There are discrepancies in the literature in regard to IL-33 and degranulation. Our finding that IL-33 is unable to stimulate degranulation is consistent with the findings of other workers [13–16] with the exception of Melendez and coworkers who reported that IL-33 induces an increase in [Ca2+]i and degranulation in mast cells [23]. We find, however, that in addition to RBL-2H3 cells and BMMC, cultured human mast cells exhibit no such responses to IL-33 under the exact conditions described by these workers. (Our unpublished data, Shoko Iwaki, Alasdair M. Gilfillan, and Michael A. Beaven). Therefore, we conclude that in the presence of low concentrations of Ag and IL-33, mast cells might undergo minimal degranulation but produce an abundance of inflammatory cytokines. As noted for basophils [17, 20], IL-33 may modestly enhance FcεRI-mediated degranulation in mast cells even though it is inactive by itself (Fig. 1). The reason for this enhancement is unclear. IL-33 had no palpable effect on FcεRI-mediated phosphorylation of PLCγ or calcium signal (Figs. 4B and 6A and B) and presumably the enhancement of degranulation was dependent on other pathways.
In conclusion, IL-33 strongly potentiates production of inflammatory cytokines by Ag, and vice versa, by mechanisms that involve synergistic interactions at the level of TAK1 and gene transcription (as depicted in Fig. 9). The synergistic pulse appears to propagate from TAK1 through the JNK/c-Jun and p38 MAP kinase/ATF-2 pathways. IL-33 is unable to initiate signals that are essential for mobilizing Ca2+, degranulation, and activation of NFAT. However, the activation of NFAT by Ag or thapsigargin provides a strong signal for enhancing cytokine production possibly by acting in combination with other transcription factors such as AP-1. IL-33 may thus markedly amplify production of cytokines when mast cells are stimulated with low concentrations of Ag with possible implications in severity of mast cell-related diseases.
Materials and Methods
Reagents
Reagents were obtained from the following sources: materials for culture medium from GIBCO/Invitrogen (Carlsbad, CA) and MediaTech, Inc (Manassas, VA) and fetal calf serum from HyClone/Thermo Scientific (Logan, VT); the DNP human serum albumin (Ag) and Ag-specific IgE from Sigma-Aldrich (St. Louis, MO); murine recombinant IL-33 from R&D Systems (Minneapolis, MN); thapsigargin from Calbiochem (Gibbstown, NJ); anti-TAK1 siRNA (ON-TARGETplus SMARTpool, Rat Map3k7) from Dharmacon/Thermo Scientific (Layfayette, CO); all primary antibodies from Cell Signaling Technology, Inc. (Danvers, MA) except for antibodies against IRAK1 and ST2 from AnaSpec, Inc. (San Jose, CA) and R&D Systems, respectively; secondary antibodies from Sigma-Aldrich or GE Healthcare/Amersham (Piscataway, NJ); enzyme inhibitors from Calbiochem except Bay 11–7082 and cyclosporin A from Biomol (Plymouth Meeting, PA) and Sigma-Aldrich, respectively; Fura-2 AM ester from Molecular Probes/Invitogen (Eugene, OR); ELISA kits from Invitrogen (Carlsbad, CA) except IL-33 from R&D systems; p-nitrophenyl N-acetyl-β-glucosaminide from Sigma-Aldrich; Procarta Cytokine Assay Kits from Panomics/Affymetrix (Santa Clara, CA); and Dual-Luciferase Reporter Assay Systems for NFAT, AP-1, and NF-κB from Promega (Madison, WI). The plasmid (NFAT/AP-1 – 3x luciferase) was obtained from Addgene (Addgene plasmid 11783) as provided by Anjana Rao and described in reference [55].
Isolation of BMMC from wild type and MyD88−/− mice
Mouse BMMC were generated from bone marrow of wild type (C57BL/6) and MyD88 knockout mice by culture for 4–8 weeks in RPMI 1640 medium supplemented with 10% fetal calf serum, 30 ng/ml of murine IL-3 (PeproTech, Rocky Hill, NJ), 4 mM of glutamine, 25 mM HEPES, 1x non-essential amino acids, 50 µM of 2-mercaptoethanol, and 100 mM of sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin. All the procedures were done under guidelines of the Institutional Animal Care and Use Committee from the National Institutes of Health, USA, and the approval of the Animal Care committee of the Federal University of Minas Gerais, Brazil. Cells were cultured at 37°C in 5% CO2 incubator and medium was changed twice a week. After 4 weeks, the cell culture consisted of >90% mature BMMC, and thereafter (4–8 weeks) used for experiments.
