Abstract
A diverse environment challenges skin to maintain temperature, hydration, and electrolyte balance while also maintaining normal immunological function. Rosacea is a common skin disease that manifests unique inflammatory responses to normal environmental stimuli. We hypothesized that abnormal function of innate immune pattern recognition could explain the enhanced sensitivity of patients with rosacea, and observed that the epidermis of patients with rosacea expressed higher amounts of Toll-like receptor 2 (TLR2) than normal patients. Increased expression of TLR2 was not seen in other inflammatory skin disorders such as atopic dermatitis or psoriasis. Overexpression of TLR2 on keratinocytes, treatment with TLR2 ligands, and analysis of TLR2-deficient mice resulted in a calcium-dependent release of kallikrein 5 from keratinocytes, a critical protease involved in the pathogenesis of rosacea. These observations show that abnormal TLR2 function may explain enhanced inflammatory responses to environmental stimuli and can act as a critical element in the pathogenesis of rosacea.
INTRODUCTION
The physical barrier of the skin forms through differentiation of keratinocytes and involves coordinated release of lamellar granule lipids such as ceramides and fatty acids (Elias and Feingold, 1992). Recent findings have shown that the physical barrier of the epidermis is linked with some innate immune functions of the skin and that both actions are interdependent. One example of this is that keratinocyte lamellar granules also transport antimicrobial peptides such as cathelicidin and its processing enzyme kallikrein 5 (KLK5), the two molecules essential to the immune function of the skin (Braff et al., 2005; Ishida-Yamamoto et al., 2005; Yamasaki et al., 2006). Further evidence of a coordination between the physical barrier and classical immune elements has come from studies in mice showing that defects in innate immune gene expression leads to defects in the physical barrier (Aberg et al., 2008). Conversely, human disorders such as atopic dermatitis illustrate how defects in elements of the physical barrier lead to abnormalities in skin immune function (McLean and Hull, 2007). Thus, components of the epidermis that maintain the physical barrier are linked to the immune functions of the epidermis, but the mechanisms that coordinate these two essential roles for the epidermis are not understood.
To function appropriately against an inherent challenge for coordinating physical barrier function and immune defense activity, the skin must have the capacity to detect potentially dangerous events such as trauma or infection. One mechanism by which the skin detects danger is through innate immune pattern recognition receptors such as the Toll-like receptors (TLRs), whose best-known role is to trigger inflammation by recognizing specific microbial products or products of host injury (Takeda et al., 2003; Jiang et al., 2005; Taylor et al., 2007). This important surveillance system must function while skin maintains normal homeostasis under conditions that are populated by a diverse collection of microbes known as the skin microbiome (Cogen et al., 2008; Grice et al., 2009). In normal individuals, commensal microbes are present on and within the epidermis and express molecules that activate TLRs (Hajjar et al., 2001; Kim et al., 2002). However, despite the abundance of these microbes in the skin microenvironment, they do not normally promote inflammation.
The skin does not tolerate the microbial or physical environment in some disease conditions. For example, abnormal sensitivity to the environment is the hallmark of rosacea, a common skin disease that is exacerbated by external triggers including UV light, heat, and a variety of microbes. These external stimuli cause individuals with this disease to develop classical symptoms that can include facial inflammation, abnormal vascular dilatation and proliferation, and formation of granulomas (Yamasaki and Gallo, 2009). Several proinflammatory systems are activated in patients with this disorder, but recent findings have shown that rosacea patients consistently show excessive activation of innate immune effector molecules. These include excess and abnormal production of cathelicidin antimicrobial peptides and increased expression and activity of the serine protease KLK5. These molecules are sufficient to reproduce many of the clinical manifestations of rosacea in mouse models (Yamasaki et al., 2007).
The enhanced sensitivity to the environment seen in patients with rosacea and abnormal expression of innate immune effector molecules provides an opportunity to further explore how keratinocytes may function beyond their role in providing a barrier. In this study, we hypothesized that keratinocytes of patients with rosacea may have abnormal function of innate immune pattern recognition molecules, which are classically thought to act as a mechanism for cells such as monocytes and macrophages that exist in a sterile environment to detect infection. We show here that patients with rosacea have enhanced expression of TLR2, and that keratinocytes with enhanced action of TLR2 respond with an increase in KLK5 that is characteristic of this disease.
