Abstract
Upon vascular injury, platelets are activated by adhesion to adhesive proteins like von Willebrand factor and collagen, or by soluble platelet agonists like ADP, thrombin, and thromboxane A2. These adhesive proteins and soluble agonists induce signal transduction via their respective receptors. The various receptor-specific platelet activation signaling pathways converge into common signaling events, which stimulate platelet shape change, granule secretion, and ultimately induce the “inside-out” signaling process leading to activation of the ligand binding function of integrin αIIbβ3. Ligand binding to integrin αIIbβ3 mediates platelet adhesion and aggregation and triggers “outside-in” signaling, resulting in platelet spreading, additional granule secretion, stabilization of platelet adhesion and aggregation, and clot retraction. It has become increasingly evident that agonist-induced platelet activation signals also crosstalk with integrin “outside-in” signals to regulate platelet responses. Platelet activation involves a series of rapid positive feedback loops that greatly amplify initial activation signals, and enable robust platelet recruitment and thrombus stabilization. Recent studies have provided novel insight into the molecular mechanisms of these processes.
Blood platelets play important roles in hemostasis, thrombosis, wound healing, atherosclerosis, inflammation, immunity, and tumor metastasis 1–4. Of these functions, the primary physiological function of platelets is to form hemostatic thrombi that prevent blood loss and maintain vascular integrity. This function must be tightly regulated, because dysregulated thrombus formation (thrombosis) causes blockage of blood vessels leading to ischemia. Thrombotic diseases such as heart attack and ischemic stroke are a leading cause of mortality in the modern world. Thus, platelets in normal circulation are in a non-adherent “resting” state, and become activated at sites of vascular injury following exposure to immobilized adhesive proteins or soluble platelet agonists. The signaling process that occurs during platelet activation can be classified into three stages: (1) The interaction of agonists with their respective platelet receptors and receptor-mediated early platelet activation signaling; (2) the intermediate common signaling events; and (3) integrin activation (inside-out signaling) and outside-in signaling. However, platelet activation is a dynamic process involving multiple feedback loops and crosstalk between different pathways. In particular, platelets rely on endogenous secondary signal amplification mechanisms and their regulation to achieve a relevant level of response to vascular injury.
In the past three decades, remarkable progress has been made in identifying the fundamental mechanisms of platelet function and signaling pathways of platelet activation, which has greatly facilitated the development of anti-platelet therapeutics for preventing and treating thrombotic diseases 1, 2. However, the agents that block fundamental platelet functions such as integrin blockers, while having potent anti-thrombotic effects, cause bleeding in ~0.5–1.5% of patients receiving such compounds. The currently used cyclooxygenase inhibitor (aspirin) and ADP receptor P2Y12 antagonists are also associated with problems such as drug-resistance and bleeding side effects. A better understanding of intracellular signaling during platelet adhesion and activation will be helpful for the development of new generations of anti-platelet therapies.
Adhesion receptor-mediated platelet activation and signaling
Platelet adhesion receptors are the key initiators of platelet activation at sites of vascular injury where platelets become exposed to adhesive proteins in the matrix or on endothelial cells (Fig. 1). Interestingly, despite significant differences in their functions and signaling pathways, several major platelet adhesion receptors share many similarities in their signal transduction mechanisms. For example, signal transduction through the glycoprotein Ib-IX-V complex (GPIb-IX), glycoprotein VI (GPVI), and integrins all involve Src family kinases (SFKs), phosphoinositide 3-kinases (PI3Ks), and the ITAM signaling pathway.
Fig. 1.
Signaling pathways of three major platelet adhesion receptors.
Collagen/GPVI-mediated platelet activation signaling
Platelets express several collagen receptors 5. Of these receptors, integrin α2β1 is important for platelet adhesion to collagen, whereas GPVI is required for collagen-induced platelet activation. GPVI is a member of the immunoglobulin superfamily and is non-covalently coupled to Fc Receptor γ chain (FcRγ) 6. Upon crosslinking of GPVI by its ligand, the immunoreceptor tyrosine-based activation motif (ITAM) (a conserved sequence, YxxL/I-X6–8-YXXL/I, originally found to be important in T cell antigen receptor signaling 7) within the FcRγ cytoplasmic domain is tyrosine phosphorylated by SFKs (mainly Lyn and Fyn) bound to the cytoplasmic domain of GPVI 8, 9. SFK activation is important for GPVI-mediated platelet activation and involves CD148, a receptor-like protein tyrosine phosphatase (PTP), which was reported to dephosphorylate the C-terminal inhibitory tyrosines of SFKs10.
ITAM phosphorylation leads to binding and activation of the tyrosine kinase Syk, which phosphorylates downstream targets such as the transmembrane adapter linker for activated T-cells (LAT) and the Src homology (SH) 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76). This induces the formation of a signaling complex including LAT, SLP-76, Btk, Gads and phospholipase Cγ2 (PLCγ2), which further activates PLCγ2 11, 12, leading to TXA2 synthesis, granule secretion, and integrin activation (Fig. 1). The PH domain of PLCγ2 also interacts with the PI3K product phosphatidylinositol 3,4,5-trisphosphate (PI 3,4,5P3), which facilitates PLCγ2 recruitment to the plasma membrane and activation 13–15. ITAM signaling is negatively regulated by signals transduced from PECAM-1 16.
VWF/GPIb-IX-mediated platelet activation
Under high shear rate flow conditions present in arteries and arterioles, initial platelet adhesion requires the binding of immobilized VWF to its platelet receptor, GPIb-IX 17–19. VWF forms a so-called “catch-bond” or “flex-bond” with the ligand binding domain of GPIb-IX 20, 21, allowing transient platelet adhesion under high shear stress. VWF/GPIb-IX interaction also induces platelet activation signaling events, leading to integrin activation and integrin-dependent stable platelet adhesion and aggregation (for a review, see ref 19). In addition, GPIb-IX binds thrombin and sensitizes platelets to low dose thrombin.
