Abstract
Salvinorin A (1), a neoclerodane diterpene from the hallucinogenic mint Salvia divinorum, is the only known naturally occurring non-nitrogenous and specific κ-opioid agonist. Some oxidative modifications of the A ring in the congeners of 1 isolated from Salvia splendens salviarin, splenolide B, splendidin and in the non-natural 8-epi-salviarin gave new derivatives, some of which were tested as agonists at opioid receptors. However, none of these compounds were active. The presence of the C-18, C-19 lactone could be at the origin of the observed lack of binding affinity.
Keywords: Salvia splendens, opioid receptors, neoclerodane diterpenes, semisynthetic derivatives
Introduction
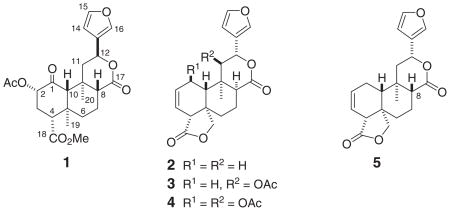
Salvinorin A (1), isolated from the sage Salvia divinorum Epling & Játiva (Labiatae) is a potent and selective κ-opioid agonist.1 Recently,2 we screened other structurally related neoclerodane diterpenoids isolated from Salvia splendens Sellow ex Roem. & Schult, as well as several semisynthetic derivatives, for binding affinity and functional activity at opioid receptors. In this communication, we describe the preparation of a series of new derivatives starting from the major diterpene constituents of S. splendens, salviarin, splenolide B, and splendidin (2–4, respectively3,4) and from the derivative 8-epi-salviarin 52,8 through several oxidative modifications of the ring A. The aim of this work was to obtain a series of new neoclerodanes (6–24) and to test their affinity to the opioid receptors and explore their structure-activity relationships. It is noteworthy that the new derivative 24 possesses identical structural parts that those of salvinorin A (1) at the C-2 (α-acetoxyl group) and C-8 (β-hydrogen) positions, that have been reported1,5–7 as some of the structural requirements for the psychoactivity of salvinorin 1.
Results and Discussion
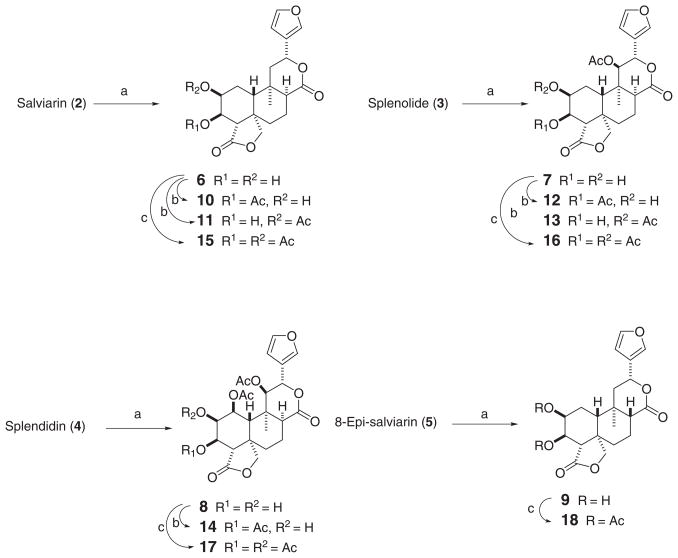
Treatment of 2–5 with osmium tetroxide (see Experimental Section) stereoselectively yielded the cis dihydroxylated derivatives 6–9, respectively. Partial acetylation of 6–8 with acetic anhydride-pyridine at room temperature for a short time gave the monoacetyl derivatives 10–14 (scheme 1). Complete acetylation of 6–9 with an excess of Ac2O-pyridine for 24 hours produced the peracetates 15–18, respectively (Scheme 1).
Scheme 1.
Reagents and conditions: (a) OsO4 (cat.), NMO, Me2CO/H2O 6:1, 24 h rt 70% (7), 72h 50°C 52% (8), 4 h rt 75% (9); (b) Ac2O/Py 1:2, rt, 15 min. 38% (10), 15% (11), 2 min. 40% (12), 15% (13), 1.5 h 50% (14); (c) Ac2O/Py 1:2, 24 h, quant.
The β-orientation of the C-2 and C-3 hydroxyl and/or acetoxyl groups in all these compounds (6–18) was supported by the following facts. The large vicinal coupling value (J = 8.0–11.1 Hz) between the H-4β and H-3 protons, observed in the 1H NMR spectra of 7–18, established that the latter proton was α-oriented, and the H-2 proton must be also α-oriented not only by its vicinal coupling value with the H-3α proton (Ja,e = 1.4–3.2 Hz) but also by the stereochemical requirements of an 1,2-dihydroxylation reaction with OsO4. NOE experiments also supported this conclusion, because irradiation at the H-3α proton signal always caused an NOE enhancement in the H-19α (pro-S) proton, thus confirming that these protons are α-oriented. The location of the acetyl group in the regioisomeric acetates (10–14) was strongly supported by the HMBC spectra, which showed correlation between the carbonyl carbon of the acetate and the proton of the acetylated position. Moreover, comparison of the chemical shifts of the H-2α and H-3α protons in each one of these derivatives (10: δ 4.17 and 5.08, 11: δ 5.20 and 3.92, 12: δ 4.18 and 5.04, 13: δ 5.29 and 3.89, 14: δ 3.92 and 5.09, respectively) further confirmed the proposed structures.
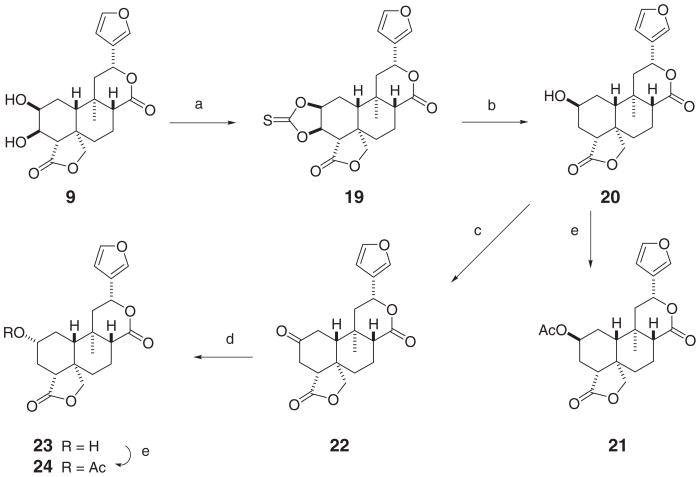
Treatment of 9 (Scheme 2) with 1,1′-thiocarbonyldiimidazole and 4-dimethylaminopyridine (4-DMAP) yielded the cyclic thiocarbonate 19, which was transformed into 20 by reaction with Bu3SnH. Acetylation of 20 by standard procedures gave the derivative 21. The location of the hydroxyl (20) or acetoxyl (21) group at the C-2β position was deduced from the multiplicities of the H-2α and H-4β protons, that appeared as a quintuplet (J = 3.1 Hz in both 20 and 21) and as a double doublet (J4β,3α = 12.6 Hz and J4β,3β = 6.3 Hz in 20, and J = 12.2 and 6.3 Hz in 21), respectively, in the 1H NMR spectra of these substances.
Scheme 2.
Reagents and conditions: (a) Im2(CS), 4-DMAP, CH2Cl2, reflux, 3.5 h, 58%; (b) Bu3SnH, AIBN, toluene, reflux, 4 h, 82%; (c) PCC, CH2Cl2, rt, 17 h, 89%; (d) NaBH4, THF, 0 °C, 15 min, α/βOH 10/1, quant; (e) Ac2O, pyridine, rt, 24 h, 100 %.