RESULTS
Elevated TLR2 expression is associated with increased KLK5 in rosacea
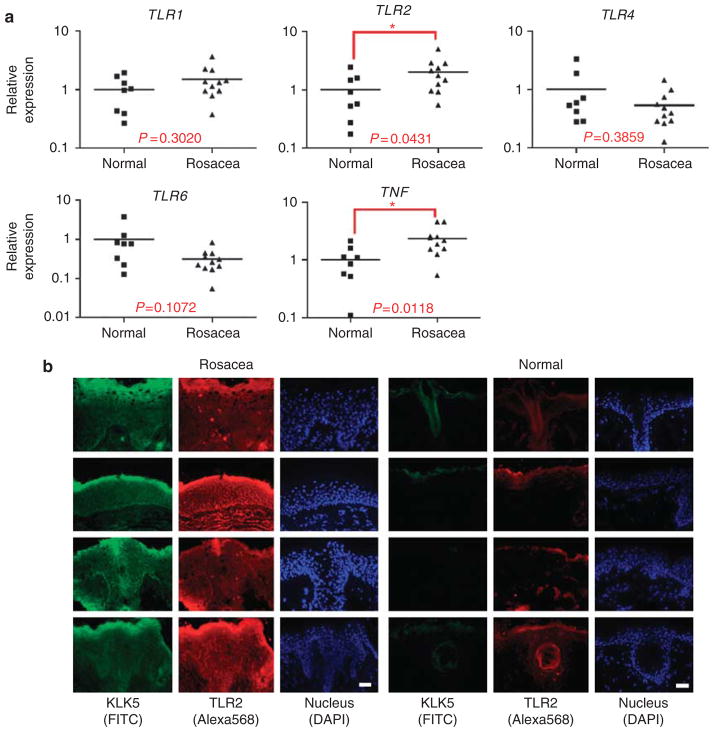
Patients with rosacea have greatly increased expression and activity of KLK5 (Yamasaki et al., 2007). This finding is thought to be an important element in the pathophysiology of this disease, as elevated epidermal serine protease activity also results in increased skin inflammation in human diseases such as Netherton’s syndrome (Chavanas et al., 2000), and mouse models show increase skin inflammation when they overexpress serine proteases (Ny and Egelrud, 2004) or lack a serine protease inhibitor (Descargues et al., 2005). As exacerbations in rosacea are associated with a wide variety of external stimuli, we hypothesized that an alteration in the capacity to detect external danger signals might correlate with abnormally high levels of KLK5 expression. To explore this, the constitutive expression of TLRs 1–9 were measured in skin obtained from rosacea patients biopsied at an untreated site in the naso-malar fold (n = 11), and these samples were compared with skin from a similar location in normal individuals (n = 8). Rosacea skin showed significantly higher TLR2 mRNA expression than normal skin (Figure 1a). Tumor necrosis factor-α, a major cytokine induced by TLR signaling, was also elevated in rosacea. The expression of other TLRs was not significantly increased in rosacea than in control skin, including TLR1, 4, and 6 (Figure 1a), and TLR3, 5, 7, 8, and 9 (data not shown). The increase in TLR2 expression was not seen in inflamed skin from other skin diseases such as atopic dermatitis (n = 7) and psoriasis (n = 5) (data not shown). Immunostaining of rosacea skin sections demonstrated that the increase in mRNA corresponded with higher TLR2 protein in the superficial epidermis (Figure 1b). Furthermore, although TLR2 expression in rosacea was throughout the epidermis and infiltrating cells in the dermis, TLR2 was limited only to superficial layers of the epidermis and hair sheath in normal skin. These observations of increased TLR2 expression and localization closely correlated with previous observations of KLK5 expression in rosacea (Yamasaki et al., 2007).
Figure 1. High Toll-like receptor 2 (TLR2) expression in rosacea skin.
(a) TLR and tumor necrosis factor-α (TNFα) mRNA in rosacea skin were compared with normal skin by quantitative real-time PCR (qPCR). Skin samples were obtained from the naso-malar fold of individuals with rosacea (n = 11) and compared with skin from a similar location in normal individuals (n = 8). The relative TLRs and TNF mRNA abundance against GAPDH levels were analyzed and normalized with the average of normal skin. *P<0.05. (b) Kallikrein 5 (KLK5) and TLR2 protein localization in rosacea and normal skin were visualized by immunohistofluorescence. KLK5 and TLR2 expression is colocalized and higher in rosacea skin. Scale bar = 500 μm.
TLR2 increases expression of KLK5 in human epidermal keratinocytes
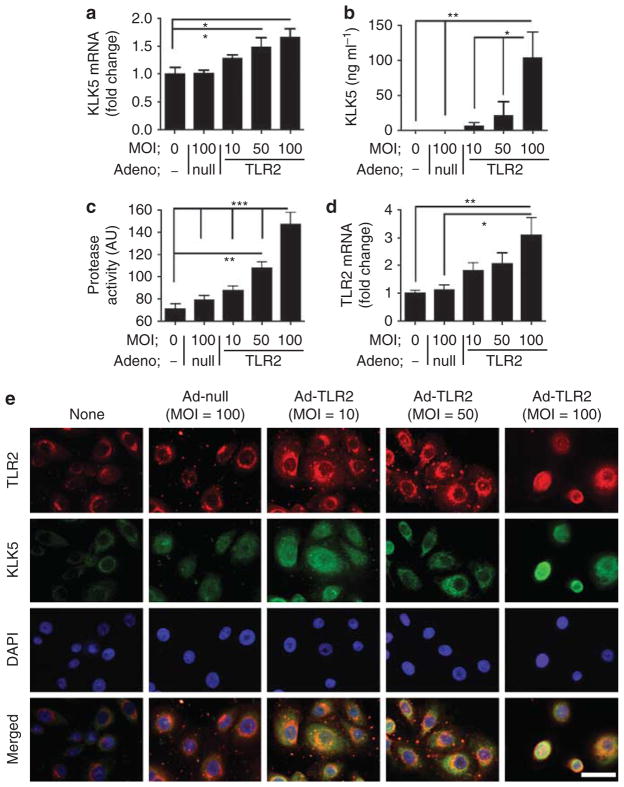
To determine if an increase in TLR2 could induce increased KLK5 release, we next employed an adenovirus vector to overexpress TLR2 in normal human epidermal keratinocytes (NHEKs). NHEKs were infected for 48 hours either with an adenovirus vector expressing TLR2 that encoded human TLR2 (Ad-TLR2), or with a control adenovirus vector (Ad-null). Treatment with Ad-TLR2 at multiplicities of infection (MOIs) from 50 to 100 slightly increased KLK5 mRNA expression whereas Ad-null treatment at the highest MOI of 100 did not affect expression of KLK5 (Figure 2a) or TLR2 (Figure 2d). However, increased expression of TLR2 induced a large relative increase in KLK5 protein in NHEK culture media (Figure 2b). Protease activity in culture media corresponded with the increase in KLK5 protein (Figure 2c), thus suggesting that the enzyme was processed to its active form. The cellular pattern of KLK expression was determined by immunostaining and showed that Ad-TLR2-treated cells at MOIs of 10 and 50 increased cytosolic KLK5, whereas Ad-TLR2 at a MOI of 100 also resulted in a nuclear staining pattern for KLK5 and TLR2 (Figure 2e).
