Abstract
Synaptic stimulation in brain slices is accompanied by changes in tissue autofluorescence, which are a consequence of changes in tissue metabolism. Autofluorescence excited by ultraviolet light has been most extensively studied, and is due to reduced pyridine nucleotides (NADH and NADPH, collectively termed NAD(P)H). Stimulation generates a characteristic compound NAD(P)H response, comprising an initial fluorescence decrease and then an overshooting increase that slowly recovers to baseline levels. Evoked NAD(P)H transients are relatively easy to record, do not require the addition of exogenous indicators and have good signal-noise ratios. These characteristics make NAD(P)H imaging methods very useful for tracking the spread of neuronal activity in complex brain tissues, however the cellular basis of synaptically-evoked autofluorescence transients has been the subject of recent debate. Of particular importance is the question of whether signals are due primarily to changes in neuronal mitochondrial function, and/or whether astrocyte metabolism triggered by glutamate uptake may be a significant contributor to the overshooting NAD(P)H fluorescence increases. This mini-review addresses the subcellular origins of NAD(P)H autofluorescence and the evidence for mitochondrial and glycolytic contributions to compound transients. It is concluded that there is no direct evidence for a contribution to NAD(P)H signals from glycolysis in astrocytes following synaptic glutamate uptake. In contrast, multiple lines of evidence, including from complimentary flavoprotein autofluorescence signals, imply that mitochondrial NADH dynamics in neurons dominate compound evoked NAD(P)H transients. These signals are thus appropriate for studies of mitochondrial function and dysfunction in brain slices, in addition to providing robust maps of postsynaptic neuronal activation following physiological activation.
Keywords: mitochondria, brain slice, metabolism, glycolysis, astrocyte, flavoprotein
1. Introduction
The pioneering work of Britton Chance and colleagues in the 1950s and 1960s established that when live tissues are illuminated with near UV wavelength light, a large portion of autofluorescence generated is due to reduced nicotinamide adenine dinucleotide (NADH) and furthermore that changes in metabolic state could be readily monitored by changes in autofluorescence intensity (Chance, 2004). Over 50 years of work has established that NADH autofluorescence signals can be effectively used to monitor neuronal activation in isolated neurons, brain slices and intact brain. The history and application of these and related autofluorescence approaches has been extensively reviewed (Anderson and Meyer, 2002; Chance, 2004; Kann and Kovacs, 2007; Mayevsky and Chance, 2007; Mayevsky and Rogatsky, 2007; Reinert et al., 2007; Tohmi et al., 2009). The present review concentrates on issues concerning the cellular basis of NADH autofluorescence transients, and mechanisms underlying their generation following synaptic stimulation. Over the years, there have developed two views on the origins of NADH changes that accompany electrical activity in brain slices: one, that changes in neuronal mitochondrial function are primarily responsible, and two, that glycolysis triggered by glutamate transport into astrocytes is a major contributor. Understanding the contributions of these two different mechanisms is important, particularly since the autofluorescence approach is being increasingly used to investigate mechanisms of neuronal and astrocyte metabolism in complex brain preparations, including questions of metabolic coupling between these cell types.
2. NADH fluorescence
Redox transitions between NADH and NAD+ are required for a multitude of metabolic processes, including many central to neuronal function. Within mitochondria, NADH serves as a principal electron donor in the electron transport chain. Oxidation of NADH to NAD+ occurs at complex I, and subsequent electron transfer is responsible for generation of the proton gradient across the inner mitochondrial membrane, which in turn is required for ATP synthesis (Nicholls and Ferguson, 2002). NADH required for this important function is obtained from reduction of NAD+ by tricarboxylic acid (TCA) cycle activity, and can also be transferred from the cytosol via shuttle systems (McKenna et al., 2006; Satrustegui et al., 2007). Glycolysis depends on NAD+ as a cofactor for glyceraldehyde phosphate dehydrogenase activity, and oxidation of cytosolic NADH by lactate dehydrogenase can maintain cytosolic NAD+ requirements required for anaerobic glycolysis.
Optical measurements of NADH/NAD+ transitions are relatively straightforward, because NADH is strongly fluorescent (particularly in mitochondrial compartments, see below) while NAD+ is non-fluorescent (Chance et al., 1962). The excitation and emission spectra of purified NADH are relatively broad (peak excitation in the near UV ~340–360nm; emission in the blue range ~430–450nm (Chance et al., 1962; Aubin, 1979; Chance et al., 1979)) and early studies confirmed that the fluorescence spectra of a range of neuronal tissues (including brain, single neurons and axons) are similar to pure reduced NADH (Chance et al., 1962; Terzuolo et al., 1966; Doane, 1967). NADH fluorescence can be effectively excited with either standard widefield epifluorescence illumination or confocal microscopes equipped with a standard UV laser source. Recent work has demonstrated effective 2 photon excitation, which can offer improved spatial resolution and penetration into thick tissues (Patterson et al., 2000; Huang et al., 2002; Zipfel et al., 2003). Autofluorescence detected with photomultiplier devices or CCD cameras is generally quite robust and stable, as long as measures are taken to minimize photobleaching and tissue damage due to excessive UV exposures. Generally, improving detection sensitivity with high NA objectives and cooled CCD cameras, and minimizing exposure rates and/or durations is helpful for long-term recordings of neuronal activity (Duchen et al., 2003). With these considerations, toxicity and bleaching can be avoided over recording periods of hours, as measured from the reproducibility of synaptically-evoked responses. Alternatively, deliberate bleaching of NADH with very high intensity UV flashes can be useful for kinetic studies of tissue NADH generation, as the enzymatic regeneration of NADH within tissues can be monitored (Combs and Balaban, 2001, 2004; Joubert et al., 2004)
From early studies of a range of tissues, it was concluded that the intensity of cellular NADH fluorescence was not uniform, but was strongly increased in the mitochondrial compartment, and quenched when located in the cytosol (Chance and Baltscheffsky, 1958).The reason(s) for enhanced mitochondrial fluorescence was not immediately established, but association with matrix proteins was considered a major contributor (Chance and Baltscheffsky, 1958; Avi-Dor et al., 1962; Estabrook, 1962). Jobsis and colleagues concluded that NADH fluorescence measured in intact cat cortex was primarily mitochondrial, and that enhancement of fluorescence was due to mitochondrial dehydrogenases (Jobsis et al., 1971). Recent work utilizing isolated mitochondria has shown that the NADH fluorescence lifetime is greatly enhanced in mitochondria and appears that this is due to binding to complex I (Blinova et al., 2005; Blinova et al., 2008). Substantially increased NADH fluorescence in the mitochondrial environment may be one reason why mitochondrial signals seem to dominate measured responses in many cases, despite the fact that NADH is involved in a multitude of extra-mitochondrial reactions (including glycolysis) occurring simultaneously throughout a cell.