An attempt to directly prepare the AcO-2α derivative 23, using a Mitsunobu reaction, starting from the compound 20, unfortunately failed. However, compound 20 was transformed into 22 by oxidation with PCC (Scheme 2). Reduction of 22 with NaBH4 at 0 °C gave a product which showed only one spot on TLC with several eluents. The 1H NMR spectrum of the reduction compound, however, revealed that it was a 10:1 mixture of the 2α-hydroxy derivative 23 and another minor constituent. Acetic anhydride-pyridine treatment of this mixture and subsequent chromatography allowed the isolation of the major acetyl derivative 24 together with a minor quantity of its C-2 epimer, the above described compound 21. The α-orientation of the substituent at C-2 in 24 was in agreement5 with the coupling values for the H-2β axial proton (Ja,a = Ja,a′ = 10.3 Hz, Ja,e = Ja,e′ = 4.3 Hz) observed in its 1H NMR spectrum.
The structures of all the new neoclerodane derivatives were supported on a complete and unambiguous assignment of the 1H and 13C NMR spectra of 7–24 and by other spectroscopic and physicochemical data (see Experimental Section).
Compounds 7 – 10, 12, and 14 – 24 were then evaluated for opioid receptor affinity as described previously.2 Unfortunately, none of these had affinity for opioid receptors (Ki > 10,000 nM). These results would confirm the key role of the orientation of furan and the C-8 configuration in the binding of neoclerodanes, such as 1, at opioid receptors. In addition, the lack of affinity by 23 and 24 would suggest that the additional lactone ring including C-18 and C-19 when compared to 1 is detrimental for binding. To distinguish between the role of the C-18, C-19 lactone moiety and that of the absolute configuration at C-12 in inhibiting the binding to opioid receptors, the corresponding C-12 epimers of 23 and 24 would need to be prepared.
Conclusion
In summary, we have provided additional methodology for the selective oxidation of neoclerodanes isolated from S. splendens. The data collected in this work indicates that the presence of the C-18, C-19 lactone may be responsible for the lack of affinity at opioid receptors. However, additional work will be needed to confirm this finding. While none of the compounds examined possesses affinity for opioid receptors, it also provides valuable insight into the nature of the affinity and activity of 1 for opioid receptors.
Experimental Section
General Experimental Procedures
Melting points were determined on a Kofler block and are uncorrected. Optical rotations were measured on a Perkin-Elmer 241 MC polarimeter. IR spectra were obtained on a Perkin-Elmer Spectrum One spectrophotometer. 1H and 13C NMR spectra were recorded in CDCl3 (7, 8, 12–18, and 21) or methanol-d4 (10, 11, 19, and 20) or 2:1 acetone-d6 – methanol-d4 (9) solution on a Varian INOVA 400 espectrometer at 400 and 100 MHz, respectively. Chemical shifts are reported in the δ scale and are referenced to residual CHCl3 (δ 7.25) or methanol (δ 3.30) signals for protons and to the solvent signals (δCHCl3 77.00, δCH3OH 49.00) for carbons. All the assignments for protons and carbons were in agreement with 2D COSY, gHSQC, gHMBC, and 1D NOESY spectra. Mass spectra were registered in the positive EI (70 eV) mode on a Hewlett-Packard 5973 instrument. Elemental analyses were conducted on a LECO CHNS-932 apparatus. Si gel Merck LiChroprep 15-25/25-40 μm 1/1 was used for flash chromatography (elution under 0.7 psi of Ar). Merck 5554 Kieselgel 60 F254 sheets were used for thin-layer chromatographic analysis. Petroleum ether (bp 50–70 °C) was used for column chromatography.
Starting Materials for Chemical Transformations
Compounds 2–4 were available from a previous work3 and 5 was obtained from 2 as described previously.2
General Procedure for the Osmylation of Compounds 2–5
0.5 mmol of the starting compounds were added to a solution of OsO4 (1 mL of a 2.5 wt. % solution in 2-methyl-2-propanol, 20.3 mg, 0.080 mmol) and 97% 4-methylmorpholine N-oxide (240 mg, 1.99 mmol) in 10 mL of acetone-water (6:1). The solution was stirred at room temperature for 4 h in the case of 2 and 5, for 24 h in the case of 3, and for 72 h at 50 °C in the case of 4. Then, the reaction was quenched by adding 1 mL of saturated aqueous solution of Na2S2O3 and the mixture was extracted with EtOAc (4×10 mL). The combined organic layers were washed with brine (10 mL) and dried over Na2SO4. Evaporation of the solvent under reduced pressure and purification by flash column chromatography (FC) yielded compound 69 from 2 (transformed, without characterization, into compounds 10, 11, and 15, see below), compound 7 from 3 (elution with 3:1 CH2Cl2-acetone: 152 mg, 70%) compound 8 from 4 (elution with 7:3 CH2Cl2-acetone: 128 mg, 52%) and compound 9 from 5 (elution with 7:3 CH2Cl2-acetone: 163 mg, 75%).
Compound 7
colorless prisms (EtOAc-petroleum ether); mp 226–230 °C; [α]D20 −46.8 (c 0.248, CHCl3); Rf (3:1 CH2Cl2-acetone) 0.30; IR (KBr) νmax 3449, 2925, 1752, 1447, 1375, 1222, 1159, 1047, 1009, 875, 729, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.51 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.8 Hz, H-16), 7.37 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.39 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.8 Hz, H-14), 5.42 (1H, d, J12β,11α = 10.8 Hz, H-12β), 5.16 (1H, d, J11α,12β = 10.8 Hz, H-11α), 4.28 (1H, d, J19a,19b = 9.4 Hz, pro-R H-19a), 4.24 (1H, dd, J19b,6β = 1.6 Hz, J19b,19a = 9.4 Hz, pro-S H-19b), 4.16 (1H, ddd, J2α,1α = 2.0 Hz, J2α,1β = 3.0 Hz, J2α,3α = 2.8 Hz, H-2α), 3.74 (1H, dd, J3α,2α = 2.8 Hz, J3α,4β = 10.0 Hz, H-3α), 2.64 (1H, br dd, J8α,6α < 0.4 Hz, J8α,7α = 4.3 Hz, J8α,7β = 3.1 Hz, H-8α), 2.61 (1H, dd, J10β,1α = 11.9 Hz, J10β,1β = 3.1 Hz, H-10β), 2.49 (1H, m, H-1β), 2.48 (1H, m, H-7β), 2.31 (1H, d, J4β,3α = 10.0 Hz, H-4β), 2.16 (3H, s, 11β-OAc), 1.88 (1H, dddd, J7α,6α = 3.1 Hz, J7α,6β =14.2 Hz, J7α,7β = 14.4 Hz, J7α,8α = 4.3 Hz, H-7α), 1.69 (1H, br dt, J6α,6β = 14.0 Hz, J6α,7α = J6α,7β = 3.0 Hz, J6α,8α < 0.4 Hz, H-6α), 1.55 (1H, ddd, J1α,1β = 14.8 Hz, J1α,2α = 2.0 Hz, J1α,10β = 11.9 Hz, H-1α), 1.28 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 14.2 Hz, J6β,7β = 3.7 Hz, J6β,19b = 1.6 Hz, H-6β), 0.89 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 177.8 (C, C-18), 169.9 (C, C-17), 169.2 (C, 11-OCOCH3), 143.9 (CH, C-15), 141.7 (CH, C-16), 121.4 (C, C-13), 108.5 (CH, C-14), 76.5 (CH, C-11), 71.3 (CH, C-12), 70.4 (CH2, C-19), 69.0 (CH, C-3), 67.6 (CH, C-2), 53.1 (CH, C-4), 49.4 (CH, C-8), 43.5 (C, C-5), 38.9 (C, C-9), 34.2 (CH, C-10), 32.3 (CH2, C-6), 27.2 (CH2, C-1), 20.7 (CH3, 11-OCOCH3), 19.7 (CH2, C-7), 18.4 (CH3, C-20); EIMS m/z 434 [M]+ (1), 392 (9), 374 (33), 325 (10), 279 (7), 203 (100), 189 (32), 176 (12), 161 (12), 110 (14), 95 (16), 81 (15); anal. C 60.69%, H 5.89%, calcd for C22H26O9, C 60.82%, H 6.03%.