Figure 2. Increased Toll-like receptor 2 (TLR2) results in increased kallikrein 5 (KLK5) expression in keratinocytes.
(a–d) TLR2 was overexpressed in normal human epidermal keratinocytes (NHEKs) by culture in the presence of increasing multiplicities of infection (MOIs) of adenovirus vector expressing TLR2 or adeno-null vector for 48 hours. (a) KLK5 mRNA and (d) TLR2 mRNA as measured by quantitative real-time PCR (qPCR). (b) KLK5 protein in the culture media measured by ELISA. (c) Protease activity of the culture media measured by zymography. *P<0.05, **P<0.01, ***P<0.01. (e) NHEKs were cultured in the presence of adenovirus vectors for 48 hours. The expression of TLR2 and KLK5 was examined by immunocytofluorescence, and nuclei were visualized with 4,6-diamidino-2-phenylindole (DAPI). Scale bar = 20 μm.
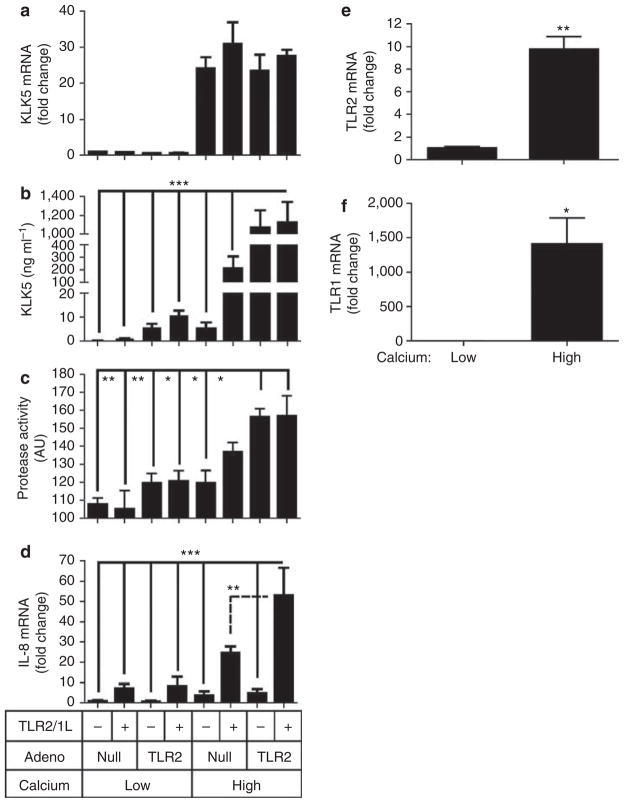
We next stimulated both null transfected and Ad-TLR2 NHEKs with the TLR2/1 ligand (TLR2/1L) Pam3Csk4, and this was done in NHEKs cultured under either low or high calcium conditions. Keratinocytes cultured in high calcium media increased TLR2 and TLR1 mRNA expression (Figure 3e and f), a phenomenon that is consistent with the increase in TLR2 expression observed in suprabasal epidermis where calcium concentration is higher than in the basal layer. KLK5 mRNA and protein in media was greatly increased by a switch to high calcium conditions alone (Figure 3a and b). The magnitude of the increase in KLK5 induced by culture in high calcium conditions minimized the much smaller KLK5 increase induced by Ad-TLR2 under low calcium, and under these conditions no significant change in KLK5 mRNA was seen with addition of the TLR2/1L. However, in high calcium, either TLR2/1L alone or infection with Ad-TLR2 induced an increase in KLK5 protein in media. Protease activity in culture media paralleled the increase of KLK5 protein (Figure 3c). In contrast to the lack of induction of KLK5 mRNA by addition of the TLR2/1L, IL-8 mRNA was significantly increased by Pam3Csk4 under all conditions (Figure 3d). Taken together, the relative lack of an increase in KLK5 mRNA despite a significant induction of KLK5 protein and protease activity suggested that the major influence of TLR2 on keratinocyte KLK5 production was post-transcriptional.
Figure 3. Increased calcium, Toll-like receptor 2 (TLR2) expression, and addition of a TLR1/2 ligand result in elevated kallikrein 5 (KLK5) and protease activity from keratinocytes.
(a–d) Normal human epidermal keratinocytes (NHEKs) were cultured in low (0.06mM) or high (1.6mM) calcium media in the presence of multiplicity of infection (MOI) = 100 of adenovirus vector expressing TLR2 (T) or null (N), and with or without a TLR1/2 ligand (Pam3CSK4: 1 μgml−1). At 48 hours after incubation, measurements were made as in Figure 2 of (a) KLK5 mRNA, (b) KLK5 protein in media, (c) protease activity in culture media, and (d) IL-8 mRNA. (e, f) NHEKs were cultured in low (0.06mM) or high (1.6mM) calcium media for 48 hours and (e) TLR2 mRNA and (f) TLR1 mRNA were measured. *P<0.05, **P<0.01, ***P<0.01.