Cell culture and experimental conditions
Cells were sensitized overnight with anti-DNP IgE (50 ng/ml), either in suspension (BMMC) or in multiwell plates (RBL-2H3 cells), in complete growth medium supplemented with fetal calf serum (10% for BMMC, 15% for RBL-2H3 cells), glutamine, antibiotic, and antimycotic agents. For each experiment, cells were washed three times in the required medium. To measure degranulation, cells were transferred to 24-well plates (0.5 × 106 cells/0.5 ml/well) in a PIPES-buffered medium (119 mM NaCl, 5 mM KCl, 5.6 mM glucose, 0.4 mM MgCl2, 25.0 mM PIPES, 40 mM NaOH, 1 M CaCl2, 10%w/v bovine serum albumin). For assay of cytokines by ELISA, cells were also transferred to 24-well plates at the same density but in complete growth medium. For immunoblotting, cells were plated in 60 mm2 dishes (2.5 × 106 cells/5 ml). Cells were incubated for 10 min at 37° C before addition of inhibitors or stimulants. Inhibitors were added 20 min before addition of stimulants.
Measurement of degranulation, cytokines, and chemokines
Degranulation was determined by a colorimetric assay of the granule marker, β-hexosaminidase, as previously described [9]. Following stimulation of cells, 10 µl of medium and cell lysates (in 0.1% Triton X) were transferred to 96-well plates for assay of β-hexosaminidase activity by measurement of release of p-nitrophenol from p-nitrophenyl N-acetyl-β-D-glucosaminide. Values were expressed as the percentage of intracellular β-hexosaminidase that was released into the medium. Statistical differences for these and all other assays were determined by ANOVA by use of the Prism software.
Individual cytokines were assayed by ELISA by use of commercial kits. Multiple cytokines was assayed simultaneously with the Procarta Protein Cytokine Assay Kit (Panomics/Affymetrix) in individual cultures (5 × 105 cells/0.5 ml). This procedure involves separation of tagged antibody-coated beads. The cytokines and chemokines assayed included mouse IL-1α, -1β, -2, -3, -4, -5, -6, -9, -12p40, -12p70, -13, -17, and -23, TNFα, TGF-β, INF-γ, GM-CSF, vascular endothelial growth factor, MIP-1α, eotaxin, MCP-1, MCP-3, keratinocyte-derived cytokine, RANTES and interferon-γ -inducible protein 10. Media samples were collected 3 h after addition of Ag. The samples were processed in the NHLBI FACS Core facility.
Immunoblotting
Following stimulation cells were washed twice with cold phosphate buffered saline and lysed in lysis buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 25 µg/ml leupeptin, 25 µg/ml aprotinin, 2 µg/ml pepstatin, protease inhibitor cocktail [1 tablet / 10 ml, Roche Applied Science, Indianapolis, IN]). Total protein content was assayed by use of the BCA™ kit (Thermo Scientific) and individual proteins were separated by SDS-PAGE. Blots were probed with the indicated primary and peroxidase-labeled secondary antibodies and visualized by chemiluminescence. Desitometry was performed with the Kodak 2000 program.
Measurement of [Ca2+]i
Changes in [Ca2+]i were monitored by use of Fura-2 AM ester as described [56]. RBL-2H3 cells and BMMC were sensitized with IgE overnight as described above and then loaded with Fura-2 AM for 30 min at 37 °C in growth medium. Cells were placed in a 96-well black culture plates (10,000 cells/well) (CulturPlate-96 F, PerkinElmer Life Sciences, Waltham, MA) in a PIPES-buffered medium (see previous section) containing 0.3 mM sulfinpyrazone after washing cells with the same medium. Fluorescence was measured at two excitation wavelengths (340 and 380 nm) and an emission wavelength of 510 nm in a Wallac Victor plate reader (PerkinElmer Life Sciences). The ratio of the fluorescence readings was calculated following subtraction of the autofluorescence of the cells.
Dual Luciferase reporter assay
RBL-2H3 cells (2 × 106 cells) were transfected with 2 µg pGL4.10 luciferase vector with or without the designated constructs along with 0.5 µg pGL4.74TK Renilla luciferase as an internal control (see Reagents). Cells (2 × 105 cells) were incubated for 18 h in 24-well plates before addition of stimulants. Four hours later cells were washed with PBS and lysed in 1 x passive lysis buffer (Promega) for assay of firefly and Renilla luciferase activities in the Wallac Victor multiplate reader. Values were calculated as the ratio of firefly/Renilla luminescence.