Compound 8
colorless plates (EtOAc – n-pentane); mp 252–255 °C, [α]D20 − 19.3 (c 0.223, CHCl3); Rf (3:1 CH2Cl2-acetone) 0.25; IR (KBr) νmax 3452, 2928, 1749, 1370, 1243, 1036, 969, 875, 737, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.47 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.8 Hz, H-16), 7.39 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.38 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.8 Hz, H-14), 5.82 (1H, d, J12β,11α = 10.9 Hz, H-12β), 5.32 (1H, d, J11α,12β = 10.9 Hz, H-11α), 5.09 (1H, dd, J1α,2α = 0.8 Hz, J1α,10β = 8.8 Hz, H-1α), 4.58 (1H, dd, J19a,6β = 2.1 Hz, J19a,19b = 8.6 Hz, pro-S H-19a), 4.25 (1H, ddd, J3α,2α = 1.4 Hz, J3α,4β = 8.0 Hz, J3α,3βOH = 6.8 Hz, H-3α), 4.20 (1H, d, J19b,19a = 8.6 Hz, pro-R H-19b), 4.12 (1H, d, J3βOH,3α = 6.8 Hz, 3β-OH), 3.85 (1H, dd, J2α,1α = 0.8 Hz, J2α,3α = 1.4 Hz, H-2α), 2.71 (1H, br dd, J8α,6α < 0.4 Hz, J8α,7α = 4.0 Hz, J8α,7β = 3.2 Hz, H-8α), 2.54 (1H, d, J4β,3α = 8.0 Hz, H-4β), 2.45 (1H, ddt, J7β,6α = J7β,8α = 3.2 Hz, J7β,6β = 3.5 Hz, J7β,7α = 14.4 Hz, H-7β), 2.27 (1H, d, J10β,1α = 8.8 Hz, H-10β), 2.03 (3H, s, 1β-OAc), 1.92 (1H, ddt, J7α,6α = J7α,8α = 4.0 Hz, J7α,6β =14.0 Hz, J7α,7β = 14.4 Hz, H-7α), 1.87 (3H, s, 11β-OAc), 1.87 (1H, br ddd, J6α,6β = 14.1 Hz, J6α,7α = 4.0 Hz, J6α,7β = 3.2 Hz, J6α,8α < 0.4 Hz, H-6α), 1.47 (1H, dddd, J6β,6α = 14.1 Hz, J6β,7α = 14.0 Hz, J6β,7β =3.5 Hz, J6β,19a = 2.1 Hz, H-6β), 1.06 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 178.5 (C, C-18), 170.1 (C, 1-OCOCH3), 169.7 (C, 11-OCOCH3), 169.3 (C, C-17), 144.2 (CH, C-15), 141.7 (CH, C-16), 121.4 (C, C-13), 108.6 (CH, C-14), 76.0 (CH, C-11), 74.4 (CH, C-2), 73.3 (CH, C-1), 73.0 (CH2, C-19), 71.1 (CH, C-12), 67.0 (CH, C-3), 49.3 (CH, C-8), 48.2 (CH, C-4), 42.9 (C, C-5), 41.9 (CH, C-10), 40.4 (C, C-9), 36.3 (CH2, C-6), 21.6 (CH3, 1-OCOCH3), 20.0 (CH3, 11-OCOCH3), 19.4 (CH2, C-7), 19.3 (CH3, C-20); EIMS m/z 492 [M]+ (9), 474 (7), 450 (46), 432 (46), 408 (30), 390 (17), 378 (21), 354 (14), 335 (20), 203 (87), 189 (100), 176 (55), 161 (29), 110 (27), 95 (42), 81 (35); anal. C 58.20%, H 5.49%, calcd for C24H28O11, C 58.53%, H 5.73%.
Compound 9
colorless rectangular plates (Me2CO – n-pentane); mp 237–240 °C; [α]D20 +28.7 (c 0.296, MeOH); Rf (3:1 CH2Cl2-acetone) 0.42; IR (KBr) νmax 3470, 3152, 3135, 2952, 1749, 1507, 1446, 1392, 1292, 1230, 1152, 1072, 1049, 1027, 991, 878, 789, 667, 604 cm−1; 1H NMR [400 MHz, 2:1 (CD3)2CO - CD3OD] δ 7.61 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.6 Hz, H-16), 7.53 (1H, t, J15,14 = J15,16 = 1.6 Hz, H-15), 6.51 (1H, dd, J14,15 = 1.6 Hz, J14,16 = 0.8 Hz, H-14), 5.54 (1H, dd, J12β,11α = 10.9 Hz, J12β,11β = 6.6 Hz, H-12β), 4.37 (1H, dd, J19a,6β = 2.0 Hz, J19a,19b = 9.4 Hz, pro-S H-19a), 4.24 (1H, d, J19b,19a = 9.4 Hz, pro-R H-19b), 4.02 (1H, ddd, J2α,1α = 2.7 Hz, J2α,1β = 4.7 Hz, J2α,3α = 2.9 Hz, H-2α), 3.73 (1H, dd, J3α,2α = 2.9 Hz, J3α,4β = 10.1 Hz, H-3α), 2.91 (1H, dd, J8β,7α = 10.3 Hz, J8β,7β = 5.3 Hz, H-8β), 2.28 (1H, dd, J10β,1α = 12.7 Hz, J10β,1β = 3.7 Hz, H-10β), 2.15 (1H, d, J4β,3α = 10.1 Hz, H-4β), 2.14 (1H, dd, J11β,11α = 13.8 Hz, J11β,12β = 6.6 Hz, H-11β), 1.85 (2H, m, H-7α and H-7β), 1.78 (2H, m, H-1β and H-11α), 1.75 (1H, m, H-6α), 1.74 (1H, ddd, J1α,1β = 14.2 Hz, J1α,2α = 2.7 Hz, J1α,10β = 12.7 Hz, H-1α), 1.34 (1H, dddd, J6β,6α = 13.5 Hz, J6β,7α = 14.0 Hz, J6β,7β = 4.0 Hz, J6β,19a = 2.0 Hz, H-6β), 0.77 (3H, s, Me-20); 13C NMR [100 MHz, 2:1 (CD3)2CO - CD3OD] δ 178.0 (C, C-18), 175.2 (C, C-17), 144.9 (CH, C-15), 141.1 (CH, C-16), 126.2 (C, C-13), 109.9 (CH, C-14), 70.8 (CH, C-12), 70.6 (CH2, C-19), 70.1 (CH, C-3), 69.5 (CH, C-2), 54.2 (CH, C-4), 47.8 (CH, C-8), 44.4 (C, C-5), 43.9 (CH2, C-11), 43.5 (CH, C-10), 37.0 (C, C-9), 35.4 (CH2, C-6), 27.7 (CH2, C-1), 20.3 (CH3, C-20), 19.5 (CH2, C-7); EIMS m/z 376 [M]+ (49), 361 (1), 265 (8), 252 (3), 238 (9), 216 (6), 171 (9), 143 (11), 131 (10), 121 (11), 105 (13), 94 (100), 81 (16), 55 (16); anal. C 63.61%, H 6.54%, calcd for C20H24O7, C 63.82%, H 6.43%.
General Procedure for Partial Acetylation: Compounds 10, 11, 12, 13, and 14
Treatment of 6 (50 mg, 0.133 mmol) with Ac2O-pyridine (1:2, 1 mL) for 15 min at room temperature followed by standard workup and purification by FC yielded compounds 10 (21 mg, 38%) and 11 (8 mg, 15%). Treatment of 7 (50 mg, 0.115 mmol) in the same conditions as for compound 6 gave, after 2 min, compounds 12 (22 mg, 40%) and 13 (8 mg, 15%). Finally, acetylation of 8 (40 mg, 0.081 mmol) for 1.5 h yielded 14 (22 mg, 50%).