TLR2 ligands increase KLK5 release dependent on calcium flux
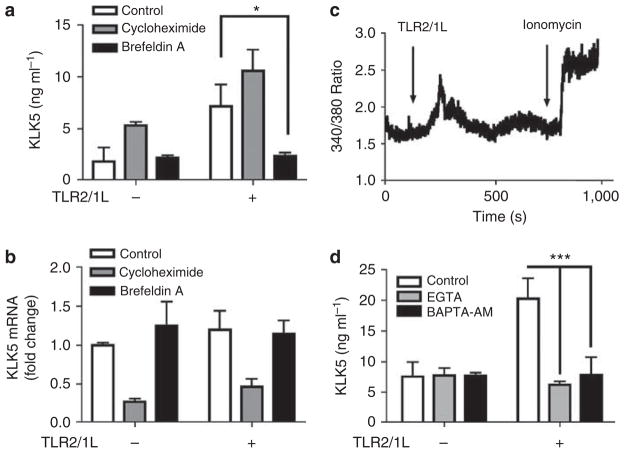
To further understand the mechanism responsible for the effects of TLR2 on KLK5 production, we next evaluated the role of new protein synthesis and lamellar body transport. Pretreatment of NHEKs with cycloheximide, an inhibitor of protein synthesis, decreased KLK5 mRNA but did not inhibit KLK5 protein release by TLR2/1L (Figure 4a and b). This suggested that the increase in KLK5 by TLR2/1L does not rely on de novo protein synthesis. In contrast, brefeldin A, an inhibitor of endoplasmic reticulum and granule transport, suppressed TLR2/1L-dependent KLK5 protein increase in culture media but not KLK5 mRNA expression. The effect of brefeldin A on KLK5 expression suggested again that the increase of KLK5 in culture media was because of post-transcriptional events such as release from keratinocyte cytoplasmic granules.
Figure 4. Toll-like receptor 2 (TLR2) ligands induce a post-transcriptional increase in kallikrein 5 (KLK5) release in a calcium-dependent manner.
(a, b) Normal human epidermal keratinocytes (NHEKs) were cultured in low calcium media and treated with cycloheximide (10 μgml−1) or brefeldin A (5 μgml−1) for 30 minutes, and stimulated with TLR2/1L (Pam3CSK4: 1 μgml−1) for a further 6 hours. Then, (b) KLK5 mRNA and (a) KLK5 protein in the culture media were measured. *P<0.05. (c) Calcium flux after TLR2/1L stimuli (Pam3CSK4: 10 μgml−1) was measured. Ionomycin was used as a positive control for the assay. (d) NHEKs were treated with EGTA (0.2mM) or BAPTA-AM (6 μM) for 30 minutes, and stimulated with TLR2/1L for a further 12 hours. KLK5 protein in the culture media was measured. ***P<0.001.
As the release of KLK5 was observed in high calcium conditions, and calcium is a stimulus of lamellar body transport in keratinocytes, we hypothesized that TLR2 activation may influence keratinocyte transmembrane calcium flux. Direct measurement of NHEK cytosolic calcium revealed that addition of a TLR2/1L resulted in a transient increase in calcium flux when cells were in high extracellular calcium conditions (2.0mM calcium; Figure 4c). This calcium flux did not occur in low calcium conditions (0.06mM calcium; data not shown), thus suggesting that TLR2 induced calcium influx across the plasma membrane and not from intracellular organelles. This event was shown to have functional relevance for KLK5 release, as depletion of extracellular calcium by EGTA, or depletion of intracellular calcium flux by BAPTA-AM, resulted in suppression of the TLR2-dependent increase in KLK5 release (Figure 4d).