3. NADPH and NAD(P)H autofluorescence
Reduced nicotinamide adenine dinucleotide phosphate (NADPH) and NADH have very similar optical properties, and therefore is expected that NADPH also contributes to some extent to total autofluorescence signals. NADPH levels in brain tissue have long been recognized to be much lower than NADH in brain tissue (Chance et al., 1962) and more recent HPLC studies have shown that NADPH/NADP levels are in the order of 10 fold lower than NADH/NAD+ levels throughout the mouse brain (midbrain, hippocampus, striatum and cortex) (Klaidman et al., 1995). In addition to lower levels, NADPH fluorescence is enhanced much less than NADH in mitochondria (Avi-Dor et al., 1962), further contributing to the dominance of NADH fluorescence in tissue measurements. In contrast to NADH, NADPH oxidation does not directly contribute to mitochondrial electron transport, and would be expected to normally make little contribution to measured autofluorescence transients during synaptic activity. While this assumption is likely to be valid for most physiological studies, changes in NADPH levels may become significant under some conditions, for example under pathologic conditions where NADPH oxidase activity may be greatly elevated (Suh et al., 2007). These considerations have led a number of groups to refer to “NAD(P)H” auofluorecence, to acknowledge the possible contribution from both reduced pyridine nucleotides (e.g. Duchen, 1992; Barbour et al., 1993; Patterson et al., 2000; Schuchmann et al., 2001; Huang et al., 2002; Kunz et al., 2002; Kann et al., 2003; Shuttleworth et al., 2003; Galeffi et al., 2007; Kahraman and Fiskum, 2007) and this terminology will be used in the following discussion of responses to nerve stimulation.
4. NAD(P)H autofluorescence transients evoked by neuronal stimulation
An early study from Lipton (Lipton, 1973) used a dual wavelength fluorimeter to record NAD(P)H autofluorescence transients following electrical stimulation of guinea pig neocortical slices. Quite intense periods of stimulation were utilized, and resulted in an initial fluorescence decrease, followed by a longer-lasting increase which gradually recovered to pre-stimulus levels (Lipton, 1973). Very similar responses can be collected with CCD-based imaging systems, and Figure 1 shows an example following stimulation of a mouse hippocampal slice. In this example, a short train of stimuli was applied to Schaffer collateral inputs (50Hz, 500ms), and fluorescence changes were extracted from a region of interest in CA1 stratum radiatum. A neuronal basis of the signal (rather than an artifactual response to strong electrical stimulation) was verified by tetrodotoxin block, and the response to this brief stimulus was also abolished by block of ionotropic glutamate receptors, implying a dependence on postsynaptic depolarization of CA1 neurons (Shuttleworth et al., 2003). It is useful to note that the signal shown here is the response to a single stimulus trial, and that averaging of multiple trials was not required to achieve a good signal/noise. The amplitude of signals (in the order of a few percent change in total fluorescence) is much greater than is usually observed with intrinsic optical signal (detected by changes in light transmission) and initial fluorescence decreases can be resolved within 100ms of the onset of the stimulus.
Figure 1.
NAD(P)H autofluorescence transient generated by synaptic stimulation in a hippocampal slice. A: general recording arrangement, with a bipolar stimulating electrode being used to deliver a brief stimulus train (50Hz, 500ms). B: Data extracted from a region of interest in stratum radiatum. Fluorescence was excited 360nm light, and emission detected >410nm. An initial fluorescence decrease was followed by a longer-lasting fluorescence increase, before slice fluorescence recovered to baseline levels. The response shown is from a single stimulus trial.
Very similar compound NAD(P)H responses following synaptic stimulation in hippocampal slices have been described by a number of groups using wide-field epifluorescence systems (e.g. Schuchmann et al., 2001; Foster et al., 2005). A 2 photon study in rat hippocampal slices also generated very similar signals, with an initial NADH fluorescence decrease followed by a long-lasting overshoot response (Kasischke et al., 2004). There are multiple cell types in the brain slice preparation, including postsynaptic dendritic processes, presynaptic terminals and astrocytes. While a number of recent imaging studies have emphasized a mitochondrial and neuronal basis for these signals (Schuchmann et al., 2001; Brennan et al., 2006), it has also been speculated that astrocytic glycolysis underlies a large component of the signals. Lipton proposed a dependence on glycogen stores and glycolytic NADH generation for responses in neocortical slices (Lipton, 1973). More recently it has been proposed that the overshooting NAD(P)H increases are due to increased astrocyte glycolysis, and that neuronal mitochondrial function is responsible only for the initial “dip” in NADH signals (Kasischke et al., 2004). Understanding the relative contributions of these two very different mechanisms is important, since subsequent work relies on this information for interpretation of NAD(P)H signals. In addition, the spatial and temporal resolution offered by NAD(P)H studies should be very helpful to inform models of brain energy metabolism and interpretation of non-invasive brain imaging signals. The discussion below supports the argument that both components of brain slice NADH signals are largely accounted for by mitochondrial, rather than cytoplasmic dynamics. While astrocytic metabolism is certainly important for normal synaptic function (and likely involves significant NAD/NADH transitions), there is currently little direct evidence that glycolysis in astrocytes contributes to measured fluorescence transients in brain slices.