Compound 10
FC eluent 97:3 CH2Cl2-acetone, Rf 0.22; colorless fine needles (EtOAc); mp 264–267 °C (decomp.); [α]D20 +19.7 (c 0.076, MeOH); IR (KBr) νmax 3575, 3440, 3003, 2925, 1785, 1742, 1721, 1455, 1372, 1237, 1202, 1183, 1131, 1075, 1028, 932, 876, 784, 691, 603 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.60 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.8 Hz, H-16), 7.50 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.54 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.8 Hz, H-14), 5.47 (1H, dd, J12β,11α = 12.5 Hz, J12β,11β = 3.3 Hz, H-12β), 5.08 (1H, dd, J3α,2α = 2.8 Hz, J3α,4β = 10.8 Hz, H-3α), 4.55 (1H, dd, J19a,6β = 1.8 Hz, J19a,19b = 9.5 Hz, pro-S H-19a), 4.47 (1H, d, J19b,19a = 9.5 Hz, pro-R H-19b), 4.17 (1H, dt, J2α,1α = J2α,3α = 2.8 Hz, J2α,1β = 3.6 Hz, H-2α), 2.58 (1H, br dd, J8α,6α < 0.3 Hz, J8α,7α = 4.6 Hz, J8α,7β = 2.2 Hz, H-8α), 2.40 (1H, d, J4β,3α = 10.8 Hz, H-4β), 2.39 (1H, dd, J10β,1α = 11.9 Hz, J10β,1β = 3.7 Hz, H-10β), 2.38 (1H, m, H-7β), 2.25 (1H, dd, J11β,11α = 14.9 Hz, J11β,12β = 3.3 Hz, H-11β), 2.08 (3H, s, 3β-OAc), 2.06 (1H, dddd, J7α,6α = 3.2 Hz, J7α,6β = 14.2 Hz, J7α,7β = 14.7 Hz, J7α,8α = 4.6 Hz, H-7α), 1.84 (1H, dd, J11α,11β = 14.9 Hz, J11α,12β = 12.5 Hz, H-11α), 1.83 (2H, m, H-1α and H-1β), 1.66 (1H, br ddd, J6α,6β = 13.7 Hz, J6α 7α = 3.2 Hz, J6α,7β = 2.9 Hz, J6α,8α < 0.3 Hz, H-6α), 1.25 (1H, dddd, J6β,6α = 13.7 Hz, J6β,7α = 14.2 Hz, J6β,7β = 3.6 Hz, J6β,19a = 1.8 Hz, H-6β), 0.97 (3H, s, Me-20); 13C NMR (100 MHz, CD3OD) δ 178.2 (C, C-18), 174.8 (C, C-17), 171.8 (C, 3-OCOCH3), 145.0 (CH, C-15), 141.3 (CH, C-16), 126.7 (C, C-13), 109.5 (CH, C-14), 72.2 (CH, C-12), 72.0 (CH, C-3), 71.4 (CH2, C-19), 67.2 (CH, C-2), 51.3 (CH, C-4), 49.9 (CH, C-8), 45.0 (C, C-5), 41.7 (CH2, C-11), 36.1 (C, C-9), 35.7 (CH, C-10), 33.9 (CH2, C-6), 27.7 (CH2, C-1), 23.0 (CH3, C-20), 20.8 (CH3, 3-OCOCH3), 20.1 (CH2, C-7); EIMS m/z 418 [M]+ (92), 400 (2), 376 (14), 375 (14), 341 (25), 265 (8), 247 (30), 203 (14), 161 (18), 145 (18), 105 (20), 94 (100), 81 (22), 55 (15); anal. C 63.31%, H 6.09%, calcd for C22H26O8, C 63.15%, H 6.26%.
Compound 11
FC eluent 97:3 CH2Cl2-acetone, Rf 0.28; colorless prisms (MeOH); mp 197–200 °C; [α]D20 +28.1 (c 0.032, MeOH); IR (KBr) νmax 3448, 3145, 3115, 2929, 1779, 1738, 1694, 1505, 1449, 1372, 1295, 1246, 1181, 1131, 1074, 1015, 875, 787, 693, 601 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.56 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.9 Hz, H-16), 7.51 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.51 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 0.8 Hz, H-14), 5.20 (1H, td, J2α,1α = 2.1 Hz, J2α,1β = J2α,3α = 3.2 Hz, H-2α), 5.18 (1H, dd, J12β,11α = 12.8 Hz, J12β,11β = 3.2 Hz, H-12β), 4.47 (1H, dd, J19a,6β = 1.8 Hz, J19a,19b = 9.6 Hz, pro-S H-19a), 4.43 (1H, d, J19b,19a = 9.6 Hz, pro-R H-19b), 3.92 (1H, dd, J3α,2α = 3.2 Hz, J3α,4β = 10.0 Hz, H-3α), 2.59 (1H, ddd, J8α,6α = 0.6 Hz, J8α,7α = 4.8 Hz, J8α,7β = 3.5 Hz, H-8α), 2.38 (1H, dddd, J7β,6α = 2.8 Hz, J7β,6β = 3.2 Hz, J7β,7α = 14.0 Hz, J7β,8α = 3.5 Hz, H-7β), 2.24 (1H, d, J4β,3α = 10.0 Hz, H-4β), 2.20 (1H, dd, J11β,11α = 15.0 Hz, J11β,12β = 3.2 Hz, H-11β), 2.16 (1H, dd, J10β,1α = 12.8 Hz, J10β,1β = 3.4 Hz, H-10β), 2.13 (3H, s, 2β-OAc), 2.08 (1H, m, H-1β), 2.01 (1H, dddd, J7α,6α = 3.6 Hz, J7α,6β = 14.8 Hz, J7α,7β = 14.0 Hz, J7α,8α = 4.8 Hz, H-7α), 1.85 (1H, ddd, J1α,1β = 14.8 Hz, J1α,2α = 2.1 Hz, J1α,10β = 12.8 Hz, H-1α), 1.84 (1H, dd, J11α,11β = 15.0 Hz, J11α,12β = 12.8 Hz, H-11α), 1.70 (1H, dddd, J6α,6β = 14.0 Hz, J6α 7α = 3.6 Hz, J6α,7β = 2.8 Hz, J6α,8α = 0.6 Hz, H-6α), 1.24 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 14.8 Hz, J6β,7β = 3.2 Hz, J6β,19a = 1.8 Hz, H-6β), 0.96 (3H, s, Me-20); 13C NMR (100 MHz, CD3OD) δ 178.1 (C, C-18), 174.5 (C, C-17), 172.2 (C, 2-OCOCH3), 145.2 (CH, C-15), 141.2 (CH, C-16), 126.4 (C, C-13), 109.4 (CH, C-14), 73.3 (CH, C-2), 72.3 (CH, C-12), 71.3 (CH2, C-19), 68.7 (CH, C-3), 55.1 (CH, C-4), 49.9 (CH, C-8), 44.6 (C, C-5), 41.6 (CH2, C-11), 37.1 (CH, C-10), 36.1 (C, C-9), 34.2 (CH2, C-6), 25.1 (CH2, C-1), 22.8 (CH3, C-20), 21.1 (CH3, 2-OCOCH3), 20.1 (CH2, C-7); EIMS m/z 418 [M]+ (59), 400 (1), 376 (7), 375 (7), 358 (11), 341 (13), 247 (22), 203 (12), 161 (18), 143 (17), 105 (20), 94 (100), 81 (26), 55 (21); anal. C 63.01%, H 6.18%, calcd for C22H26O8, C 63.15%, H 6.26%.