Microbial stimulation of KLK5 is dependent on TLR2
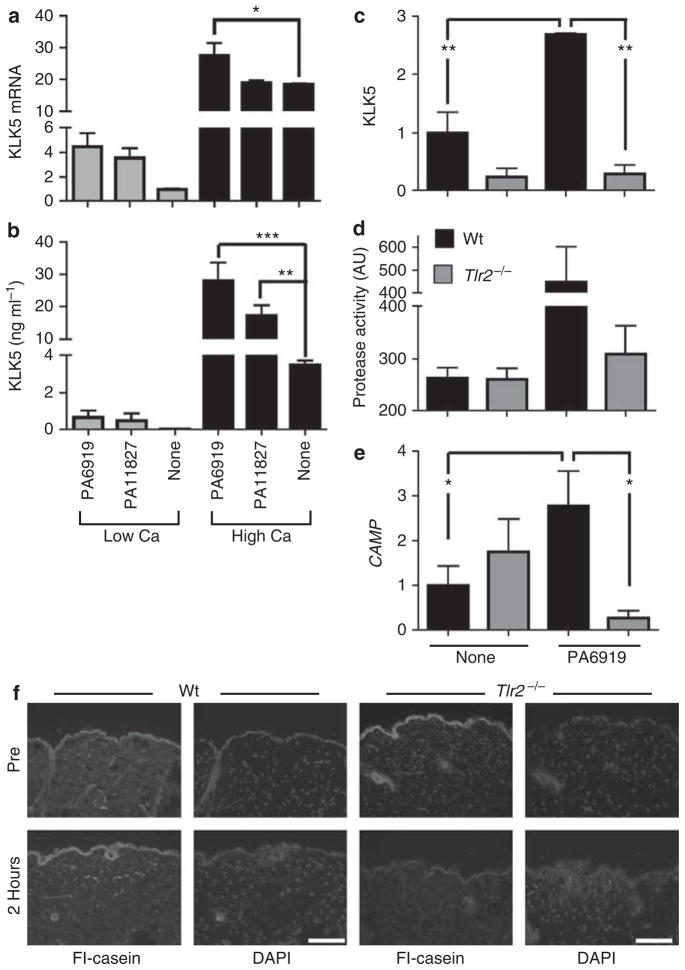
To explore the physiological relevance of the increase in TLR2 seen in keratinocytes of rosacea skin, we next examined if common commensal microbes found on human facial skin could influence KLK5 release in a TLR2-dependent manner. This would therefore associate the known pathological effects of increased KLK5 activity with a natural ligand for TLR2 (Kim et al., 2002). Propionibacterium are the most abundant microbial inhabitants of facial skin (Grice et al., 2009), and we selected two clinical isolates of Propionibacterium acnes (ATCC no. 11827 and 6919) to explore their influence on the expression of KLK5. Similar to previous observations made by TLR2 overexpression or with use of purified TLR2/1L, P. acnes only slightly increased KLK5 mRNA (Figure 5a), but significantly increased KLK5 protein when cultured in high calcium conditions (Figure 5b). Furthermore, skin from Tlr2−/− mice did not increase Klk5 and protease activity in response to P. acnes (Figure 5c and d). An increase in serine protease activity induced by chemical disruption of the stratum corneum was also found to be decreased in Tlr2−/− mice (Figure 5f). Furthermore, induction of the murine gene Camp (cathelicidin antimicrobial peptide) was abolished in Tlr2−/− mice (Figure 5e).
Figure 5. Propionibacterium acnes stimulate kallikrein 5 (KLK5) expression and protease activity through Toll-like receptor 2 (TLR2).
(a, b) P. acnes (1 × 106 CFU per ml) were added to normal human epidermal keratinocytes (NHEKs) cultured in low or high calcium media for 24 hours, and then (a) KLK5 mRNA and (b) KLK5 protein in the culture media were measured. (c–e) Skin explants from Tlr2−/− (gray) and Wt (black) mice were treated with P. acnes 6919 ex vivo. At 24 hours after incubation, (c) KLK5 mRNA, (d) protease activity in the culture media, and (e) Camp mRNA were measured. *P<0.05, **P<0.01, ***P<0.001. (f) Skin samples from Wt and Tlr2−/− mice were biopsied before and 2 hours after chemical irritation. In situ zymography visualized protease activity. Scale bar = 100 μm.
DISCUSSION
Rosacea is a common inflammatory skin disease that affects 3% of the population aged >30 years, and is characterized by facial erythema, papulopustules, and telangiectasia (Straus, 1991; Kelly, 1996; Crawford et al., 2004). Disease severity in rosacea is exacerbated by a variety of external stimuli, and involves aberrant expression of KLK5 and cathelicidin (Yamasaki et al., 2007). In this study we asked why patients with rosacea are abnormally reactive to the environment and respond with excessive KLK5 expression. We found that keratinocytes in rosacea skin have higher TLR2 expression compared with normal skin. Subsequent analysis of the functional consequences of this observation revealed that the expression of TLR2 in keratinocytes, or exposure to TLR2 ligands, enhances KLK5 production and protease activity. These data provide the first molecular explanation for the enhanced sensitivity of facial skin of patients with this disease, and provide further insight into systems that trigger innate inflammatory responses.
The findings of this study suggest that the capacity of TLR2 to increase release of KLK5 from keratinocytes is strongly dependent on calcium. Previous work has shown that calcium influences keratinocyte differentiation (Menon et al., 1985; Mauro et al., 1998). Direct observations of extracellular calcium concentrations in whole skin show that they are low in the basal epidermis, increased in mid epidermis, and highest in stratum granulosum. KLK5 is normally detectable only in differentiated keratinocytes at the layer of granular and cornified cells of the epidermis in vivo, the region of highest calcium concentrations. Thus, a potential role for calcium in control of KLK5 production is suggested by the colocalization of these molecules. The data here confirmed that calcium is a stimulus for increased KLK5 transcription, and are in agreement with a recent previous publication from our group (Morizane et al., 2010), but go on to make the important new observation that the functional release of KLK5 and total serine protease activity in the epidermis is controlled by the action of TLR2. Supporting this conclusion, we show for the first time to our knowledge that TLR2 ligands induce enhanced calcium flux in epidermal keratinocytes and that the increase in KLK5 release occurs in a calcium-dependent manner. This response reveals a less-known function for TLR2, as the vast majority of previous observations following activation of TLR2 have focused on de novo mRNA transcription after encountering pathogen-associated molecules. The implications of these observations extend beyond understanding a disease process such as rosacea, and suggest a role for the skin microbiome in influencing normal keratinocyte function during epidermal barrier development. Such an observation would complement recent observations of a role for the microbiome and TLR2 in controlling epidermal inflammation following injury (Lai et al., 2009), and evidence that signaling through TLR2 enhances bronchial epithelial cell growth and epithelial wound repair (Shaykhiev et al., 2008).