5. Discrimination between mitochondrial and cytoplasmic origins of NADH signals
Evidence that metabolic NAD(P)H transients generated by glutamate originate in mitochondria (rather than the cytosol) has come from studies of specialized glial cells (Muller cells), isolated from the salamander retina. These cells produce significant NAD(P)H fluorescence increases following glutamate application (Barbour et al., 1993) and it was subsequently shown that the increases are restricted to regions in distal processes that are enriched in mitochondria (Poitry et al., 2000). Furthermore, localized application of a mitochondrial respiration inhibitor produced fluorescence increases and led to the conclusion that recorded NAD(P)H fluorescence increases during glutamate stimulation are predominately mitochondrial (Poitry et al., 2000).
In contrast to the heterogenous distribution of mitochondria in Muller cells, mitochondria are more widely distributed throughout neurons and astrocytes, and thus higher resolution imaging is required to test directly whether NAD(P)H dynamics derive from predominantly mitochondrial, rather than cytoplasmic compartments. Confocal or 2 photon imaging (Patterson et al., 2000; Zipfel et al., 2003) is particularly useful for this task and such studies are most informative when imaging is combined with pharmacological approaches to verify mitochondrial origin by evaluation of maximal oxidation and reduction with mitochondrial uncouplers and respiration inhibitors (Duchen et al., 2003). A recent 2 photon imaging study has demonstrated punctuate NADH autofluorescence in dendrites of isolated Purkinje neurons (Hayakawa et al., 2005). It was concluded that biphasic NAD(P)H transients following K+ depolarization were due to mitochondrial NADH dynamics, since responses were restricted to the bright punctate structures and were not observed in adjacent cytosol (Hayakawa et al., 2005). In addition, NAD(P)H fluorescence in these structures increased with the mitochondrial complex I inhibitor rotenone and decreased with the mitochondrial uncoupler FCCP (Hayakawa et al., 2005).
The extent to which NAD(P)H transients occur within mitochondria and the cytosol in astrocytes is not yet clear. A large portion of the astrocytic NAD+ pool is attributed to the cytosol (Alano et al., 2007) and 2 photon imaging of acutely-prepared hippocampal slices revealed NADH autofluorescence co-localized with the mitochondrial marker cytochrome oxidase in astrocytes, but also showed more distributed fluorescence within these cells that was attributed to cytosolic NADH (Kasischke et al., 2004). In that initial study of slices acutely prepared from young rats, it was concluded that it is possible to identify astrocytes within the neuropil solely by means of bright intrinsic NADH autofluorescence (Kasischke et al., 2004). However subsequent work suggests that this is not a reliable criterion. A more recent 2 photon study in similar rat hippocampal slices demonstrates bright resting NAD(P)H fluorescence in both identified neurons and astrocytes (Gordon et al., 2008), and it was noted that selectively enhanced astrocyte autofluorescence was not seen when the neocortex of adult mice was imaged with 2 photon microscopy (Takano et al., 2007). It is not yet clear whether these differences might involve developmental changes in astrocyte NADH fluorescence, or have some other explanation. Thus high resolution imaging of individual astrocytes at different developmental stages (together with manipulation of mitochondrial function) would be very helpful to determine under what conditions NAD(P)H autofluorescence is selectively elevated in astrocytes, and to what extent this originates from mitochondrial and/or cytosolic compartments.
In initial 2 photon studies, the spatial resolution available was not high enough to distinguish between possible mitochondrial and cytoplasmic sources within individual astrocytes during synaptic stimulation (Kasischke et al., 2004), however cytosolic NAD(P)H increases have been concluded following metabotropic glutamate receptor activation in perivascular astrocytes (Gordon et al., 2008).
6. Comparison with flavoprotein autofluorescence to test mitochondrial origin
When most tissues are excited with blue light (~420–480nm) the predominant fluorescence emission recorded at green wavelengths is due to endogenous flavoproteins. Flavoproteins include enzymes that utilize a flavin adenine nucleotide (redox couple FADH2/FAD+) for activation. In contrast to the situation with NADH, the reduced form (FADH2) is non-fluorescent and it is the oxidized form (FAD+) that fluoresces. Flavoprotein signals have historically been used much less than NADH signals in studies of neuronal activation, partly due to concerns about hemoglobin absorption (Mayevsky and Rogatsky, 2007). However flavoprotein autofluorescence has recently been demonstrated to have significant utility for studies of neuronal activation (see (Reinert et al., 2007; Shibuki et al., 2007; Tohmi et al., 2009), and the signals generated are assumed to be due to mitochondrial dynamics.
Early work concluded that multiple flavoproteins are involved in mitochondrial electron transfer and are thereby coupled to NADH utilization by the electron transport chain (Scholz et al., 1969). FADH2 is generated from succinate in the TCA cycle, and oxidized via coenzyme Q and complex II in the electron transport chain. The flavoprotein lipoamide dehydrogenase (LipDH) is in equilibrium with NADH/NAD+ transitions at complex I and makes a significant contribution to tissue flavoprotein autofluorescence (Scholz et al., 1969; Kunz et al., 2002; Rocheleau et al., 2004). FADH2/FAD+ transitions are not directly involved in glycolysis, and early studies demonstrated that flavoprotein metabolic signals could be more direct measures of oxidative metabolism and less contaminated by cytosolic processes than NAD(P)H measurements. For example, in studies of rat liver, it was shown that inhibition of the respiratory chain with rotenone or anoxia produced the expected decrease in flavoprotein fluorescence and increase in NADH fluorescence. However, subsequent addition of pyruvate decreased NADH signals (due to cytosolic lactate dehydrogenase activity, see below), with negligible effect on flavoprotein signals (Scholz et al., 1969). These observations were consistent with flavoprotein signals serving as selective marker of the mitochondrial redox state (Scholz et al., 1969).