There has been evidence that GPIb-IX is associated with the ITAM receptors Fcγ receptor IIA (FcγRIIA) 22 or FcRγ 23. Genetic deletion of ITAM signaling molecules, such as FcRγ, Syk, LAT, SLP76 and Btk, abolishes the TXA2- and secretion-dependent second wave of platelet aggregation induced by VWF/botrocetin in washed mouse platelets 24, 25. However, loss of FcRγ and LAT does not appear to affect GPIb-IX-dependent integrin activation and TXA2 synthesis 24, 26, both of which involve the mitogen-activated protein kinase (MAPK) pathway 27–29. Similarly, Syk is not required for GPIb-IX- and integrin-dependent stable platelet adhesion to VWF under shear stress 30. Considering the importance of the ITAM pathway in granule secretion and integrin outside-in signaling, it likely functions as an important signal amplification mechanism in GPIb-IX signaling.
The cytoplasmic domain of the GPIbα chain reportedly interacts with SFKs and PI3Ks 23, 31, which are important for transmitting the “early” activation signals from GPIb-IX 24, 26, 31–33, leading to calcium elevation 34 and integrin activation independent of other receptors 26, 30, 33. The SFK Lyn is required for activation of PI3K and its downstream effector Akt, leading to integrin activation 24, 30, 33. Interestingly, VWF/GPIb-IX interaction induces elevation of intracellular cGMP levels 35, 36 and sequential activation of cGMP–dependent protein kinase (PKG) and the MAPKs, p38 and extracellular stimuli responsive kinase (ERK) 27, 28, 35. The cGMP signaling pathway is activated downstream from the Lyn/PI3K/Akt pathway 30, 33, which is known to activate nitric oxide (NO) synthase (NOS). NO may be important for VWF-induced cGMP elevation 35, 36, although SFK-dependent, NO-independent soluble guanylyl cyclase activation has been proposed by one group 37. A role for the PKG/MAPK signaling pathway in GPIb-IX-mediated integrin activation has been shown using inhibitors and knockout mice 27, 28, 35. Together, these data reveal that the Lyn/PI3K/Akt/NO/cGMP/PKG/MAPK signaling pathway plays an important role in GPIb-IX-mediated platelet activation. It is important to note that the role of NO and cGMP in platelet activation is biphasic 35. The low concentrations of NO/cGMP synthesized during platelet activation are stimulatory whereas high concentrations of NO and cGMP inhibit platelet activation. The biphasic role of the NO/cGMP pathway may serve to stimulate robust hemostatic thrombus formation at sites of vascular injury while preventing overgrowth of the thrombus.
Platelet activation and signaling mediated by G protein-coupled receptors
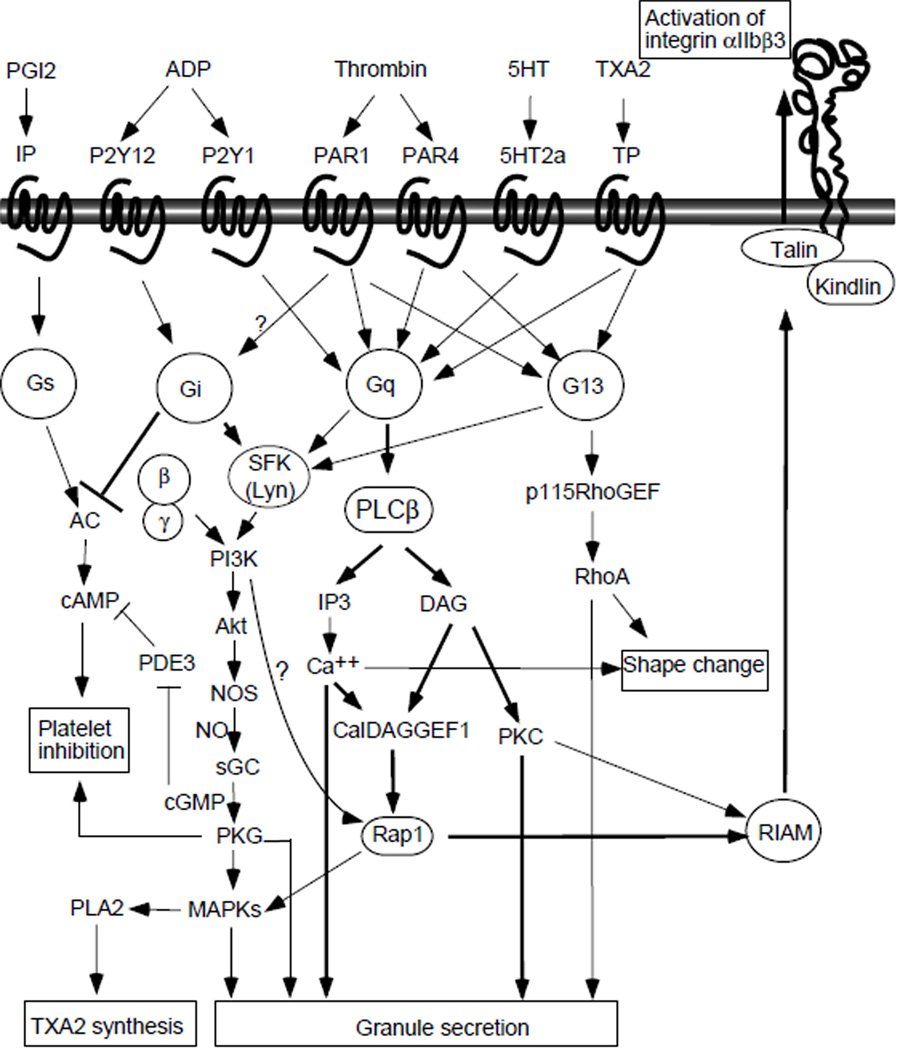
A variety of soluble platelet agonists are released from damaged cells (e.g. ADP), produced during coagulation (e.g. thrombin) and inflammation (e.g. platelet-activating factor (PAF)), and enriched in atherosclerotic plaques (e.g. lysophosphatidic acid (LPA)), and they play a critical role in platelet activation and thrombus formation 38. Equally important, soluble platelet agonists, such as TXA2, ADP, and serotonin, are released from stimulated platelets, which serve to amplify platelet activation and recruit circulating platelets. These agonists activate platelets via G-protein-coupled receptors (GPCR), a family of seven-transmembrane domain receptors that transmit signals through heterotrimeric G proteins (Fig 2).