We observed elevated TLR2 expression in rosacea skin and in keratinocytes cultured in high calcium media (Figure 3e). This increase in TLR2 expression by calcium may explain reason why keratinocytes in high calcium media showed better responses to TLR2/1 ligands. From a functional perspective, it is also logical that increased TLR2 in suprabasal epidermis would be beneficial to detect microbes at the surface. However, some reports have shown increased TLR2 expression in basal cells of normal human epidermis (Baker et al., 2003; Pivarcsi et al., 2003; Begon et al., 2007). Three possibilities could explain the reason for these diverse observations: body site from where skin specimens were taken, antibodies used, and methods of immunodetection. Our samples were taken from the faces of normal and rosacea individuals, a significant difference from previous studies. Regarding the antibodies, we used monoclonal antibodies against TLR2. A close examination of previous publications using this antibody shows that TLR2 localized in the suprabasal area of normal epidermis and in hair follicle, similar to our results (Pivarcsi et al., 2003). In contrast, Baker et al. (2003) used polyclonal antibodies when detecting TLR2 at the basal layer. Finally, in our work we used an immunofluorescence staining technique and set the exposure time such that signals were not saturated. Under these conditions, the abundance of TLR2 in normal skin is barely detected, and this is in upper epidermis. With longer exposure times, one can see staining in the entire epidermis of normal skin as previously reported (Begon et al., 2007). Thus, our findings may be consistent with the distribution of TLR2 previously reported, but now more specifically shows relative expression in facial skin.
Although microbes have long been thought to have a role in the pathology of rosacea, studies have shown contradictory results regarding microbial colonization and the associated incidence of rosacea (Buechner, 2005; Yamasaki and Gallo, 2009). We showed here that TLR2 overexpression by adenovirus vector system in normal human keratinocytes increased TLR2 expression 2- to 3-fold. Similarly, stimulation with the TLR2/1L Pam3CSK4 enhanced KLK5 expression, and elevated TLR2 2.0±1.2-fold more than normal skin. In addition, P. acnes, the most abundant of the facial skin commensal microbes (Roth and James, 1988), could induce KLK5 and cathelicidin in a TLR2-dependent manner. Therefore, elevated TLR2 expression could be a cause of aberrant expression of both KLK5 and cathelicidin, both of which are important in rosacea (Yamasaki et al., 2007). Also, although it is known that KLK5, as well as cathelicidin, are localized and transported in the lamellar granule system in keratinocytes (Braff et al., 2005; Ishida-Yamamoto et al., 2005), the mechanisms that are involved in lamellar granule formation and release are not fully understood. Our data explain why rosacea skin is potentially susceptible to a wide variety of microbial molecules because of the relatively promiscuous nature of TLR2 recognition. Therefore, rosacea patients might respond more than normals, although bacterial diversity and quantities are similar between rosacea and normal skin. Thus, our findings suggest a molecular basis for increased microbial sensitivity. These results also show how many different microbes could contribute to the disease in different individuals and may explain previous contradictory studies attempting to isolate a single microbe as associated with the disease.
In summary, in this study we show that increased TLR2 expression and a subsequent broad increased susceptibility to innate immune stimuli may explain the aberrant expression of cathelicidin and KLK in rosacea. A proper balance between pattern recognition receptors and the external and internal ligands are necessary to maintain homeostasis of healthy skin and the cutaneous barrier (Yamasaki and Gallo, 2008; Lai and Gallo, 2009). In the case of this disease, the recognition of a specific role for TLR2 therefore offers greater insight toward an appropriate therapy.
MATERIALS AND METHODS
Human skin sampling and RNA collection
All sample acquisition including the skin biopsies was approved by the Committee on Investigations Involving Human Subjects of the University of California, San Diego, and was conducted according to the Declaration of Helsinki Principles. Informed written consent was obtained from all study subjects. Skin samples were obtained and stored as previously written (Yamasaki et al., 2007). For the gene expression analysis, frozen sections (10 of 10 μm) were collected from each sample, and total RNA was extracted by TRIzol Reagent (Invitrogen, Carlsbad, CA). Quantitative reverse transcriptase-PCR was performed as written below.
Immunohistofluorescence
For immunohistofluorescence, frozen sections (6 μm) or keratinocytes cultured on chamber slides were fixed with ice-cold aceton, and incubated with mouse monoclonal antibody to TLR2 (TL2.1; Abcam, Cambridge, MA) and biotinylated goat antibody to KLK (R&D Systems, Minneapolis, MN) at 4 °C overnight. After washing with phosphate-buffered saline, AlexaFluor568-conjugated goat antibody to mouse IgG (Molecular Probes/Invitrogen, Carlsbad, CA) and FITC-conjugated streptoavidin were used as secondary antibodies, respectively. Sections were mounted in ProLong Gold Anti-Fade reagent with 4,6-diamidino-2-phenylindole (Molecular Probes/Invitrogen). Images were obtained using an Olympus BX41 fluorescent microscope (Scientific Instrument Company, Temecula, CA).