Compound 12
FC eluent 90:10 CH2Cl2-acetone, Rf 0.33; colorless prisms (EtOAc – n-pentane); mp 157–160 °C; [α]D20 −48.6 (c 0.111, CHCl3); IR (KBr) νmax 3463, 3145, 2927, 1779, 1745, 1506, 1447, 1376, 1223, 1161, 1040, 1012, 933, 875, 729, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.49 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.9 Hz, H-16), 7.39 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.39 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 0.8 Hz, H-14), 5.39 (1H, d, J12β,11α = 10.7 Hz, H-12β), 5.18 (1H, d, J11α,12β = 10.7 Hz, H-11α), 5.04 (1H, dd, J3α,2α = 2.8 Hz, J3α,4β = 10.8 Hz, H-3α), 4.32 (1H, dd, J19a,6β = 1.9 Hz, J19a,19b = 9.6 Hz, pro-S H-19a), 4.27 (1H, d, J19b,19a = 9.6 Hz, pro-R H-19b), 4.18 (1H, ddd, J2α,1α = 3.0 Hz, J2α,1β = 4.6 Hz, J2α,3α = 2.8 Hz, H-2α), 2.66 (1H, br dd, J8α,6α < 0.5 Hz, J8α,7α = 4.1 Hz, J8α,7β = 2.4 Hz, H-8α), 2.64 (1H, dd, J10β,1α = 10.0 Hz, J10β,1β = 2.8 Hz, H-10β), 2.48 (2H, m, H-1β and H-7β), 2.44 (1H, d, J4β,3α = 10.8 Hz, H-4β), 2.16 (3H, s, 3β-OAc), 1.96 (3H, s, 11β-OAc), 1.90 (1H, ddt, J7α,6α = J7α,8α = 4.1 Hz, J7α,6β =14.2 Hz, J7α,7β = 14.6 Hz, H-7α), 1.75 (1H, br ddd, J6α,6β = 14.0 Hz, J6α,7α = 4.0 Hz, J6α,7β = 3.1 Hz, J6α,8α < 0.5 Hz, H-6α), 1.66 (1H, ddd, J1α,1β = 13.8 Hz, J1α,2α = 3.0 Hz, J1α,10β = 10.0 Hz, H-1α), 1.32 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 14.2 Hz, J6β,7β = 3.9 Hz, J6β,19a = 1.9 Hz, H-6β), 0.92 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 174.8 (C, C-18), 169.8 (C, 3-OCOCH3), 169.7 (C, C-17), 169.1 (C, 11-OCOCH3), 143.9 (CH, C-15), 141.5 (CH, C-16), 121.4 (C, C-13), 108.5 (CH, C-14), 76.4 (CH, C-11), 71.4 (CH, C-12), 69.9 (CH, C-3), 69.6 (CH2, C-19), 66.6 (CH, C-2), 49.6 (CH, C-4), 49.3 (CH, C-8), 43.9 (C, C-5), 38.9 (C, C-9), 34.0 (CH, C-10), 32.1 (CH2, C-6), 27.5 (CH2, C-1), 20.9 (C, 3-OCOCH3), 20.7 (CH3, 11-OCOCH3), 19.7 (CH2, C-7), 18.4 (CH3, C-20); EIMS m/z 476 [M]+ (5), 458 (4), 434 (9), 416 (45), 398 (17), 374 (14), 356 (16), 338 (16), 325 (18), 203 (100), 189 (44), 110 (16), 95 (19), 81 (17); anal. C 60.29%, H 5.78%, calcd for C24H28O10, C 60.50%, H 5.92%.
Compound 13
FC eluent 90:10 CH2Cl2-acetone, Rf 0.40; amorphous, white solid; [α]D20 −38.9 (c 0.072, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.43 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 1.9 Hz, H-16), 7.40 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.36 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 1.0 Hz, H-14), 5.29 (1H, ddd, J2α,1α = 2.7 Hz, J2α,1β = 4.8 Hz, J2α,3α = 3.1 Hz, H-2α), 5.15 (1H, d, J11α,12β = 10.9 Hz, H-11α), 5.10 (1H, d, J12β,11α = 10.9 Hz, H-12β), 4.30 (1H, d, J19a,19b = 9.4 Hz, pro-R H-19a), 4.23 (1H, dd, J19b,6β = 1.9 Hz, J19b,19a = 9.4 Hz, pro-S H-19b), 3.89 (1H, ddd, J3α,2α = 3.1 Hz, J3α,4β = 9.4 Hz, J3α,3βOH = 4.3 Hz, H-3α), 2.70 (2H, m, H-1β and H-7β), 2.67 (1H, br dd, J8α,6α < 0.5 Hz, J8α,7α = 4.7 Hz, J8α,7β = 2.5 Hz, H-8α), 2.44 (1H, d, J3βOH,3α = 4.3 Hz, 3β-OH), 2.42 (1H, dd, J10β,1α = 12.9 Hz, J10β,1β = 3.9 Hz, H-10β), 2.31 (1H, d, J4β,3α = 9.4 Hz, H-4β), 2.19 (3H, s, 2β-OAc), 1.96 (3H, s, 11β-OAc), 1.92 (1H, dddd, J7α,6α = 4.1 Hz, J7α,6β = 14.2 Hz, J7α,7β = 14.4 Hz, J7α,8α = 4.7 Hz, H-7α), 1.80 (1H, br ddd, J6α,6β = 14.2 Hz, J6α,7α = 4.1 Hz, J6α,7β = 3.2 Hz, J6α,8α < 0.5 Hz, H-6α), 1.72 (1H, ddd, J1α,1β = 15.2 Hz, J1α,2α = 2.7 Hz, J1α,10β = 12.9 Hz, H-1α), 1.35 (1H, tdd, J6β,6α = J6β,7α = 14.2 Hz, J6β,7β = 3.8 Hz, J6β,19b = 1.9 Hz, H-6β), 0.92 (3H, s, Me-20); EIMS m/z 476 [M]+ (17), 434 (16), 416 (32), 398 (17), 374 (11), 338 (11), 325 (22), 203 (100), 189 (33), 176 (14), 110 (16), 95 (22), 81 (18); C24H28O10, Mr 476.
Compound 14
FC eluent 90:10 CH2Cl2-acetone, Rf 0.25; colorless prisms (EtOAc – n-pentane); mp 190–193 °C, [α]D20 −20.5 (c 0.341, CHCl3); IR (KBr) νmax 3451, 3015, 2943, 1745, 1506, 1444, 1370, 1239, 1143, 1039, 970, 899, 875, 737, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.46 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 1.8 Hz, H-16), 7.39 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.38 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 1.0 Hz, H-14), 5.81 (1H, d, J12β,11α = 10.9 Hz, H-12β), 5.34 (1H, d, J11α,12β = 10.9 Hz, H-11α), 5.13 (1H, dd, J1α,2α = 0.8 Hz, J1α,10β = 8.8 Hz, H-1α), 5.09 (1H, dd, J3α,2α = 1.9 Hz, J3α,4β = 8.7 Hz, H-3α), 4.63 (1H, dd, J19a,6β = 2.1 Hz, J19a,19b = 8.7 Hz, pro-S H-19a), 4.14 (1H, d, J19b,19a = 8.7 Hz, pro-R H-19b), 3.92 (1H, ddd, J2α,1α = 0.8 Hz, J2α,3α = 1.9 Hz, J2α,2βOH = 3.7 Hz, H-2α), 3.68 (1H, d, J2βOH,2α = 3.7 Hz, 3β-OH), 2.90 (1H, d, J4β,3α = 8.7 Hz, H-4β), 2.71 (1H, ddd, J8α,6α = 1.0 Hz, J8α,7α = 4.2 Hz, J8α,7β = 3.3 Hz, H-8α), 2.45 (1H, ddt, J7β,6α = J7β,8α = 3.3 Hz, J7β,6β = 3.7 Hz, J7β,7α = 14.6 Hz, H-7β), 2.36 (1H, d, J10β,1α = 8.8 Hz, H-10β), 2.14 (3H, s, 3β-OAc), 2.04 (3H, s, 1β-OAc), 1.92 (1H, ddt, J7α,6α = J7α,8α = 4.2 Hz, J7α,6β = 14.0 Hz, J7α,7β = 14.6 Hz, H-7α), 1.88 (3H, s, 11β-OAc), 1.83 (1H, dddd, J6α,6β = 14.2 Hz, J6α,7α = 4.1 Hz, J6α,7β = 3.3 Hz, J6α,8α = 1.0 Hz, H-6α), 1.47 (1H, dddd, J6β,6α = 14.2 Hz, J6β,7α = 14.0 Hz, J6β,7β = 3.7 Hz, J6β,19a = 2.1 Hz, H-6β), 1.08 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 174.4 (C, C-18), 170.5 (C, 3-OCOCH3), 169.8 (C, 1-OCOCH3), 169.6 (C, 11-OCOCH3), 169.0 (C, C-17), 144.2 (CH, C-15), 141.7 (CH, C-16), 121.4 (C, C-13), 108.6 (CH, C-14), 76.0 (CH, C-11), 73.0 (CH, C-1), 72.5 (CH, C-2), 72.0 (CH2, C-19), 71.0 (CH, C-12), 68.5 (CH, C-3), 49.2 (CH, C-8), 45.4 (CH, C-4), 42.9 (CH, C-10), 41.9 (C, C-5), 40.4 (C, C-9), 36.2 (CH2, C-6), 21.5 (CH3, 1-OCOCH3), 20.8 (CH3, 3-OCOCH3), 20.0 (CH3, 11-OCOCH3), 19.5 (CH3, C-20), 19.3 (CH2, C-7); EIMS m/z 534 [M]+ (4), 516 (1), 492 (32), 474 (48), 450 (19), 432 (18), 415 (19), 372 (16), 354 (23), 337 (24), 277 (16), 219 (17), 204 (100), 203 (97), 202 (46), 189 (100), 176 (68), 161 (31), 110 (28), 95 (43), 81 (38); anal. C 58.19%, H 5.41%, calcd for C26H30O12, C 58.42%, H 5.66%.