Fig. 2.
GPCR-coupled platelet activation signaling.
The heterotrimeric G proteins consist of three subunits; α, β and γ, which bind to GPCRs in a α/β/γ complex. Upon receptor ligation, the α subunit is converted from a GDP-bound form to the active GTP-bound form. Activated Gα subunits dissociate from the receptor and from the β/γ complex and interact with specific downstream targets to transmit GPCR signals 38. The β/γ complex can also interact with and activate downstream effectors, including PI3Kγ 39. Based on the similarity of α subunits, G proteins can be divided into 4 subfamilies: Gq/G11, G12/G13, Gi/Go/Gz, and Gs, each of which is coupled to selective receptors and downstream effectors (Fig. 2) 38. Platelets express Gq, G12/13, Gi/z, and Gs. G proteins in platelets are coupled to agonist receptors that stimulate platelet activation, with the exception of Gs, which is coupled to receptors for physiological platelet inhibitors (PGI2 and adenosine) that mediate inhibitory signals by stimulating adenylyl cyclase-dependent cAMP synthesis (Fig. 2). Thrombin-induced platelet activation is mediated via a dual system of G protein coupled protease-activated receptors (PAR): PAR1 and PAR4 in humans 40, and PAR3 and PAR4 in mice 41. PAR3 appears to sensitize PAR4 to thrombin42, 43. PAR1 and PAR4 directly couple to Gq and G12/13 41, and possibly Gi 44, 45. TXA2 activates platelets via the TXA2 receptor TP, which couples to Gq and G13 46, 47. Serotonin recognizes the Gq-coupled receptor 5HT2A 38. ADP induces platelet activation via P2Y1 (Gq-coupled) and P2Y12 (Gi-coupled) 38, 48. The epinephrine receptor (α2) in platelets is reportedly coupled to Gz, another Gi subtype 49.
Gq-mediated signaling
Gq has been shown to transmit cellular signals mainly through its interaction and stimulation of phospholipase C-β (PLCβ). Gq signaling is important for GPCR-stimulated platelet granule secretion, integrin activation, and consequent platelet aggregation 50. Deletion of Gq causes defects in platelet secretion and aggregation in response to a variety of agonists including thrombin, ADP, TXA2 analog U46619, and even collagen (probably due to the dependence of the collagen signaling pathway on TXA2) 50. In addition, Gq is important in ADP-induced platelet shape change 50, probably by stimulating calcium/calmodulin- and/or RhoA-dependent contractile signaling 51.
Gi-mediated signaling
Although Gq is required for platelet activation induced by GPCR agonists, it is neither sufficient for platelet aggregation induced by ADP nor for optimal platelet response induced by TXA2 or low dose thrombin. The Gi-coupled ADP receptor, P2Y12 52, 53, is also required for ADP-induced platelet activation and promotes platelet activation induced by TXA2 and low dose thrombin 45, 54. However, it remains controversial whether the thrombin receptors are coupled to Gi directly or indirectly via P2Y12 44, 45. The role of Gi-coupled receptors in promoting platelet activation is consistent with the inhibitory effect of Gi on cAMP synthesis, which relieves the inhibitory effect of cAMP-dependent protein kinase on platelet activation. Importantly, P2Y12-coupled Gi is a major mechanism responsible for the activation of PI3K, particularly β/γ subunit-activated PI3Kγ, in platelets 55, 56 and subsequent activation of the small GTPase Rap1b 57, 58, a critical mediator of integrin activation.
G13 signaling
Platelets express both Gα12 and Gα13 59; however, only Gα13 knockout platelets show reduced and unstable platelet aggregation induced by low dose thrombin and the TXA2 analog U46619. Gα13 knockout platelets have a reduction in granule secretion induced by U46619, but not thrombin 60. Shape change induced by these agonists is also reduced in Gα13 knockout platelets. GTP-bound Gα13 interacts with and activates guanine nucleotide exchange factors (GEF) for the small G protein RhoA, such as p115RhoGEF, which subsequently converts RhoA into the active GTP-bound form 61. RhoA activates Rho kinase, which phosphorylates and inhibits myosin light chain (MLC) phosphatase 62, thus enhancing MLC phosphorylation and MLC-dependent contraction. G13 therefore stimulates platelet shape change and granule secretion 62. Interestingly, Gα13 deficiency causes more dramatic defects in platelet adhesion and in hemostasis and thrombosis in vivo, relative to its effect on integrin activation, aggregation and granule secretion in vitro, suggesting an additional role of Gα13 in platelet function 60. Indeed, Gα13 binds to the cytoplasmic domain of integrin β3 and plays a critical role in integrin outside-in signaling 63.
Common platelet activation signaling and amplification pathways
Although the initial signaling mechanisms of various platelet receptors differ, they ultimately converge into common intracellular signaling events. In particular, almost all agonists induce activation of PLC. For example, PLCγ and PLCβ are activated by the ITAM pathway and the Gq pathway, respectively 64. PLC catalyzes the hydrolysis of phosphatidylinositol biphosphate (PIP2) to release inositol trisphosphate (IP3) and diacyglycerol (DAG), which activate calcium mobilization and PKC respectively. Intracellular calcium and DAG together also activate Calcium and DAG-regulated guanine nucleotide exchange factor 1 (CalDAG-GEF1), a Rap1 guanine nucleotide exchange factor important in integrin signaling 65.
Calcium signaling
The critical role of cytosolic calcium in platelet activation and function has been known for many years. Agonist-induced calcium elevation is mainly induced by IP3 receptor-mediated release of calcium from intracellular stores and store-operated calcium entry from outside of platelets 66, 67. A role for store-independent calcium entry has also been shown 66. Canonical transient potential channels (TRPC) and the calcium release-activated channel (CRAC) (Orai1) have been shown to mediate calcium entry 66, 67. Elevation of calcium levels activates multiple signaling events and molecules including actin-myosin interaction, protein kinase C (PKC), calmodulin, nitric oxide synthases (NOS), and calcium-dependent proteases. Recently, CalDAG-GEFI has been shown to mediate several important Ca2+ responses, including Rap1 activation, ERK activation, TXA2 synthesis, and granule secretion 67. Calcium elevation also positively regulates SFKs and the PI3K/Akt signaling pathway 68.