Quantitative reverse transcriptase-PCR
Complementary DNA was synthesized from RNA by the iScript complementary DNA Synthesis Kit (Bio-Rad, Hercules, CA) as described by the manufacture’s protocol. TaqMan Gene Expression Assays (Applied Biosystems, Carlsbad, CA) were used to analyze expression of human KLK5 (assay ID: Hs00202752_m1), mouse Klk5 (assay ID: Mm01203811_m1), human TLR1 (assay ID: Hs00413978_m1), human TLR2 (assay ID: Hs00610101_m1), human TLR4 (assay ID: Hs00152937_m1), human TLR6 (assay ID: Hs00271977_s1), and human tumor necrosis factor (assay ID: Hs00174128_m1) as described by the manufacturer’s instructions. Cathelicidin gene Camp was detected by probe: FAM- CAGAGG ATTGTGACTTCA-MGB; primers: 5′-CTTCACCAGCCCGTCCTTC-3′ and 5′-CCAGGACGACACAGCAGTCA-3′. GAPDH mRNA was detected by probe: VIC-CATCCATGACAACTTTGGTA-MGB; primers: 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGGTCATGAGT CCTTCCACG-3′, and was used as an internal control to validate RNA for each sample. Each mRNA expression was calculated as relative expression to GAPDH mRNA, and all data are presented as fold change against each control (mean of normal skins or nonstimulated cells). All the experiments were performed at least three times, and the data are presented as mean and SEM of one representative result.
Cells, media, and reagents
NHEKs were cultured as previously written (Yamasaki et al., 2007). Cycloheximide, brefeldin A, EGTA, BAPTA-AM, and Fura-2AM (Fura-2-acetoxymethyl ester) were purchased from Sigma (St Louis, MO). Pam3CSK4 was purchased from Invivogen (San Diego, CA). Ionomycin was purchased from EMD Chemicals (Gibbstown, NJ). Coating Matrix was purchased from Invitrogen.
In vitro cell stimulation and sample collection
NHEKs were grown to subconfluence in a 24-well or 12-well flat bottom plate (Corning Incorporated Life Sciences, Lowell, MA). The eight-well chamber slides (Nunc International, Rochester, NY) were used for the immunocytofluorescence experiments. For the experiment, media was removed from the cells and replaced with media containing stimulants at the indicated concentrations. All stimulations were done in EpiLife media (Invitrogen) with growth supplement. Cells were allowed to incubate with the described condition for 24 hours, and media was then removed and spun down at 1,000 × g for 10 minutes at 4 °C to remove any debris. Cell media was stored at −20 °C until analysis. RNA was extracted after supernatant collection using TRIzol reagent (Invitrogen). RNA was stored at −80 °C.
Keratinocyte differentiation was induced by stepping up calcium concentration in cultured media (high Ca media). NHEKs were cultured with basal EpiLife media with 0.06mM Ca2+ until confluent, followed by culturing in EpiLife media with 1.0mM Ca2+ for 2 days and 1.6mM for 2 days (total 4 days). After four days of high calcium condition, NHEKs change morphology, have granules, and increase expressions of keratins 1 and 10, filaggrin, and involucrin genes that relate to epidermal keratinocyte differentiation (Read and Watt, 1988; Boisseau et al., 1992; Morizane et al., 2010).
Adenovirus vector
Adenovirus vector expressing TLR2 (Ad-CMV-TLR2) and control vector (Ad-CMV-null) were obtained from Vector Biolabs (Philadelphia, PA). The number of NHEKs were counted using hemocytometer, and MOI of adenovirus vector was calculated from dividing plaque-forming unit by NHEK cell number. NHEKs were incubated with adenovirus vector for 24 hours to optimize protein induction from genes derived by adenovirus vector. In case of treating with Pam3CSK4 (1 μgml−1, InvivoGen), Pam3CSK4 was added at 24 hours after adenovirus infection, and NHEKs were incubated for another 24 hours.
ELISA
KLK5 protein in NHEK cultured media was measured by ELISA as previously written (Morizane et al., 2010).
Protease assay
Protease activity in cultured media was monitored with EnzChek Protease Assay Kit (Molecular Probes/Invitrogen) as previously written (Yamasaki et al., 2007).
Measurement of [Ca2+]cyt
NHEKs were used at passage 5. Measurements were taken as previously described (Firth et al., 2009). Briefly, cells were seeded on coated 25-mm circular coverslips 24 hours before measurements. Loading with Fura-2AM, washing, and perfusion steps were performed in a solution containing 140mM NaCl, 5mM KCl, 10mM glucose, 10mM HEPES, and either 0.06mM CaCl2 or 2mM CaCl2 as described, with the final solution adjusted to pH 7.4. Cells were loaded with 3 μM Fura-2AM for 30 minutes in the dark at room temperature. Coverslides were then placed in a recording cell chamber on the stage of an inverted Nikon microscope (Eclipse/TE 200, Melville, NY) with the TE-FM epifluorescence attachment. The cells were then washed for 30 minutes at 32 °C to wash away extracellular dye and to permit intracellular cleavage of Fura-2AM to active Fura-2. Following the wash step, cells were continuously perfused with solutions lacking any treatment, or solutions containing Pam3CSK4 or ionomycin as described. Fluorescence from peripheral cytoplasmic regions from cells and background fluorescence were monitored continuously using a fluorescence microscopy system. Data were collected at 1-second intervals using Nikon UV-Fluor objectives (excitation 340 and 380nm with xenon lamp, emission 520 nm) using × 40 magnification.
Preparation of bacteria
P. acnes ATCC 6919 and ATCC 11827 were cultured as previously written (Nakatsuji et al., 2009). P. acnes barely grow in keratinocyte cultured media because of aerobic condition and insufficient nutrition for them, and the number of P. acnes was not changed during the incubation period with keratinocytes for 24–48 hours.