General Procedure for Complete Acetylation: Compounds 15, 16, 17 and 18
Treatment of compounds 6–9 (0.05 mmol) with Ac2O-pyridine (1:2, 400 μL) for 24 h at room temperature followed by standard workup and purification by FC yielded quantitatively the acetyl derivatives 15–18, respectively.
Compound 15
FC eluent 97:3 CH2Cl2-acetone, Rf 0.45; colorless prisms (EtOAc – n-pentane); mp 283–286 °C; [α]D20 +25.7 (c 0.167, CHCl3); IR (KBr) νmax 3139, 2926, 1784, 1746, 1726, 1449, 1366, 1293, 1247, 1179, 1132, 1054, 1028, 1008, 923, 875, 802, 785, 691, 604 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.41 (2H, m, H-15 and H-16), 6.38 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 1.0 Hz, H-14), 5.43 (1H, ddd, J2α,1α = 2.4 Hz, J2α,1β = 4.8 Hz, J2α,3α = 3.1 Hz, H-2α), 5.17 (1H, dd, J12β,11α = 12.7 Hz, J12β,11β = 3.2 Hz, H-12β), 5.05 (1H, dd, J3α,2α = 3.1 Hz, J3α,4β = 11.1 Hz, H-3α), 4.33 (2H, s, H2-19), 2.53 (1H, ddt, J7β,6α = J7β,8α = 2.6 Hz, J7β,6β = 3.4 Hz, J7β,7α = 14.7 Hz, H-7β), 2.47 (1H, br dd, J8α,6α < 0.4 Hz, J8α,7α = 4.5 Hz, J8α,7β = 2.6 Hz, H-8α), 2.38 (1H, d, J4β,3α = 11.1 Hz, H-4β), 2.22 (1H, dd, J10β,1α = 13.6 Hz, J10β,1β = 2.9 Hz, H-10β), 2.17 (1H, dd, J11β,11α = 15.0 Hz, J11β,12β = 3.2 Hz, H-11β), 2.13 (3H, s, 2β-OAc), 2.04 (3H, s, 3β-OAc), 1.93 (1H, ddd, J1β,1α = 15.2 Hz, J1β,2α = 4.8 Hz, J1β,10β = 2.9 Hz, H-1β), 1.91 (1H, dddd, J7α,6α = 2.8 Hz, J7α,6β = 14.3 Hz, J7α,7β = 14.7 Hz, J7α,8α = 4.5 Hz, H-7α), 1.78 (1H, m, H-6α), 1.75 (1H, dd, J11α,11β = 15.0 Hz, J11α,12β = 12.7 Hz, H-11α), 1.74 (1H, ddd, J1α,1β = 15.2 Hz, J1α,2α = 2.4 Hz, J1α,10β = 13.6 Hz, H-1α), 1.36 (1H, ddd, J6β,6α = 13.8 Hz, J6β,7α = 14.3 Hz, J6β,7β = 3.4 Hz, H-6β), 0.95 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 174.0 (C, C-18), 171.4 (C, C-17), 170.0 (C, 3-OCOCH3), 169.9 (C, 2-OCOCH3), 144.0 (CH, C-15), 139.5 (CH, C-16), 124.5 (C, C-13), 108.1 (CH, C-14), 70.4 (CH, C-12), 69.5 (CH2, C-19), 67.9 (CH, C-3), 67.8 (CH, C-2), 49.8 (CH, C-4), 48.8 (CH, C-8), 43.5 (C, C-5), 40.9 (CH2, C-11), 35.7 (CH, C-10), 35.0 (C, C-9), 32.5 (CH2, C-6), 24.6 (CH2, C-1), 22.9 (CH3, C-20), 21.1 (CH3, 2-OCOCH3), ), 20.6 (CH3, 3-OCOCH3), 19.1 (CH2, C-7); EIMS m/z 460 [M]+ (100), 418 (46), 400 (5), 375 (6), 357 (62), 341 (52), 247 (31), 203 (15), 145 (14), 94 (47), 81 (14); anal. C 62.81%, H 6.20%, calcd for C24H28O9, C 62.60%, H 6.13%.
Compound 16
FC eluent 95:5 CH2Cl2-acetone, Rf 0.35; colorless quadrangular plaques (EtOAc-petroleum ether); mp 260–263 °C; [α]D20 −37.9 (c 0.269, CHCl3); IR (KBr) νmax 3145, 2936, 1776, 1750, 1747, 1447, 1373, 1229, 1186, 1161, 1139, 1057, 876, 735, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.41 (1H, dd, J16,14 = 0.9 Hz, J16,15 = 1.8 Hz, H-16), 7.39 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.35 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.9 Hz, H-14), 5.44 (1H, dt, J2α,1α = J2α,3α = 3.1 Hz, J2α,1β = 4.8 H, H-2α), 5.16 (1H, d, J11α,12β = 10.8 Hz, H-11α), 5.10 (1H, d, J12β,11α = 10.8 Hz, H-12β), 5.02 (1H, dd, J3α,2α = 3.1 Hz, J3α,4β = 11.0 Hz, H-3α), 4.33 (1H, dd, J19a,6β = 1.6 Hz, J19a,19b = 9.5 Hz, pro-S H-19a), 4.28 (1H, d, J19b,19a = 9.5 Hz, pro-R H-19b), 2.68 (1H, br dd, J8α,6α < 0.5 Hz, J8α,7α = 4.1 Hz, J8α,7β = 2.8 Hz, H-8α), 2.41 (1H, dd, J10β,1α = 13.2 Hz, J10β,1β = 3.3 Hz, H-10β), 2.50 (2H, m, H-1β and H-7β), 2.40 (1H, d, J4β,3α = 11.0 Hz, H-4β), 2.17 (3H, s, 3β- or 2β-OAc), 2.03 3H, s, 2β- or 3β-OAc), 1.95 (3H, s, 11β-OAc), 1.92 (1H, dddd, J7α,6α = 3.9 Hz, J7α,6β = 13.8 Hz, J7α,7β = 14.4 Hz, J7α,8α = 4.1 Hz, H-7α), 1.78 (2H, m, H-1α and H-6α), 1.34 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 13.8 Hz, J6β,7β = 4.0 Hz, J6β,19a = 1.6 Hz, H-6β), 0.92 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 173.8 (C, C-18), 170.1 (C, 3-OCOCH3), 170.0 (C, 2-OCOCH3), 169.5 (C, C-17), 168.9 (C, 11-OCOCH3), 144.1 (CH, C-15), 141.2 (CH, C-16), 121.2 (C, C-13), 108.3 (CH, C-14), 76.1 (CH, C-11), 71.5 (CH, C-12), 69.4 (CH2, C-19), 67.9 (CH, C-2), 67.5 (CH, C-3), 49.8 (CH, C-4), 49.2 (CH, C-8), 43.9 (C, C-5), 38.9 (C, C-9), 35.6 (CH, C-10), 32.1 (CH2, C-6), 25.9 (CH2, C-1), 21.1 (C, 2- or 3-OCOCH3), 20.7 (CH3, 3- or 2-OCOCH3), 20.6 (CH3, 11-OCOCH3), 19.7 (CH2, C-7), 18.2 (CH3, C-20); EIMS m/z 518 [M]+ (9), 476 (46), 458 (60), 434 (18), 416 (18), 398 (50), 374 (21), 357 (22), 338 (29), 325 (18), 203 (100), 202 (99), 189 (84), 161 (22), 110 (25), 95 (31), 81 (29); anal. C 60.41%, H 5.62%, calcd for C26H30O11, C 60.23%, H 5.83%.