From these considerations, transitions between FADH2 and FAD+ are expected to be inverted with respect to NADH/NAD+ fluorescence changes, if the transients have a mitochondrial origin (reviewed in Duchen et al., 2003). For these reasons, comparison of the two signals have been used as diagnostic tests of mitochondrial involvement in a range of preparations including responses to depolarization of isolated neurons (Duchen, 1992) and spreading depolarizations in brain slices (Hepp et al., 2005; Gerich et al., 2006). Comparison of NADH and flavoprotein signals has also been applied as a diagnostic test for the basis of responses to synaptic stimulation. In mouse hippocampal slices, brief trains of synaptic stimulation produced flavoprotein autofluorecence transients that were very similar to NAD(P)H transients, but inverted in sign. Thus a sharp flavoprotein fluorescence increase was observed immediately following the onset of stimulation, and followed by a longer lasting fluorescence decrease before levels returned towards baseline (Shuttleworth et al., 2003), consistent with mitochondrial origin for both signals. The same relationship was observed when much longer trains of synaptic stimulation were used (Brennan et al., 2006), and when responses to cumulative stimulation protocols were assessed (Brennan et al., 2007). Thus, over a relatively wide range of stimulus parameters that have been tested so far with dual NAD(P)H/flavoprotein imaging, it appears that mitochondrial dynamics are responsible for slice transients following synaptic glutamate release. Figure 2 illustrates pathways that could couple postsynaptic depolarization and ion flux to mitochondrial autofluorescence transients.
Figure 2.
Model to illustrate possible coupling between postsynaptic neuronal activation and mitochondrial autofluorescence signals, based in part on observations in (Shuttleworth et al., 2003; Brennan et al., 2006). Glutamate release and activation of both AMPA and NMDA subtypes of glutamate receptors results in substantial ATP consumption, as ATP-dependent pumps restore resting cytosolic Na+ and Ca2+ levels. ADP/ATP ratio changes can couple to increases in mitochondrial electron transport, thereby underlying initial NAD(P)H fluorescence decreases. Mitochondrial Ca2+ accumulation can trigger TCA cycle activity, but this effect appears to make little contribution to NAD(P)H fluorescence increases following synaptic stimulation in hippocampal slices. Overshooting NADH increases from TCA cycle stimulation are instead suggested to be stimulated by ADP/ATP ratio decreases. Increases in substrate availability could also contribute to overshooting NAD(P)H increases. Mitochondrial flavoprotein signals are inverted with respect to NAD(P)H increases, as FADH2 is oxidized at complex II to generate fluorescent FAD+, and also because of flavoprotein transitions associated with NADH oxidation at complex 1 (see section 6). Key: 1) Na+/K+/ATPase, 2) voltage-dependent Na+ channel, 3) AMPA subtype glutamate receptor, 4) NMDA type glutamate receptor, 5) voltage-dependent Ca2+ channel, 6) plasma membrane Ca2+ATPase, 7) mitochondrial Ca2+ uniporter, 8) adenine nucleotide transpoATP/ADP translocator.
7. Pharmacological tests to distinguish between mitochondrial and glycolytic contributions
Mitochondrial metabolism can be disrupted with a range of inhibitors, including agents selective for complexes of the electron transport chain (e.g. rotenone) or ATP synthesis (oligomycin) or agents that dissipate the mitochondrial inner membrane potential (e.g. FCCP) (Nicholls and Ferguson, 2002; Foster et al., 2006). In cases where such agents have been tested on autofluorescence signals generated by stimulation of neurons or glia, results are consistent with a mitochondrial source of signals (e.g. Poitry et al., 2000; Schuchmann et al., 2001; Kosterin et al., 2005). However the effects of disruption of mitochondrial activity can be complicated in brain slice preparations, including depolarization and disruption of transmitter release. Thus it is necessary that effects of mitochondrial inhibitors are coupled with measures of postsynaptic activation, to ensure that the stimulus has not been modified.
Astrocytic mitochondrial function can be selectively prevented by treatment with fluoroacetate or fluorocitrate (Swanson and Graham, 1994; Fonnum et al., 1997). These agents irreversibly inhibit the activity of the TCA enzyme aconitase, and selectivity for astrocytes can be achieved because of preferential expression of acetate transporters in this cell type (Waniewski and Martin, 1998). However since long exposures at relatively high concentrations are usually required, this protocol must be carried out with caution to limit inhibitor uptake into neurons. In addition, disruption of normal glutamine-glutamate cycling can lead to loss of transmitter availability, and significant decrements in postsynaptic responses (Bacci et al., 2002; Lee et al., 2005). A preliminary report studying synaptically-evoked responses in cerebellum has shown that fluoroacetate decreased the sustained phase of flavoprotein autofluorescence transients and suggested a role for astrocytic mitochondrial function (Reinert et al., 2007). As noted by the authors, additional studies are required because it is possible that the inhibitor was disrupting neuronal function (Reinert et al., 2007), and further studies with fluoroacetate should be very interesting to address possible contributions of astrocyte mitochondrial metabolism to compound autofluorescence signals.
Glycolysis can be effectively blocked by membrane permeable inhibitors such as 2-deoxyglucose (2-DG) and iodoacetate. However interpretation of the effects of these agents can be complicated by downstream consequences on mitochondrial function. For example, a strong inhibition of mitochondrial NAD(P)H transients might be expected with 2-DG, since mitochondrial metabolism relies on products of glycolysis (i.e. pyruvate) for generation of NADH by mitochondrial TCA cycle function. In a recent study this problem was addressed by providing preparations with exogenous pyruvate to sustain mitochondrial metabolism during pharmacological block of glycolysis (Brennan et al., 2006). Under these conditions there was still evidence for metabolic compromise, since synaptic potentials were reduced by extracellular accumulation of adenosine and presynaptic A1 receptor activation. When this was compensated for, postsynaptic activation was maintained and evoked NAD(P)H transients were unaffected. This implies that all components of compound synaptic NAD(P)H transients could be completely sustained by mitochondrial metabolism, without a requirement for glycolysis (Brennan et al., 2006).It is acknowledged that supply of low pyruvate concentrations to support oxidative metabolism could artificially shift metabolism from glycolytic to oxidative processes. For this reason, it was noteworthy that comparison of NADH and flavoprotein responses (see above) under the same stimulation conditions (without supplemental pyruvate) supported the conclusion that both components of NADH responses were unlikely to be glycolytic in murine brain slices (Brennan et al., 2006).