Mouse skin sampling and ex vivo bacteria stimulation
All animal procedures were approved by the institutional animal care and use committee of the Veterans Affairs San Diego Healthcare System. After shaving with an electric hair clipper, full-thickness back skins were obtained from Tlr2−/− mice (Takeuchi et al., 1999) and Wt C57Bl/6 mice using 6-mm punch biopsy. Fat was removed by scissors, and the skins were washed in 70% ethanol for 2 minutes, rinsed in phosphate-buffered saline for 2 minutes, and submerged in DMEM media supplemented with 1% heat-inactivated fetal calf serum. Then, 106 CFU per ml of live P. acnes 6919 was applied, and the skins were incubated in humidified incubator supplemented with 5% CO2. After 24 hours, cultured media and RNA from the skin were collected.
In situ zymography
The mouse skins were obtained by punch biopsies before and 2 hours after skin barrier abrasion by treating with Nair for 3 minutes and wiping off hairs and Nair (Church & Dwight, Princeton, NJ) with alcohol swab. The skins were fresh frozen in Tissue-Tek optimal cutting temperature compound (Electron Microscopy Sciences, Hatfield, PA), and were kept in −80 °C. In situ zymography were performed as previously written (Yamasaki et al., 2007). Representative data from the three mice are shown.
Statistical analysis
All the experiments were performed at least three times, and the data are presented as mean and SEM of one representative results. One-way analysis of variance analyzed by GraphPad Prism 4 (GraphPad Software, La Jolla, CA) determined significance unless otherwise stated. Mann–Whitney U-test was used to compare gene expression between rosacea and normal skin. P<0.05 was considered significant.
Acknowledgments
We thank Anke Leichtle and Allen F Ryan for providing skin of Tlr2−/− mice, and Amy L Firth and Jason X Yuan for their assistance regarding measurements of intracellular calcium concentrations in keratinocytes. The NIH grants NIH R01-AR052728, NIH R01-AI052453, and a VA Merit Award to RLG, and grants from the National Rosacea Society (to KY and RLG) supported this work.
Abbreviations
- KLK
kallikrein
- MOI
multiplicity of infection
- NHEK
normal human epidermal keratinocyte
- TLR
Toll-like receptor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Aberg KM, Man MQ, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–25. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Ovigne JM, Powles AV, et al. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–9. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- Begon E, Michel L, Flageul B, et al. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol. 2007;17:497–506. doi: 10.1684/ejd.2007.0264. [DOI] [PubMed] [Google Scholar]
- Boisseau AM, Donatien P, Surleve-Bazeille JE, et al. Production of epidermal sheets in a serum free culture system: a further appraisal of the role of extracellular calcium. J Dermatol Sci. 1992;3:111–20. doi: 10.1016/0923-1811(92)90044-c. [DOI] [PubMed] [Google Scholar]
- Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- Buechner SA. Rosacea: an update. Dermatology. 2005;210:100–8. doi: 10.1159/000082564. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–2. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158:442–55. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327–41. doi: 10.1016/j.jaad.2004.03.030. quiz 42–4. [DOI] [PubMed] [Google Scholar]
- Descargues P, Deraison C, Bonnart C, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992;11:176–82. [PubMed] [Google Scholar]
- Firth AL, Yau J, White A, et al. Chronic exposure to fibrin and fibrinogen differentially regulates intracellular Ca2+ in human pulmonary arterial smooth muscle and endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L979–86. doi: 10.1152/ajplung.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, O’Mahony DS, Ozinsky A, et al. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–9. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Deraison C, Bonnart C, et al. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124:360–6. doi: 10.1111/j.0022-202X.2004.23583.x. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Kelly A. P. Acne and related disorders. In: Sams W, Lynch P, editors. Principles and Practice of Dermatology. Churchill Livingstone; New York: 1996. pp. 801–18. [Google Scholar]
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–41. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Tolllike receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–82. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–41. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro T, Bench G, Sidderas-Haddad E, et al. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol. 1998;111:1198–201. doi: 10.1046/j.1523-1747.1998.00421.x. [DOI] [PubMed] [Google Scholar]
- McLean WH, Hull PR. Breach delivery: increased solute uptake points to a defective skin barrier in atopic dermatitis. J Invest Dermatol. 2007;127:8–10. doi: 10.1038/sj.jid.5700609. [DOI] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–12. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- Morizane S, Yamasaki K, Kabigting FD, et al. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010;130:1297–306. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Fang JY, et al. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480–8. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny A, Egelrud T. Epidermal hyperproliferation and decreased skin barrier function in mice overexpressing stratum corneum chymotryptic enzyme. Acta Derm Venereol. 2004;84:18–22. doi: 10.1080/00015550310005924. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A, Bodai L, Rethi B, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–30. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- Read J, Watt FM. A model for in vitro studies of epidermal homeostasis: proliferation and involucrin synthesis by cultured human keratinocytes during recovery after stripping off the suprabasal layers. J Invest Dermatol. 1988;90:739–43. doi: 10.1111/1523-1747.ep12560940. [DOI] [PubMed] [Google Scholar]
- Roth RR, James WD. Microbial ecology of the skin. Annu Rev Microbiol. 1988;42:441–64. doi: 10.1146/annurev.mi.42.100188.002301. [DOI] [PubMed] [Google Scholar]
- Shaykhiev R, Behr J, Bals R. Microbial patterns signaling via Toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS ONE. 2008;3:e1393. doi: 10.1371/journal.pone.0001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus J. Rosacea and acne rosacea. In: Orkin M, Maibach H, Dahl M, editors. Dermatology. Prentice Hall International; New Jersey: 1991. pp. 337–8. [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. doi: 10.1684/ejd.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–80. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]