Protein kinase C
Platelets express several isoforms of the PKC family, including the classical (or conventional) PKC isoforms α and β (calcium and DAG-dependent), the novel PKC isoforms δ, θ, and η (DAG-dependent, calcium-independent) and an atypical PKC isoform ζ (calcium- and DAG-independent) 69–73. Another novel PKC, PKC ε, is detectable in mouse platelets but not in human platelets 74. Classical PKCs, particularly PKC α, play a critical and general role in platelet granule secretion and secretion-dependent aggregation. PKC α has also been shown to regulate Rap1 and integrin signaling in a reconstituted CHO cell model 75. PKC δ and θ promote dense granule secretion in response to thrombin receptor agonists 69, 71, 72; however, their roles in GPVI-mediated secretion and aggregation are controversial. PKC δ has been reported to negatively regulate GPVI-induced dense granule secretion 69, 72 or to have no effect 73. PKC θ has been shown to promote GPVI-dependent platelet granule secretion and aggregation by one group 71, but to negatively regulate GPVI-mediated granule secretion and platelet activation by others 76, 77. Pleckstrin is a major PKC substrate and may possibly be involved in cytoskeleton regulation 78.
Signals leading to granule secretion and secretion-dependent signal amplification
A common platelet response to all agonists is the secretion of granule contents. Platelets contain three major types of granules, α-granules (containing adhesion proteins such as fibrinogen, VWF, coagulation and fibrinolytic factors, cytokines, growth factors, and adhesion receptors), dense granules (containing nucleotides like ADP, ATP and GTP, serotonin, histamine, pyrophosphates, divalent cations, etc.), and lysozomes (containing a host of proteolytic enzymes) 79. Granule secretion plays critical roles in amplifying platelet activation, in recruitment of circulating platelets into aggregates, and is important for thrombus stabilization 79, 80. Thus, it can be considered a signaling amplification mechanism. Granule secretion also plays important roles in inflammation, atherosclerosis, host defense, wound healing, angiogenesis, and malignancy 81. Granule secretion requires fusion between plasma and granule (vesicle) membranes, which is mediated by protein complexes of vesicle soluble N-ethylmaleimide-sensitive fusion protein attachment receptors (v-SNAREs) proteins (mainly VAMP-8 in platelets) and plasma membrane (target)-SNAREs (t-SNAREs) (mainly syntaxin and SNAP-23 in platelets), as reviewed elsewhere 79. The interaction between SNARE proteins is regulated by their phosphorylation and involves small GTPases such as Rab27. There are multiple signaling events and pathways that are important in stimulating granule secretion: (1) calcium signaling; (2) PKC-dependent phosphorylation and regulation of SNARE complexes 70; (3) integrin outside-in signaling, (4) TXA2 generation, which is important in granule secretion induced by ADP, VWF, and collagen; (5) signaling via the small GTPases Rac-1 and RhoA 82, 83, (6) activation of SFKs, particularly Lyn 84, 85; (7) the PI3K/Akt signaling pathway 56, 86–89; (8) the NO/cGMP/PKG pathway 90, 91; and (9) the signaling pathways of MAPK isoforms p38, ERK, and JNK 92, 93. Recent studies suggest that the SFK Lyn activates the PI3K/Akt pathway 85. PI3K and Akt mediate granule secretion primarily by activating the NO/cGMP/PKG pathway, which stimulates granule secretion through the activation of MAPKs and phosphorylation of SNARE proteins 90–92.
Integrin signaling
Inside-out signaling
Platelets express integrins αIIbβ3 (fibrinogen receptor), αvβ3 (vitronectin receptor), α2β1 (collagen receptor), α5β1 (fibronectin receptor), and α6β1 (laminin receptor). These integrins share similar signal transduction mechanisms. The most abundant integrin in platelets, αIIbβ3, is normally kept in a “resting” or low affinity state in circulating platelets, but transforms into a high affinity “activated” state following platelet activation. Activated αIIbβ3, by binding to its ligands (fibrinogen, VWF and many matrix proteins containing RGD-like sequences), mediates stable platelet adhesion, platelet aggregation, and thrombus formation. The integrin-proximal intracellular signaling mechanism that induces changes in the extracellular ligand binding domain of integrins from a “low affinity” state to the “activated” state is referred to as “inside-out” signaling 1, 94. It has been shown that inside-out signaling requires the binding of talin and kindlins to the cytoplasmic domain of β3 95–97. The relationship between talin and kindlins in inside-out signaling is still being clarified. The binding sites in the β3 cytoplasmic domain for talin and kindlins appear distinct. Talin binds to the membrane proximal region and the membrane-proximal NXXY motif of β3 98–100, whereas kindlins bind to the sequences around the C-terminal NXXY motif 96, 97. Recent studies support the hypothesis that kindlins regulate talin-integrin interaction and cooperate with talin to stimulate inside-out signaling 97. The binding of talin head domain to β3 appears to be sufficient to trigger disruption of the interaction between the membrane proximal regions of the cytoplasmic domains of αIIb and β3, and conformational changes in αIIbβ3 that propagate to the extracellular ligand binding domain, transforming integrin αIIbβ3 into the “active” conformation95, 101, 102. It has been proposed that the change of conformation in αIIbβ3 from a bent form to an extended form results in the activation of the ligand binding function of the integrin 1. A possible role of integrin transmembrane domain interactions in this process has also been suggested 103.