Conclusions that glycolysis is not involved in the widely detected NAD(P)H transients may seem difficult to reconcile with earlier studies that used exogenous pyruvate to provide positive evidence for glycolysis in brain slice NADH responses. For example, in the initial study of synaptic stimulation in guinea-pig cortical slices (Lipton, 1973) the addition of pyruvate to the superfusate during a long-lasting NADH overshoots caused a prompt fluorescence decrease. Since pyruvate is oxidized to lactate in a process oxidizizng NADH in the cytosol, this was interpreted to suggest that the overshoot was due to cytosolic NADH production (Lipton, 1973). This result was replicated more recently in rat hippocampal slices (Foster et al., 2005). An alternative explanation for this result comes from consideration of the fact that NADH measurements using single photon techniques are a measure of total fluorescence from all sources. Thus an increase in mitochondrial NADH fluorescence (generated by synaptic stimulation) may appear to be reversed by a sudden decrease in background cytosolic fluorescence following addition of exogenous pyruvate, even though the cytosolic signal was not changing due to synaptic stimulation. Complex effects of exogenous pyruvate were observed in a study of murine hippocampal slices, where low concentrations increased total NAD(P)H fluorescence (attributed to increased substrate availability for TCA cycle activity) while higher pyruvate concentrations produced a NAD(P)H decrease (Brennan et al., 2006). Thus the effects of pyruvate on NAD(P)H levels can be complex (see above) and combination with effects of selective inhibitors is helpful for evaluating contributions of glycolysis to compound responses.
8. Possible cellular compartments involved in mitochondrial responses
Mitochondrial NADH transients could theoretically originate from multiple compartments within a brain slice, including nerve terminals, postsynaptic neurons and astrocytes. Under many stimulation conditions, it is likely that metabolic consequences of postsynaptic depolarizations make the dominant contribution to both components of compound responses (initial dip, and overshoot), without significant contributions from astrocytes or nerve terminals.
An influential early study in dorsal root ganglion neurons provided clear evidence that isolated neurons could generate compound NADH transients following direct depolarization (Duchen, 1992). The recordings were made from single neurons, and when driven through the patch electrode similar responses were recorded, implying that they were not contaminated by signals from other cell types. The pharmacology of the responses was consistent with a mitochondrial origin, implying that changes in neuronal mitochondrial function is responsible for both phases (initial dip and overshoots) of evoked responses (Duchen, 1992).
In brain slices, a similar dependency of neuronal depolarization can be deduced from the effects of inhibitors of glutamate receptors. With brief trains of electrical stimulation, block of AMPA receptors leads to a partial block of postsynaptic responses and partial reduction of NADH signals, but a combination of NMDA and AMPA receptor block results in abolition of postsynaptic responses, and block of the NAD(P)H transients (Shuttleworth et al., 2003). Presynaptic glutamate release and astrocyte uptake is not blocked by these antagonists and metabolism in these two latter compartments should not be blocked, implying that postsynaptic depolarization is likely sufficient to explain both components of NAD(P)H transients. In subsequent work with much longer stimulus durations, a small NAD(P)H signal was found that was not blocked by iontropic receptor block. This appears to be due in part to metabotropic glutamate receptor activation, and not due to astrocytic glutamate uptake since it was not prevented by selective transport inhibitor TBOA (Brennan et al., 2006). These same data suggest that presynaptic elements only make a small contribution, although it is possible that this contributes somewhat to the inotropic receptor insensitive response during long stimulus trains.
Presynaptic mitochondrial function can contribute to compound NADH transients in some preparations. Mitochondria can be localized at a high concentration in presynaptic terminals (e.g. Rowland et al., 2000) and have been implicated under some circumstances in the modulation of presynaptic Ca2+ dynamics (Tang and Zucker, 1997; Zucker, 1999). Presynaptic Ca2+ and Na+ extrusion are also energy requiring processes, and presynaptic NADH/NAD+ transitions might therefore be expected, particularly with intense presynaptic stimulus trains. Presynaptic NADH transients with both oxidizing and reducing phases have recently been documented in a preparation of neurosecretory terminals (Kosterin et al., 2005), and responses that are predominantly oxidizing were described recently in lizard motor nerve terminals following long periods of high frequency stimulation (Talbot et al., 2007). Within the CNS, parallel fiber stimulation also leads to some pre-synaptic NADH transients (Diez-Garcia et al., 2005), however it does appear that these are relatively small, and signals following synaptic stimulation in the cerebellum are dominated by postsynaptic responses (Diez-Garcia et al., 2005).