Supplementary Material
Phosphorylation of TAK1 by Ag. RBL-2H3 cells, sensitized with Ag-specific IgE overnight, were stimulated or not (ns) with the indicated concentration (ng/ml) of Ag or 70 pg/ml IL-33 for 30 min. Western blots were prepared from cell lysates for detection of phosphorylated TAK1 (Thr 187). The blots were representative of two such experiments.
Effects of the TAK1 inhibitor, 5Z-7-oxozeaenol, on degranulation and production of TNFα and IL-6 in RBL-2H3 cells. Cells were exposed to the indicated concentrations of 5Z-7-oxozeaenol for 10 min before stimulation with 3 ng/ml Ag and 70 pg/ml IL-33 for 15 min for measurement of release of the granule marker, β-hexosaminidase, or 3 h for measurement of production of TNFα and IL-6. Data are mean and SEM of values from 3 separate cultures.
Acknowledgments
This work was supported by the Intramural Research Program, NHLBI, National Institutes of Health M.V.A, S. I., and M. A. B); National Institutes of Health grant RO1 TW006612 (GRIP) (M.V.A.); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil (M.V.A., C.R., R.T.G., J.R.C-M.). M. V. A. performed much of this work while on leave from the Faculty of Medicine, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil.
The authors also thank Dr. Mauro M. Texeira, Department of Biochemistry and Immunology, Federal University of Minas Gerais, Brazil for suggestions and initial supply of IL-33 and Leigh Samsel, FACS Core Facility, National Heart, Lung and Blood Institute for assistance with the Affymetrix cytokine assay system.
Abbreviations
- Akt
protein kinase B
- Bcl10
B cell lymphoma 10
- BMMC
bone marrow–derived mast cells
- [Ca2+]i
concentration of free cytosolic Ca2+
- IKK
inhibitor of the NF-κB kinase complex
- IRAK
IL-1 receptor-associated kinase
- PK
protein kinase
- PL
phospholipase
- RBL-2H3
rat basophilic leukemia mast cell
- TAK1
TGFβ-activated kinase1
- TIR
Toll/interleukin-1 receptor
- TRAF6
TNF receptor-associated factor 6
Footnotes
Disclosure
All authors declare that they have no competing financial interests.
References
- 1.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur.J.Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat.Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn HS. and Gilfillan,A.M., G protein-coupled receptors and the modification of FceRI-mediated mast cell activation. Immunol.Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FceRI and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: Recent Advances. Annu.Rev.Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 6.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat.Rev.Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 7.Rivera J. and Gilfillan,A.M., Molecular regulation of mast cell activation. J.Allergy Clin.Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Abramson J, Pecht I. Regulation of the mast cell response to the type 1 Fce receptor. Immunol.Rev. 2007;217:231–254. doi: 10.1111/j.1600-065X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells: Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J.Biol.Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 10.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Crit.Rev.Immunol. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalesnikoff J, Baur N, Leitges M, Hughes MR, Damen JE, Huber M, Krystal G. SHIP negatively regulates IgE + antigen-induced IL-6 production in mast cells by inhibiting NFkB activity. J Immunol. 2002;168:4737–4746. doi: 10.4049/jimmunol.168.9.4737. [DOI] [PubMed] [Google Scholar]
- 12.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FceRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 13.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J.Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 14.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 15.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FceRI signals. J.Leukoc.Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 16.Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1-family member IL-33. Blood. 2008 doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38a–dependent pathway. J.Leukoc.Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int.Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 20.Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J.Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 21.Silver MR, Margulis A, Wood N, Goldman SJ, Kasaian M, Chaudhary D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm.Res. 2010;59:207–218. doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 22.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc.Natl.Acad.Sci.U.S.A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pushparaj PN, Tay HK, H'ng SC, Ptman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc.Nat.Acad.Sci.USA. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc.Natl.Acad.Sci.U.S.A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat.Rev.Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 26.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin.Exp.Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 27.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc.Natl.Acad.Sci.U.S.A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu D, Jiang HR, Li Y, Pushparaj PN, Kurowska-Stolarska M, Leung BP, Mu R, Tay HK, McKenzie AN, McInnes IB, Melendez AJ, Liew FY. IL-33 exacerbates autoantibody-induced arthritis. J.Immunol. 2010;184:2620–2626. doi: 10.4049/jimmunol.0902685. [DOI] [PubMed] [Google Scholar]