Recent studies suggest that CalDAG-GEF1 and its downstream target, Rap1, play an important role in inside-out signaling 65, 104, providing a possible link between these signaling events and integrin inside-out signaling. CalDAG-GEF1 converts Rap1, a member of the Ras family of small GTPases, from the GDP-bound form to the active GTP-bound form, which interacts with the Rap1-GTP-interating adaptor molecule (RIAM). The role of CalDAG-GEF1/Rap1 in integrin inside-out signaling is consistent with the data that RIAM promotes αIIbβ3-talin interaction and integrin activation 105. The predominant Rap1 isoform expressed in platelets is Rap1b. However, platelets lacking Rap1b 104 or CalDAG-GEF1 65 show only partial defects in αIIbβ3-dependent platelet aggregation, suggesting that neither Rap1b, nor CalDAG-GEFI is fully responsible for inside-out signaling. It remains to be determined whether other isoforms of CalDAG-GEF and Rap1 or alternative pathways are also important in inside-out signaling.
Outside-in signaling
Ligand binding to integrin αIIbβ3 not only mediates platelet adhesion and aggregation, but also initiates a series of intracellular signaling events (“outside-in” signaling), leading to platelet spreading, granule secretion, stable adhesion, and clot retraction 106. Following ligand binding, integrins undergo “a ligand-induced conformational change” that can be propagated outside-in to the cytoplasmic domain 107. However, although ligand-induced conformational changes of αIIbβ3 occur upon the binding of multimeric macromolecular ligands such as fibrinogen or monomeric peptide ligands such as RGDS, significant cellular response only occurs with multimeric macromolecular ligands, suggesting that ligand-induced receptor clustering may be important for transmitting outside-in signals. To date, the most proximal signaling event that has been found to occur following integrin ligation is the binding of the G protein subunit Gα13 to the cytoplasmic domain of β3 63. The interaction of Gα13 with β3 stimulates the activation of SFKs 63, particularly β3-bound c-Src 108, and thus initiates SFK-dependent signals required for outside-in signaling. SFKs mediate outside-in signaling through the following mechanisms: (1) SFK-mediated phosphorylation of the two NXXY motifs in the cytoplasmic domain of β3 is critically important for outside-in signaling 109. Phosphorylation at Y747 negatively regulates talin binding 110. Phosphorylation at Y759 protects β3 from calpain cleavage 111. β3 tyrosine phosphorylation may also promote β3 interaction with intracellular molecules such as myosin heavy chain and adapter protein SHC 112. (2) c-Src phosphorylates and activates a major RhoA GTPase activating protein (GAP), p190RhoGAP, which inactivates RhoA 113. By this mechanism, the β3-bound c-Src mediates transient RhoA inhibition during the early phase of platelet spreading on fibrinogen 63, 114. Inhibition of c-Src or deletion of the c-Src binding site in β3 abolishes integrin-mediated cell spreading in CHO cells and platelets 114, 115, which can be reversed by RhoA inhibitors, suggesting that c-Src-mediated, transient RhoA inhibition is critical for integrin outside-in signaling leading to platelet spreading. Interestingly, the c-Src-mediated inhibition of RhoA requires the binding of c-Src to a specific site at the C-terminus of β3 that is sensitive to cleavage by a calcium-dependent protease (calpain) 114. Following thrombus formation and coagulation, cleavage of β3 by calpain at this site abolishes the interaction of c-Src with β3, which relieves the inhibitory effect of β3-bound c-Src on RhoA, leading to activation of RhoA and clot retraction 114. (3) SFKs activates Syk 108. In human platelets, this can be mediated through phosphorylation of FcγRIIA 116, which recruits Syk into the integrin signaling complex. Syk activation may also involve its interaction with the β3 cytoplasmic domain 117. Syk facilitates the assembly of a SLP76/LAT/Btk/Vav complex that mediates activation of PLCγ2 and subsequent platelet activation events in a manner analogous to the GPVI-mediated ITAM signaling pathway116–118.
Crosstalk between GPCR signaling and integrin outside-in signaling
Integrin outside-in signaling amplifies platelet responses to GPCR agonists. Conversely, GPCR signaling promotes integrin outside-in signaling. For example, platelet spreading on fibrinogen is greatly enhanced when platelets are treated with GPCR agonists. This is because GPCRs not only induce integrin activation, but also directly regulate integrin outside-in signaling. In particular, GPCR-mediated activation of Gα13, while not directly responsible for integrin activation, greatly enhances the interaction of Gα13 with β3, which is required for outside-in signaling 63. Importantly, the GPCR/Gα13 and integrin outside-in signaling pathways coordinate with each other to dynamically regulate RhoA-dependent signaling in platelets. The ability of these two signaling pathways to crosstalk and dynamically regulate RhoA-dependent signaling is critical for the processes of shape change, granule secretion, spreading, and clot retraction in platelets (Fig. 3).
Fig. 3.
Gα13-dependent crosstalk between GPCR signaling and integrin outside-in signaling in regulating RhoA activity in platelets. Adapted from Gong et al63.
Conclusion
Significant progress has been made in recent years in our understanding of platelet signal transduction during adhesion and activation. As a result, we now face an increasingly complex signaling network in platelets and new frontiers to be explored. Many new opportunities for discovery lie in the molecular details of the apparently well-defined signaling pathways. With the goal of fighting thrombotic and hemorrhagic diseases in mind, it is intriguing to know whether further dissection of the molecular mechanisms of integrin signaling may lead to the development of new inhibitors that specifically inhibit outside-in signaling-mediated amplification of platelet activation and platelet recruitment without blocking ligand binding function of integrins critically important in hemostasis. Also, the importance of the crosstalk between various adhesion receptor signaling pathways and G-protein-coupled signaling pathways is increasingly evident. Understanding the crosstalk between these pathways may provide insight into the phenomena of "resistance" to existing platelet inhibitors, and may allow for the development of new therapeutics that are more effective in treating thrombosis with less bleeding side effects. Finally, elucidation of platelet signaling pathways that contribute to the functions of platelets in events beyond hemostasis and thrombosis, such as those discussed in other articles in this series, may reveal new therapeutic targets for the treatment of disorders such as inflammatory diseases, atherosclerosis, and cancer.
Acknowledgments
Sources of funding
This work was supported by grants, HL062350, HL068819, and HL080264 (X.D.) from National Heart, Lung and Blood Institute, and by the NIH/National Center for Research Resources Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease Grant P20 RR021954 (Z.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
X.D. (University of Illinois) holds patents relevant to the topic of this review.