Compound 17
FC eluent 95:5 CH2Cl2-acetone, Rf 0.27; colorless fine plates (EtOAc – n-pentane); mp 285–287 °C, [α]D20 −29.9 (c 0.267, CHCl3); IR (KBr) νmax 3145, 2932, 1752, 1436, 1371, 1233, 1162, 1043, 958, 875, 738, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.47 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 1.8 Hz, H-16), 7.40 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.39 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 1.0 Hz, H-14), 5.80 (1H, d, J12β,11α = 11.0 Hz, H-12β), 5.34 (1H, d, J11α,12β = 11.0 Hz, H-11α), 5.22 (2H, m, H-2α and H-3α), 5.09 (1H, br d, J1α,2α < 0.4 Hz, J1α,10β = 8.8 Hz, H-1α), 4.45 (1H, dd, J19a,6β = 2.1 Hz, J19a,19b = 9.1 Hz, pro-S H-19a), 4.21 (1H, d, J19b,19a = 9.1 Hz, pro-R H-19b), 2.88 (1H, d, J4β,3α = 8.7 Hz, H-4β), 2.73 (1H, ddd, J8α,6α = 1.2 Hz, J8α,7α = 4.0 Hz, J8α,7β = 3.4 Hz, H-8α), 2.48 (1H, dddd, J7β,6α = 2.9 Hz, J7β,6β = 3.8 Hz, J7β,7α = 14.4 Hz, J7β,8α = 3.4 Hz, H-7β), 2.42 (1H, d, J10β,1α = 8.8 Hz, H-10β), 2.09 (6H, s, 2β-OAc and 3β-OAc), 2.06 (3H, s, 1β-OAc), 1.93 (1H, ddt, J7α,6α = J7α,8α = 4.0 Hz, J7α,6β = 14.0 Hz, J7α,7β = 14.4 Hz, H-7α), 1.87 (3H, s, 11β-OAc), 1.85 (1H, dddd, J6α,6β = 14.7 Hz, J6α,7α = 4.1 Hz, J6α,7β = 2.9 Hz, J6α,8α = 1.2 Hz, H-6α), 1.52 (1H, dddd, J6β,6α = 14.2 Hz, J6β,7α = 14.0 Hz, J6β,7β = 3.8 Hz, J6β,19a = 2.1 Hz, H-6β), 1.07 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 173.3 (C, C-18), 170.4 (C, 3- or 2-OCOCH3), 169.6 (C, 11-OCOCH3), 169.1 (C, C-17), 168.7 (C, 1-OCOCH3), 168.1 (C, 2- or 3-OCOCH3), 144.3 (CH, C-15), 141.7 (CH, C-16), 121.3 (C, C-13), 108.5 (CH, C-14), 75.9 (CH, C-11), 71.7 (CH2, C-19), 71.36 (2CH, C-1 and C-2), 71.0 (CH, C-12), 66.5 (CH, C-3), 49.3 (CH, C-8), 45.7 (CH, C-4), 43.0 (C, C-5), 41.7 (CH, C-10), 40.4 (C, C-9), 36.3 (CH2, C-6), 21.4 (CH3, 1-OCOCH3), 20.7 (CH3, 3- or 2-OCOCH3), 20.4 (CH3, 2- or 3-OCOCH3), 20.0 (CH3, 11-OCOCH3), 19.4 (CH3, C-20), 19.2 (CH2, C-7); EIMS m/z 576 [M]+ (2), 534 (23), 516 (52), 492 (24), 474 (35), 456 (25), 431 (18), 371 (22), 354 (28), 337 (32), 204 (94), 203 (96), 202 (53), 189 (100), 176 (50), 161 (26), 110 (23), 95 (33), 81 (27); anal. C 58.29%, H 5.73%, calcd for C28H32O13, C 58.33%, H 5.59%.
Compound 18
FC eluent 95:5 CH2Cl2-acetone, Rf 0.47; amorphous, vitreous solid; [α]D20 +32.0 (c 0.147, CHCl3); IR (KBr) νmax 3148, 2948, 1781, 1746, 1506, 1373, 1227, 1180, 1155, 1060, 1020, 875, 805, 738, 602 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.45 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.7 Hz, H-16), 7.42 (1H, t, J15,14 = J15,16 = 1.7 Hz, H-15), 6.40 (1H, dd, J14,15 = 1.7 Hz, J14,16 = 0.8 Hz, H-14), 5.46 (1H, ddd, J2α,1α = 2.3 Hz, J2α,1β = 4.8 Hz, J2α,3α = 3.1 Hz, H-2α), 5.37 (1H, dd, J12β,11α = 10.4 Hz, J12β,11β = 7.1 Hz, H-12β), 5.05 (1H, dd, J3α,2α = 3.1 Hz, J3α,4β = 11.0 Hz, H-3α), 4.31 (1H, dd, J19a,6β = 2.0 Hz, J19a,19b = 9.5 Hz, pro-S H-19a), 4.24 (1H, d, J19b,19a = 9.5 Hz, pro-R H-19b), 2.66 (1H, dd, J8β,7α = 11.9 Hz, J8β,7β = 3.9 Hz, H-8β), 2.41 (1H, d, J4β,3α = 11.0 Hz, H-4β), 2.13 (3H, s, 2β-OAc), 2.04 (1H, m, H-7β), 2.03 (3H, s, 3β-OAc), 2.02 (1H, m, H-7α), 2.00 (1H, m, H-6α), 1.95 (1H, dd, J10β,1α = 13.5 Hz, J10β,1β = 2.8 Hz, H-10β), 1.88 (3H, m, H-1β, H-11α, and H-11β), 1.76 (1H, ddd, J1α,1β = 15.2 Hz, J1α,2α = 2.3 Hz, J1α,10β = 13.5 Hz, H-1α), 1.38 (1H, tdd, J6β,6α = J6β,7α = 13.6 Hz, J6β,7β = 3.8 Hz, J6β,19a = 2.0 Hz, H-6β), 0.82 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 174.1 (C, C-18), 172.9 (C, C-17), 169.9 (C, 3-OCOCH3), 169.7 (C, 2-OCOCH3), 144.0 (CH, C-15), 139.7 (CH, C-16), 124.0 (C, C-13), 108.5 (CH, C-14), 69.8 (CH, C-12), 69.5 (CH2, C-19), 68.0 (CH, C-3), 67.6 (CH, C-2), 49.7 (CH, C-4), 47.2 (CH, C-8), 44.3 (CH, C-10), 43.6 (C, C-5), 43.2 (CH2, C-11), 36.2 (C, C-9), 34.3 (CH2, C-6), 25.1 (CH2, C-1), 21.0 (CH3, 2-OCOCH3), 20.6 (CH3, 3-OCOCH3), 19.7 (CH3, C-20), 18.5 (CH2, C-7); EIMS m/z 460 [M]+ (34), 445 (1), 418 (14), 400 (4), 376 (6), 358 (19), 234 (15), 220 (15), 188 (20), 143 (15), 94 (100), 81 (14); anal. C 62.33%, H 5.97%, calcd for C24H28O9, C 62.60%, H 6.13%.