9. NADH and the astrocyte neuron lactate shuttle hypothesis
The astrocyte neuron lactate shuttle hypothesis was formulated to address inconsistencies between glucose metabolism and oxygen consumption during neuronal activation, and differential distributions of transporters for metabolites between neurons and astrocytes. A resulting model suggests that glucose metabolism in astrocytes generates lactate which is shuttled to neurons to be used as a primary oxidative fuel (Magistretti and Pellerin, 1999). This hypothesis has generated valuable discussion about aspects of neuronal glial interaction, and recently it was suggested that NADH autofluorescence imaging provided evidence for this model in brain slices (Kasischke et al., 2004; Pellerin and Magistretti, 2004). The experimental work showed biphasic NADH transients in rodent hippocampal slices utilizing 2 photon imaging (Kasischke et al., 2004). Responses to long trains of presynaptic stimulation were analyzed to determine which pixels showed initial NADH decreases and which included NADH overshoots. It was concluded that initial dip and overshoot responses were spatially segregated, across the entire slice. The magnification used for these stimulation studies was too low to allow for the cellular sources of these different responses to be directly demonstrated and co-localization of evoked signals with astrocyte markers (e.g. GFP driven by the GFAP promoter) was not performed. However, the authors used an assumption that bright NADH fluorescence was sufficient to identify astrocytes and that dim areas should therefore represent dendritic compartments. On this basis, it was suggested that the initial dip is due to mitochondrial activity in dendrites and that this is followed by overshooting NADH increases in astrocytes, due to glycolysis triggered by glutamate uptake (Kasischke et al., 2004). This interesting suggestion was not directly tested in the initial report, but subsequent work suggests that this sequence of events does not underlie compound NAD(P)H transients in slice. In a series of studies using identical stimuli, it was shown that NAD(P)H overshoots were not reduced by selective block of glycolysis, or inhibition of glutamate uptake into astrocytes (Brennan et al., 2006). In addition, the relationship of evoked flavoprotein and NAD(P)H transients was consistent with the large body of accumulated evidence that NAD(P)H signals are dominated by mitochondrial (rather than cytosolic) sources (discussed in section 1 above).
The reasons for the spatial segregation of areas of NADH dips and overshoots described by (Kasischke et al., 2004) are not yet known. It will be interesting to perform direct tests to determine whether overshoot signals are indeed restricted to astrocytes, for example by co-registration of evoked NADH responses with astrocyte localization in the same preparations. However as discussed above, previous studies with isolated neurons and the pharmacology of evoked responses in slice studies suggest that neuronal depolarization is responsible for both the dip and overshoot components. If this is indeed the case, subcellular compartmentalization of dip and overshoot responses seems unlikely, and other factors including small image shifts during extended stimulation may contribute to apparent spatial segregation of signals.
A recent study testing the effects of an inhibitor of monocarboxylate transporers (α-cyano-4-hydroxycinnamate, 4-CIN) on synaptic responses in hippocampal slices sheds additional light on the question of astrocyte-neuron metabolic shuttling (Galeffi et al., 2007). This study identified concentrations of 4-CIN that should prevent neuronal lactate uptake, but have little effect on mitochondrial metabolism. Within this concentration range, 4-CIN selectively reduced NAD(P)H overshoot responses, which supports the idea that lactate uptake (perhaps following release from astrocytes) could contribute significantly to mitochondrial NADH increases in neurons. This observation was also taken to provide additional evidence against the idea that astrocytic glycolysis is responsible for NAD(P)H overshoots in slices (Galeffi et al., 2007).
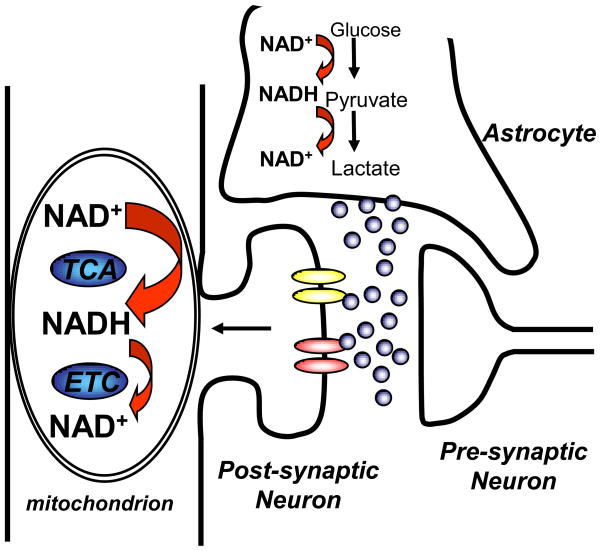
Taken together, it seems reasonable to conclude that NADH autofluorescence transients are not a useful monitor of astrocyte metabolism during synaptic activation. Astrocyte glycolysis may be stimulated to high levels following glutamate uptake, but be completely invisible because of tight coupling between glycolysis and lactate production in astrocytes. Thus equivalent amounts of NADH generated in astrocytes by glycolysis following glutamate uptake should be oxidized by lactate dehydrogenase, with no net accumulation of fluorescent NADH. Even if there were some transient excess of NADH produced in astrocytes, it seems likely that they are completely masked by much larger fluorescence signals generated by NADH dynamics in neuronal mitochondria (Figure 3).
Figure 3.
Model to explain predominant NAD(P)H signals in neurons, rather than astrocytes, during synaptic signaling. It is likely that glutamate release triggers significant metabolic activation in both postsynaptic neurons and astrocytes. Glutamate activation of neuronal ionotropic receptors leads to significant mitochondrial activation, involving initial NADH decreases and subsequent overshooting NADH fluorescence increases (see Figure 2). These NADH dynamics in neurons are readily detectable, in part due to enhanced fluorescence of NADH in the mitochondrial compartment. Astrocyte glycolysis is likely activated following glutamate uptake, but any NADH increases from glycolysis in the cytosolic compartment appear difficult to detect, perhaps due to 1) small amplitude of fluorescence increases generated by NAD+/NADH transitions in the cytosolic compartment (see section 2) and/or 2) concomitant decreases in cytosolic NADH due to lactate generation.