References
- 1.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth SS, Woulfe DS, Weitz JI, Gachet C, Conley PB, Goodman SG, Roe MT, Kuliopulos A, Moliterno DJ, French PA, Steinhubl SR, Becker RC. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009;29:449–457. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- 3.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328:562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- 5.Clemetson KJ, Clemetson JM. Platelet collagen receptors. Thromb Haemost. 2001;86:189–197. [PubMed] [Google Scholar]
- 6.Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor gamma-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 7.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 8.Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J Exp Med. 1998;188:267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, Hibbs ML, Dunn AR, Lowell CA, Watson SP. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–4253. [PubMed] [Google Scholar]
- 10.Senis YA, Tomlinson MG, Ellison S, Mazharian A, Lim J, Zhao Y, Kornerup KN, Auger JM, Thomas SG, Dhanjal T, Kalia N, Zhu JW, Weiss A, Watson SP. The tyrosine phosphatase CD148 is an essential positive regulator of platelet activation and thrombosis. Blood. 2009;113:4942–4954. doi: 10.1182/blood-2008-08-174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 12.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 13.Pasquet JM, Bobe R, Gross B, Gratacap MP, Tomlinson MG, Payrastre B, Watson SP. A collagen-related peptide regulates phospholipase Cgamma2 via phosphatidylinositol 3-kinase in human platelets. Biochem J. 1999;342:171–177. [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe N, Nakajima H, Suzuki H, Oda A, Matsubara Y, Moroi M, Terauchi Y, Kadowaki T, Suzuki H, Koyasu S, Ikeda Y, Handa M. Functional phenotype of phosphoinositide 3-kinase p85alpha-null platelets characterized by an impaired response to GP VI stimulation. Blood. 2003;102:541–548. doi: 10.1182/blood-2002-11-3327. [DOI] [PubMed] [Google Scholar]
- 15.Gilio K, Munnix IC, Mangin P, Cosemans JM, Feijge MA, van der Meijden PE, Olieslagers S, Chrzanowska-Wodnicka MB, Lillian R, Schoenwaelder S, Koyasu S, Sage SO, Jackson SP, Heemskerk JW. Non-redundant roles of phosphoinositide 3-kinase isoforms alpha and beta in glycoprotein VI-induced platelet signaling and thrombus formation. J Biol Chem. 2009;284:33750–33762. doi: 10.1074/jbc.M109.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 17.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 18.Lopez JA, Dong JF. Structure and function of the glycoprotein Ib-IX-V complex. Curr Opin Hematol. 1997;4:323–329. doi: 10.1097/00062752-199704050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Du X. Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Curr Opin Hematol. 2007;14:262–269. doi: 10.1097/MOH.0b013e3280dce51a. [DOI] [PubMed] [Google Scholar]
- 20.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, Lopez JA, Cruz MA, Dong JF, McIntire LV, McEver RP, Zhu C. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118:3195–3207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Zhang CZ, Zhang X, Springer TA. A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature. 2010;466:992–995. doi: 10.1038/nature09295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullam PM, Hyun WC, Szollosi J, Dong J, Foss WM, Lopez JA. Physical proximity and functional interplay of the glycoprotein Ib-IX-V complex and the Fc receptor FcgammaRIIA on the platelet plasma membrane. J Biol Chem. 1998;273:5331–5336. doi: 10.1074/jbc.273.9.5331. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Suzuki-Inoue K, Satoh K, Asazuma N, Yatomi Y, Berndt MC, Ozaki Y. Role of Fc receptor gamma-chain in platelet glycoprotein Ib-mediated signaling. Blood. 2001;97:3836–3845. doi: 10.1182/blood.v97.12.3836. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Pestina TI, Berndt MC, Jackson CW, Gartner TK. Botrocetin/VWF-induced signaling through GPIb-IX-V produces TxA2 in an alphaIIbbeta3- and aggregation-independent manner. Blood. 2005;106:2750–2756. doi: 10.1182/blood-2005-04-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108:2596–2603. doi: 10.1182/blood-2006-01-011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood. 2004;103:3403–3411. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J Biol Chem. 2001;276:42226–42232. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood. 2006;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–3414. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Stojanovic A, Hay N, Du X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood. 2008;111:658–665. doi: 10.1182/blood-2007-04-085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mu FT, Cranmer SL, Andrews RK, Berndt MC. Functional association of phosphoinositide-3-kinase with platelet glycoprotein Ibalpha, the major ligand-binding subunit of the glycoprotein Ib-IX-V complex. J Thromb Haemost. 2010;8:324–330. doi: 10.1111/j.1538-7836.2009.03672.x. [DOI] [PubMed] [Google Scholar]
- 32.Yap CL, Anderson KE, Hughan SC, Dopheide SM, Salem HH, Jackson SP. Essential role for phosphoinositide 3-kinase in shear-dependent signaling between platelet glycoprotein Ib/V/IX and integrin alpha(IIb)beta(3) Blood. 2002;99:151–158. doi: 10.1182/blood.v99.1.151. [DOI] [PubMed] [Google Scholar]
- 33.Yin H, Liu J, Li Z, Berndt MC, Lowell CA, Du X. Src family tyrosine kinase Lyn mediates VWF/GPIb-IX-induced platelet activation via the cGMP signaling pathway. Blood. 2008;112:1139–1146. doi: 10.1182/blood-2008-02-140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzucato M, Pradella P, Cozzi MR, De Marco L, Ruggeri ZM. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibalpha mechanoreceptor. Blood. 2002;100:2793–2800. doi: 10.1182/blood-2002-02-0514. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, Hofmann F, Du X. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell. 2003;112:77–86. doi: 10.1016/s0092-8674(02)01254-0. [DOI] [PubMed] [Google Scholar]
- 36.Riba R, Oberprieler NG, Roberts W, Naseem KM. Von Willebrand factor activates endothelial nitric oxide synthase in blood platelets by a glycoprotein Ib-dependent mechanism. J Thromb Haemost. 2006;4:2636–2644. doi: 10.1111/j.1538-7836.2006.02195.x. [DOI] [PubMed] [Google Scholar]