- 29.Hsu C-L, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS.One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol.Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 31.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J.Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 32.Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc.Natl.Acad.Sci.USA. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer G, Lipsky BP, Smithgall MD, Meininger D, Siu S, Talabot-Ayer D, Gabay C, Smith DE. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine. 2008;42:358–364. doi: 10.1016/j.cyto.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Gadina M, Jefferies CA. IL-33: a sheep in wolf's clothing? Sci.STKE. 2007;2007:e31. doi: 10.1126/stke.3902007pe31. [DOI] [PubMed] [Google Scholar]
- 35.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat.Rev.Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IkB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol.Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuyama Y, Okazaki H, Tamemoto H, Kimura H, Kamata Y, Nagatani K, Nagashima T, Hayakawa M, Iwamoto M, Yoshio T, Tominaga SI, Minota S. Increased Levels of Interleukin 33 in Sera and Synovial Fluid from Patients with Active Rheumatoid Arthritis. J.Rheumatol. 2009 doi: 10.3899/jrheum.090492. [DOI] [PubMed] [Google Scholar]
- 41.Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J.Immunol. 1998;161:4866–4874. [PubMed] [Google Scholar]
- 42.Razin E, Szallasi Z, Kazanietz MG, Blumberg PM, Rivera J. Protein kinase C-b and C-e link the mast cell high-affinity receptor for IgE to the expression of c-fos and c-jun. Proc.Natl.Acad.Sci.USA. 1994;91:7722–7726. doi: 10.1073/pnas.91.16.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, Kato RM, Kang S, Patrone L, Wall R, Teitell M, Leitges M, Kawakami T, Rawlings DJ. PKC-b controls IkB kinase lipid raft recruitment and activation in response to BCR signaling. Nat.Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 44.Hayden MS, Ghosh S. Shared principles in NF-kB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Cissel DS, Fraundorfer PF, Beaven MA. Thapsigargin-induced secretion is dependent on activation of a cholera toxin-sensitive and a phosphatidylinositol-3-kinase-regulated phospholipase D in a mast cell line. J.Pharmacol.Exp.Ther. 1998;285:110–118. [PubMed] [Google Scholar]
- 46.Mascarell L. and Truffa-Bachi,P., New aspects of cyclosporin a mode of action: from gene silencing to gene up-regulation. Mini.Rev.Med.Chem. 2003;3:205–214. doi: 10.2174/1389557033488150. [DOI] [PubMed] [Google Scholar]
- 47.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J.Biol.Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 48.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat.Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 49.Klemm S, Gutermuth J, Hültner L, Sparwasser T, Behrendt H, Peschel C, Mak TW, Jakob T, Ruland J. The Bcl10-Malt1 complex segregates FceRI-mediated nuclear factor-kB activation and cytokine production from mast cell degranulation. J.Exp.Med. 2006;203:337–347. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol.Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 51.Loots GG. and Ovcharenko,I., rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–W221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loots GG, Ovcharenko I. Mulan: multiple-sequence alignment to predict functional elements in genomic sequences. Methods Mol.Biol. 2007;395:237–254. [PMC free article] [PubMed] [Google Scholar]
- 53.Mácian F, López-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 54.de Lumley M, Hart DJ, Cooper MA, Symeonides S, Blackburn JM. A biophysical characterisation of factors controlling dimerisation and selectivity in the NF-kB and NFAT families. J.Mol.Biol. 2004;339:1059–1075. doi: 10.1016/j.jmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 55.Macian F, Garcia-Rodriguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of FceRI-mediated mast cell activation by Kit. J.Biol.Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phosphorylation of TAK1 by Ag. RBL-2H3 cells, sensitized with Ag-specific IgE overnight, were stimulated or not (ns) with the indicated concentration (ng/ml) of Ag or 70 pg/ml IL-33 for 30 min. Western blots were prepared from cell lysates for detection of phosphorylated TAK1 (Thr 187). The blots were representative of two such experiments.
Effects of the TAK1 inhibitor, 5Z-7-oxozeaenol, on degranulation and production of TNFα and IL-6 in RBL-2H3 cells. Cells were exposed to the indicated concentrations of 5Z-7-oxozeaenol for 10 min before stimulation with 3 ng/ml Ag and 70 pg/ml IL-33 for 15 min for measurement of release of the granule marker, β-hexosaminidase, or 3 h for measurement of production of TNFα and IL-6. Data are mean and SEM of values from 3 separate cultures.