Conclusions
A long history of NAD(P)H measurements in brain tissues has confirmed the utility of this autofluorescence approach for studies of mitochondrial dysfunction and metabolic stress, as well as providing a useful optical measure of the spread of coordinated neuronal activity. When glutamate is released from presynaptic nerve terminals, the dominant response is due to NADH/NAD+ transitions in neuronal mitochondria, following activation of postsynaptic glutamate receptors. In contrast, there is not yet direct evidence that changes in metabolism due to either presynaptic glutamate release or astrocyte glycolysis make significant contributions to these compound NAD(P)H signals. These conclusions simplify the interpretation of mapping studies following focal electrical stimulation, but do not rule out the possibility that NAD(P)H signals could be of significant utility for studies of coupling between mitochondrial and glycolytic compartments under pathological conditions or during changes in oxygen availability (e.g. Gordon et al., 2008; Bickler et al., 2009). Of particular value will be additional high resolution imaging studies, especially when combined with selective pharmacological inhibition of mitochondrial or glycolytic metabolism in neurons and astrocytes.
Acknowledgments
Supported by NIH grant NS051288. Dr JA Connor is thanked for his helpful comments on the manuscript.
Grant information: Supported by NIH grant NS051288
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res. 2007;85:3378–3385. doi: 10.1002/jnr.21479. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Meyer FB. In vivo fluorescent imaging of NADH redox state in brain. Methods Enzymol. 2002;352:482–494. doi: 10.1016/s0076-6879(02)52042-5. [DOI] [PubMed] [Google Scholar]
- Aubin JE. Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem. 1979;27:36–43. doi: 10.1177/27.1.220325. [DOI] [PubMed] [Google Scholar]
- Avi-Dor Y, Olson JM, Doherty MD, Kaplan NO. Fluorescence of pyridine nucleotides in mitochondria. J Biol Chem. 1962;237:7. [Google Scholar]
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. doi: 10.1152/jn.00665.2001. [DOI] [PubMed] [Google Scholar]
- Barbour B, Magnus C, Szatkowski M, Gray PT, Attwell D. Changes in NAD(P)H fluorescence and membrane current produced by glutamate uptake into salamander Muller cells. J Physiol. 1993;466:573–597. [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Gray J, McKleroy W. Inositol 1,4,5-triphosphate receptors and NAD(P)H mediate Ca2+ signaling required for hypoxic preconditioning of hippocampal neurons. Neuroscience. 2009;160:51–60. doi: 10.1016/j.neuroscience.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova K, Carroll S, Bose S, Smirnov AV, Harvey JJ, Knutson JR, Balaban RS. Distribution of mitochondrial NADH fluorescence lifetimes: steady-state kinetics of matrix NADH interactions. Biochemistry. 2005;44:2585–2594. doi: 10.1021/bi0485124. [DOI] [PubMed] [Google Scholar]
- Blinova K, Levine RL, Boja ES, Griffiths GL, Shi ZD, Ruddy B, Balaban RS. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry. 2008;47:9636–9645. doi: 10.1021/bi800307y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. Modulation of the amplitude of NAD(P)H fluorescence transients after synaptic stimulation. J Neurosci Res. 2007 doi: 10.1002/jnr.21288. [DOI] [PubMed] [Google Scholar]
- Chance B. Mitochondrial NADH redox state, monitoring discovery and deployment in tissue. Methods Enzymol. 2004;385:361–370. doi: 10.1016/S0076-6879(04)85020-1. [DOI] [PubMed] [Google Scholar]
- Chance B, Baltscheffsky H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958;233:736–739. [PubMed] [Google Scholar]
- Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979;254:4764–4771. [PubMed] [Google Scholar]
- Combs CA, Balaban RS. Direct imaging of dehydrogenase activity within living cells using enzyme-dependent fluorescence recovery after photobleaching (ED-FRAP) Biophys J. 2001;80:2018–2028. doi: 10.1016/S0006-3495(01)76172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CA, Balaban RS. Enzyme-dependent fluorescence recovery after photobleaching of NADH: in vivo and in vitro applications to the study of enzyme kinetics. Methods Enzymol. 2004;385:257–286. doi: 10.1016/S0076-6879(04)85015-8. [DOI] [PubMed] [Google Scholar]
- Diez-Garcia J, Matsushita S, Mutoh H, Nakai J, Ohkura M, Yokoyama J, Dimitrov D, Knopfel T. Activation of cerebellar parallel fibers monitored in transgenic mice expressing a fluorescent Ca2+ indicator protein. Eur J Neurosci. 2005;22:627–635. doi: 10.1111/j.1460-9568.2005.04250.x. [DOI] [PubMed] [Google Scholar]
- Doane MG. Fluorometric measurement of pyridine nucleotide reduction in the giant axon of the squid. J Gen Physiol. 1967;50:2603–2632. doi: 10.1085/jgp.50.11.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992;283 (Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–389. doi: 10.1016/s0076-6879(03)61019-0. [DOI] [PubMed] [Google Scholar]
- Estabrook RW. Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles. Anal Biochem. 1962;4:231–245. doi: 10.1016/0003-2697(62)90006-4. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21:106–113. [PubMed] [Google Scholar]
- Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuroscience. 2005;132:645–657. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Foster KA, Galeffi F, Gerich FJ, Turner DA, Muller M. Optical and pharmacological tools to investigate the role of mitochondria during oxidative stress and neurodegeneration. Prog Neurobiol. 2006;79:136–171. doi: 10.1016/j.pneurobio.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Foster KA, Sadgrove MP, Beaver CJ, Turner DA. Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J Neurochem. 2007;103:2449–2461. doi: 10.1111/j.1471-4159.2007.04939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich FJ, Hepp S, Probst I, Muller M. Mitochondrial inhibition prior to oxygen-withdrawal facilitates the occurrence of hypoxia-induced spreading depression in rat hippocampal slices. J Neurophysiol. 2006;96:492–504. doi: 10.1152/jn.01015.2005. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Nemoto T, Iino M, Kasai H. Rapid Ca2+-dependent increase in oxygen consumption by mitochondria in single mammalian central neurons. Cell Calcium. 2005;37:359–370. doi: 10.1016/j.ceca.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hepp S, Gerich FJ, Muller M. Sulfhydryl oxidation reduces hippocampal susceptibility to hypoxia-induced spreading depression by activating BK channels. J Neurophysiol. 2005;94:1091–1103. doi: 10.1152/jn.00291.2005. [DOI] [PubMed] [Google Scholar]