- 37.Gambaryan S, Kobsar A, Hartmann S, Birschmann I, Kuhlencordt PJ, Muller-Esterl W, Lohmann SM, Walter U. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost. 2008;6:1376–1384. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 38.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 39.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 40.Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci U S A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 42.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 43.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 44.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Foster C, Lecchi A, Quinton TM, Prosser DM, Jin J, Cattaneo M, Kunapuli SP. Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling. Blood. 2002;99:3629–3636. doi: 10.1182/blood.v99.10.3629. [DOI] [PubMed] [Google Scholar]
- 46.Knezevic I, Borg C, Le Breton GC. Identification of Gq as one of the G-proteins which copurify with human platelet thromboxane A2/prostaglandin H2 receptors. J Biol Chem. 1993;268:26011–26017. [PubMed] [Google Scholar]
- 47.Djellas Y, Manganello JM, Antonakis K, Le Breton GC. Identification of Galpha13 as one of the G-proteins that couple to human platelet thromboxane A2 receptors. J Biol Chem. 1999;274:14325–14330. doi: 10.1074/jbc.274.20.14325. [DOI] [PubMed] [Google Scholar]
- 48.Ohlmann P, Laugwitz KL, Nurnberg B, Spicher K, Schultz G, Cazenave JP, Gachet C. The human platelet ADP receptor activates Gi2 proteins. Biochem J. 1995;312:775–779. doi: 10.1042/bj3120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Wu J, Kowalska MA, Dalvi A, Prevost N, O'Brien PJ, Manning D, Poncz M, Lucki I, Blendy JA, Brass LF. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc Natl Acad Sci U S A. 2000;97:9984–9989. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 51.Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- 52.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 53.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul BZ, Jin J, Kunapuli SP. Molecular mechanism of thromboxane A(2)-induced platelet aggregation. Essential role for p2t(ac) and alpha(2a) receptors. J Biol Chem. 1999;274:29108–29114. doi: 10.1074/jbc.274.41.29108. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in PI3Kgamma-deficient mice. Faseb J. 2001;15:2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Zhang G, Le Breton GC, Gao X, Malik AB, Du X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J Biol Chem. 2003;278:30725–30731. doi: 10.1074/jbc.M301838200. [DOI] [PubMed] [Google Scholar]
- 57.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277:23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 58.Lova P, Paganini S, Hirsch E, Barberis L, Wymann M, Sinigaglia F, Balduini C, Torti M. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the Gi-dependent activation of platelet Rap1B. J Biol Chem. 2003;278:131–138. doi: 10.1074/jbc.M204821200. [DOI] [PubMed] [Google Scholar]
- 59.Offermanns S, Laugwitz KL, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- 61.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 62.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, Kozasa T, Du X. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin "outside-in" signaling. Science. 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 65.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 66.Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood. 2002;100:2801–2811. doi: 10.1182/blood-2002-03-0723. [DOI] [PubMed] [Google Scholar]
- 67.Bergmeier W, Stefanini L. Novel molecules in calcium signaling in platelets. J Thromb Haemost. 2009;7 Suppl 1:187–190. doi: 10.1111/j.1538-7836.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 68.Xiang B, Zhang G, Liu J, Morris AJ, Smyth SS, Gartner TK, Li Z. A G-independent mechanism mediating Akt phosphorylation in platelets. J Thromb Haemost. 2010;8:2032–2041. doi: 10.1111/j.1538-7836.2010.03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murugappan S, Tuluc F, Dorsam RT, Shankar H, Kunapuli SP. Differential role of protein kinase C delta isoform in agonist-induced dense granule secretion in human platelets. J Biol Chem. 2004;279:2360–2367. doi: 10.1074/jbc.M306960200. [DOI] [PubMed] [Google Scholar]
- 70.Konopatskaya O, Gilio K, Harper MT, Zhao Y, Cosemans JM, Karim ZA, Whiteheart SW, Molkentin JD, Verkade P, Watson SP, Heemskerk JW, Poole AW. PKCalpha regulates platelet granule secretion and thrombus formation in mice. J Clin Invest. 2009;119:399–407. doi: 10.1172/JCI34665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagy B, Jr, Bhavaraju K, Getz T, Bynagari YS, Kim S, Kunapuli SP. Impaired activation of platelets lacking protein kinase C-theta isoform. Blood. 2009;113:2557–2567. doi: 10.1182/blood-2008-07-169268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chari R, Getz T, Nagy B, Jr, Bhavaraju K, Mao Y, Bynagari YS, Murugappan S, Nakayama K, Kunapuli SP. Protein kinase C[delta] differentially regulates platelet functional responses. Arterioscler Thromb Vasc Biol. 2009;29:699–705. doi: 10.1161/ATVBAHA.109.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pula G, Schuh K, Nakayama K, Nakayama KI, Walter U, Poole AW. PKCdelta regulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035–4044. doi: 10.1182/blood-2006-05-023739. [DOI] [PubMed] [Google Scholar]
- 74.Pears CJ, Thornber K, Auger JM, Hughes CE, Grygielska B, Protty MB, Pearce AC, Watson SP. Differential roles of the PKC novel isoforms, PKCdelta and PKCepsilon, in mouse and human platelets. PLoS One. 2008;3:e3793. doi: 10.1371/journal.pone.0003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 76.Hall KJ, Harper MT, Gilio K, Cosemans JM, Heemskerk JW, Poole AW. Genetic analysis of the role of protein kinase Ctheta in platelet function and thrombus formation. PLoS One. 2008;3:e3277. doi: 10.1371/journal.pone.0003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harper MT, Poole AW. Protein kinase Ctheta negatively regulates store-independent Ca2+ entry and phosphatidylserine exposure downstream of glycoprotein VI in platelets. J Biol Chem. 2010;285:19865–19873. doi: 10.1074/jbc.M109.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lian L, Wang Y, Flick M, Choi J, Scott EW, Degen J, Lemmon MA, Abrams CS. Loss of pleckstrin defines a novel pathway for PKC-mediated exocytosis. Blood. 2009;113:3577–3584. doi: 10.1182/blood-2008-09-178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren Q, Ye S, Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Curr Opin Hematol. 2008;15:537–541. doi: 10.1097/MOH.0b013e328309ec74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reed GL, Fitzgerald ML, Polgar J. Molecular mechanisms of platelet exocytosis: insights into the "secrete" life of thrombocytes. Blood. 2000;96:3334–3342. [PubMed] [Google Scholar]
- 81.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang JS, Dong L, Kozasa T, Le Breton GC. Signaling through G(alpha)13 switch region I is essential for protease-activated receptor 1-mediated human platelet shape change, aggregation, and secretion. J Biol Chem. 2007;282:10210–10222. doi: 10.1074/jbc.M605678200. [DOI] [PubMed] [Google Scholar]
- 83.Akbar H, Kim J, Funk K, Cancelas JA, Shang X, Chen L, Johnson JF, Williams DA, Zheng Y. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. J Thromb Haemost. 2007;5:1747–1755. doi: 10.1111/j.1538-7836.2007.02646.x. [DOI] [PubMed] [Google Scholar]
- 84.Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gamma-thrombin. Blood. 2002;99:2442–2447. doi: 10.1182/blood.v99.7.2442. [DOI] [PubMed] [Google Scholar]
- 85.Li Z, Zhang G, Liu J, Stojanovic A, Ruan C, Lowell CA, Du X. An important role of the SRC family kinase Lyn in stimulating platelet granule secretion. J Biol Chem. 2010;285:12559–12570. doi: 10.1074/jbc.M109.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovacsovics TJ, Bachelot C, Toker A, Vlahos CJ, Duckworth B, Cantley LC, Hartwig JH. Phosphoinositide 3-kinase inhibition spares actin assembly in activating platelets but reverses platelet aggregation. J Biol Chem. 1995;270:11358–11366. doi: 10.1074/jbc.270.19.11358. [DOI] [PubMed] [Google Scholar]
- 87.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004;113:441–450. doi: 10.1172/JCI20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104:1703–1710. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stojanovic A, Marjanovic JA, Brovkovych VM, Peng X, Hay N, Skidgel RA, Du X. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem. 2006;281:16333–16339. doi: 10.1074/jbc.M512378200. [DOI] [PubMed] [Google Scholar]
- 90.Li Z, Zhang G, Marjanovic JA, Ruan C, Du X. A platelet secretion pathway mediated by cGMP-dependent protein kinase. J Biol Chem. 2004;279:42469–42475. doi: 10.1074/jbc.M401532200. [DOI] [PubMed] [Google Scholar]
- 91.Randriamboavonjy V, Schrader J, Busse R, Fleming I. Insulin induces the release of vasodilator compounds from platelets by a nitric oxide-G kinase-VAMP-3-dependent pathway. J Exp Med. 2004;199:347–356. doi: 10.1084/jem.20030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113:893–901. doi: 10.1182/blood-2008-05-155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adam F, Kauskot A, Nurden P, Sulpice E, Hoylaerts MF, Davis RJ, Rosa JP, Bryckaert M. Platelet JNK1 is involved in secretion and thrombus formation. Blood. 2010;115:4083–4092. doi: 10.1182/blood-2009-07-233932. [DOI] [PubMed] [Google Scholar]
- 94.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 96.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 97.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 99.Patil S, Jedsadayanmata A, Wencel-Drake JD, Wang W, Knezevic I, Lam SC. Identification of a talin-binding site in the integrin beta(3) subunit distinct from the NPLY regulatory motif of post-ligand binding functions. The talin n-terminal head domain interacts with the membrane- proximal region of the beta(3) cytoplasmic tail. J Biol Chem. 1999;274:28575–28583. doi: 10.1074/jbc.274.40.28575. [DOI] [PubMed] [Google Scholar]
- 100.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 101.Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, Qin J. A Structural Mechanism of Integrin alpha(IIb)beta(3) "Inside-Out" Activation as Regulated by Its Cytoplasmic Face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 102.Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA, Sligar SG, Taylor KA, Ginsberg MH. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188:157–173. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li R, Mitra N, Gratkowski H, Vilaire G, Litvinov R, Nagasami C, Weisel JW, Lear JD, DeGrado WF, Bennett JS. Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science. 2003;300:795–798. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 104.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 106.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104(6):1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 107.Leisner TM, Wencel-Drake JD, Wang W, Lam SC. Bidirectional transmembrane modulation of integrin alphaIIbbeta3 conformations. J Biol Chem. 1999;274:12945–12949. doi: 10.1074/jbc.274.18.12945. [DOI] [PubMed] [Google Scholar]
- 108.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, Lowell CA, Shattil SJ. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157(2):265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 110.Anthis NJ, Haling JR, Oxley CL, Memo M, Wegener KL, Lim CJ, Ginsberg MH, Campbell ID. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J Biol Chem. 2009;284:36700–36710. doi: 10.1074/jbc.M109.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xi X, Flevaris P, Stojanovic A, Chishti A, Phillips DR, Lam SC, Du X. Tyrosine phosphorylation of the integrin beta 3 subunit regulates beta 3 cleavage by calpain. J Biol Chem. 2006;281:29426–29430. doi: 10.1074/jbc.C600039200. [DOI] [PubMed] [Google Scholar]
- 112.Jenkins AL, Nannizzi-Alaimo L, Silver D, Sellers JR, Ginsberg MH, Law DA, Phillips DR. Tyrosine phosphorylation of the beta3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- 113.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 114.Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, Du X. A molecular switch that controls cell spreading and retraction. J Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ablooglu AJ, Kang J, Petrich BG, Ginsberg MH, Shattil SJ. Antithrombotic effects of targeting alphaIIbbeta3 signaling in platelets. Blood. 2009;113:3585–3592. doi: 10.1182/blood-2008-09-180687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Woodside DG, Obergfell A, Leng L, Wilsbacher JL, Miranti CK, Brugge JS, Shattil SJ, Ginsberg MH. Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr Biol. 2001;11:1799–1804. doi: 10.1016/s0960-9822(01)00565-6. [DOI] [PubMed] [Google Scholar]
- 118.Abtahian F, Bezman N, Clemens R, Sebzda E, Cheng L, Shattil SJ, Kahn ML, Koretzky GA. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–6949. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]