- Huang S, Heikal AA, Webb WW. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein. Biophys J. 2002;82:2811–2825. doi: 10.1016/S0006-3495(02)75621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis FF, O’Connor M, Vitale A, Vreman H. Intracellular redox changes in functioning cerebral cortex. I. Metabolic effects of epileptiform activity. J Neurophysiol. 1971;34:735–749. doi: 10.1152/jn.1971.34.5.735. [DOI] [PubMed] [Google Scholar]
- Joubert F, Fales HM, Wen H, Combs CA, Balaban RS. NADH enzyme-dependent fluorescence recovery after photobleaching (ED-FRAP): applications to enzyme and mitochondrial reaction kinetics, in vitro. Biophys J. 2004;86:629–645. doi: 10.1016/S0006-3495(04)74141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman S, Fiskum G. Anoxia-induced changes in pyridine nucleotide redox state in cortical neurons and astrocytes. Neurochem Res. 2007;32:799–806. doi: 10.1007/s11064-006-9206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kann O, Schuchmann S, Buchheim K, Heinemann U. Coupling of neuronal activity and mitochondrial metabolism as revealed by NAD(P)H fluorescence signals in organotypic hippocampal slice cultures of the rat. Neuroscience. 2003;119:87–100. doi: 10.1016/s0306-4522(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Klaidman LK, Leung AC, Adams JD., Jr High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal Biochem. 1995;228:312–317. doi: 10.1006/abio.1995.1356. [DOI] [PubMed] [Google Scholar]
- Kosterin P, Kim GH, Muschol M, Obaid AL, Salzberg BM. Changes in FAD and NADH fluorescence in neurosecretory terminals are triggered by calcium entry and by ADP production. J Membr Biol. 2005;208:113–124. doi: 10.1007/s00232-005-0824-x. [DOI] [PubMed] [Google Scholar]
- Kunz D, Winkler K, Elger CE, Kunz WS. Functional imaging of mitochondrial redox state. Methods Enzymol. 2002;352:135–150. doi: 10.1016/s0076-6879(02)52014-0. [DOI] [PubMed] [Google Scholar]
- Lee J, Tommerdahl M, Favorov OV, Whitsel BL. Optically recorded response of the superficial dorsal horn: dissociation from neuronal activity, sensitivity to formalin-evoked skin nociceptor activation. J Neurophysiol. 2005;94:852–864. doi: 10.1152/jn.00976.2004. [DOI] [PubMed] [Google Scholar]
- Lipton P. Effects of membrane depolarization on nicotinamide nucleotide fluorescence in brain slices. Biochem J. 1973;136:999–1009. doi: 10.1042/bj1360999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion. 2007;7:330–339. doi: 10.1016/j.mito.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Rogatsky GG. Mitochondrial function in vivo evaluated by NADH fluorescence: from animal models to human studies. Am J Physiol Cell Physiol. 2007;292:C615–640. doi: 10.1152/ajpcell.00249.2006. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71:399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics. Elsevier Science; 2002. [Google Scholar]
- Patterson GH, Knobel SM, Arkhammar P, Thastrup O, Piston DW. Separation of the glucose-stimulated cytoplasmic and mitochondrial NAD(P)H responses in pancreatic islet beta cells. Proc Natl Acad Sci U S A. 2000;97:5203–5207. doi: 10.1073/pnas.090098797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroscience. Let there be (NADH) light. Science. 2004;305:50–52. doi: 10.1126/science.1100428. [DOI] [PubMed] [Google Scholar]
- Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M. Mechanisms of glutamate metabolic signaling in retinal glial (Muller) cells. J Neurosci. 2000;20:1809–1821. doi: 10.1523/JNEUROSCI.20-05-01809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert KC, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging in the cerebellar cortex in vivo. J Neurosci Res. 2007;85:3221–3232. doi: 10.1002/jnr.21348. [DOI] [PubMed] [Google Scholar]
- Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J Biol Chem. 2004;279:31780–31787. doi: 10.1074/jbc.M314005200. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrustegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- Scholz R, Thurman RG, Williamson JR, Chance B, Bucher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem. 1969;244:2317–2324. [PubMed] [Google Scholar]
- Schuchmann S, Kovacs R, Kann O, Heinemann U, Buchheim K. Monitoring NAD(P)H autofluorescence to assess mitochondrial metabolic functions in rat hippocampal-entorhinal cortex slices. Brain Res Brain Res Protoc. 2001;7:267–276. doi: 10.1016/s1385-299x(01)00080-0. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Hishida R, Kitamura H, Takahashi H, Tohmi M. Coupling of brain function and metabolism: Endogenous flavoprotein fluorescence imaging of neural activities by local changes in energy metabolism. Handbook of Neurochemistry and Molecular Biology Brain Energetics Integration of Molecular and Cellular Processes. 2007:321–342. [Google Scholar]
- Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Graham SH. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 1994;664:94–100. doi: 10.1016/0006-8993(94)91958-5. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Talbot J, Barrett JN, Barrett EF, David G. Stimulation-induced changes in NADH fluorescence and mitochondrial membrane potential in lizard motor nerve terminals. J Physiol. 2007;579:783–798. doi: 10.1113/jphysiol.2006.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Terzuolo CA, Chance B, Handelman E, Rossini L, Schmelzer P. Measurements of reduced pyridine nucleotides in a single neuron. Biochim Biophys Acta. 1966;126:361–372. doi: 10.1016/0926-6585(66)90073-2. [DOI] [PubMed] [Google Scholar]
- Tohmi M, Takahashi K, Kubota Y, Hishida R, Shibuki K. Transcranial flavoprotein fluorescence imaging of mouse cortical activity and plasticity. J Neurochem. 2009;109(Suppl 1):3–9. doi: 10.1111/j.1471-4159.2009.05926.x. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]