Preparation of the Thiocarbonate 19 from Compound 9
A solution of compound 9 (100 mg, 0.266 mmol), 95 % 1,1′-thiocarbonyldiimidazole (165 mg, 0.879 mmol), and 4-DMAP (6 mg, 0.049 mmol) in dry CH2Cl2 (10 mL) was heated to reflux under Ar for 3.5 h. Then, the solvent was removed in vacuo and the solid residue was purified by FC (90:10 CH2Cl2-acetone as eluent) to give pure 19 (64 mg, 58%): colorless quadrangular plates (MeOH); mp 277−280 °C; [α]D20 +76.2 (c 0.021, pyridine); IR (KBr) νmax 3153, 3126, 2939, 1774, 1750, 1509, 1445, 1354, 1222, 1185, 1155, 1143, 1068, 1030, 876, 823, 603 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.60 (1H, dd, J16,14 = 0.9 Hz, J16,15 = 1.9 Hz, H-16), 7.51 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.51 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 0.9 Hz, H-14), 5.52 (1H, dd, J12β,11α = 11.0 Hz, J12β,11β = 6.5 Hz, H-12β), 4.91 (1H, ddd, J2α,1α = 3.0 Hz, J2α,1β = 2.7 Hz, J2α,3α = 4.7 Hz, H-2α), 4.47 (1H, dd, J19a,6β = 2.0 Hz, J19a,19b = 9.6 Hz, pro-S H-19a), 4.37 (1H, d, J19b,19a = 9.6 Hz, pro-R H-19b), 3.99 (1H, dd, J3α,2α = 4.7 Hz, J3α,4β = 9.8 Hz, H-3α), 2.96 (1H, dd, J8β,7α = 11.4 Hz, J8β,7β = 4.4 Hz, H-8β), 2.53 (1H, d, J4β,3α = 9.8 Hz, H-4β), 2.30 (1H, dt, J1β,1α = 15.3, J1β2α = J1β,10β = 2.7 Hz, H-1β), 2.18 (1H, dd, J10β,1α = 13.8 Hz, J10β,1β = 2.7 Hz, H-10β), 2.13 (1H, dd, J11β,11α = 13.8 Hz, J11β,12β = 6.5 Hz, H-11β), 1.98 (1H, ddd, J1α,1β = 15.3 Hz, J1α,2α = 3.0 Hz, J1α,10β = 13.8 Hz, H-1α), 1.93 (1H, m, H-6α), 1.90 (1H, dd, J11α,11β = 13.8 Hz, J11α,12β = 11.0 Hz, H-11α), 1.88 (2H, m, H-7α and H-7β), 1.43 (1H, dddd, J6β,6α = 13.6 Hz, J6β,7α = 13.9 Hz, J6β,7β = 4.7 Hz, J6β,19a = 2.0 Hz, H-6β), 0.83 (3H, s, Me-20); 13C NMR (100 MHz, CD3OD) δ 183.4 (C, C=S), 177.9 (C, C-18), 176.4 (C, C-17), 145.2 (CH, C-15), 141.4 (CH, C-16), 125.9 (C, C-13), 109.8 (CH, C-14), 82.2 (CH, C-2), 79.5 (CH, C-3), 71.6 (CH2, C-19), 71.0 (CH, C-12), 57.4 (CH, C-4), 47.7 (CH, C-8), 44.2 (C, C-5), 43.9 (CH2, C-11), 43.3 (CH, C-10), 37.2 (C, C-9), 35.6 (CH2, C-6), 24.4 (CH2, C-1), 20.1 (CH3, C-20), 19.6 (CH2, C-7); EIMS m/z 418 [M]+ (47), 403 (1), 374 (4), 358 (3), 322 (4), 307 (4), 280 (9), 217 (6), 105 (13), 94 (100), 81 (14), 55 (15); anal. C 60.01%, H 5.43%, S 7.31%, calcd for C21H22O7S, C 60.28%, H 5.30%, S 7.66%.
Preparation of Compound 20 from Compound 19
A solution of 19 (60 mg, 0.143 mmol), 120 μL (126 mg, 0.433 mmol) of 97% n-Bu3SnH, and 2,2′-azobis(2-methylpropionitrile) (AIBN, 5 mg, 0.03 mmol) in toluene (5 mL) was heated to reflux under Ar for 4 h. Then, the mixture was evaporated to a small volume and partitioned between acetonitrile and n-hexane (5 mL each). The acetonitrile layer was washed with n-hexane (4×5 mL) to remove the residual tin compounds. The acetonitrile phase was distilled in vacuo and the residue was purified by FC (80:20 CH2Cl2-acetone as eluent) to give pure 20 (42 mg, 82%): colorless fine needles (EtOAc-petroleum ether); mp 224–227 °C; [α]D20 +9.6 (c 0.146, MeOH); Rf (80:20 CH2Cl2-acetone) 0.32; IR (KBr) νmax 3474, 3152, 3136, 2950, 2574, 1745, 1507, 1445, 1379, 1293, 1230, 1165, 1152, 1053, 1027, 986, 878, 790, 672, 602 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.58 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 2.0 Hz, H-16), 7.51 (1H, t, J15,14 = J15,16 = 2.0 Hz, H-15), 6.49 (1H, dd, J14,15 = 2.0 Hz, J14,16 = 1.0 Hz, H-14), 5.51 (1H, dd, J12β,11α = 11.0 Hz, J12β,11β = 6.6 Hz, H-12β), 4.49 (1H, dd, J19a,6β = 2.0 Hz, J19a,19b = 9.4 Hz, pro-S H-19a), 4.32 (1H, d, J19b,19a = 9.4 Hz, pro-R H-19b), 4.21 (1H, quint, J2α,1α = J2α,1β = J2α,3α = J2α,3β = 3.1 Hz, H-2α), 2.91 (1H, dd, J8β,7α = 10.8 Hz, J8β,7β = 5.0 Hz, H-8β), 2.35 (1H, dd, J4β,3α = 12.6 Hz, J4β,3β = 6.3 Hz, H-4β), 2.26 (1H, dd, J10β,1α = 11.7 Hz, J10β,1β = 4.7 Hz, H-10β), 2.15 (1H, dd, J11β,11α = 14.0 Hz, J11β,12β = 6.6 Hz, H-11β), 2.00 (1H, dddd, J3β,1β = 1.8 Hz, J3β,2α = 3.1 Hz, J3β,3α = 14.4 Hz, J3β,4β = 6.3 Hz, H-3β), 1.90 (2H, m, H-7α and H-7β), 1.85 (1H, m, H-6α), 1.81 (1H, dd, J11α,11β = 14.0 Hz, J11α,12β = 11.0 Hz, H-11α), 1.71 (1H, m, H-1β), 1.68 (2H, m, H-1α and H-3α), 1.36 (1H, dddd, J6β,6α = 13.8 Hz, J6β,7α = 13.3 Hz, J6β,7β = 4.2 Hz, J6β,19a = 2.0 Hz, H-6β), 0.80 (3H, s, Me-20); 13C NMR (100 MHz, CD3OD) δ 181.3 (C, C-18), 176.8 (C, C-17), 145.2 (CH, C-15), 141.3 (CH, C-16), 126.2 (C, C-13), 109.8 (CH, C-14), 71.6 (CH, C-12), 71.2 (CH2, C-19), 65.7 (CH, C-2), 48.3 (CH, C-8), 47.1 (CH, C-4), 44.2 (CH, C-10), 44.1 (C, C-5), 43.9 (CH2, C-11), 37.4 (C, C-9), 35.6 (CH2, C-6), 32.4 (CH2, C-3), 29.0 (CH2, C-1), 20.4 (CH3, C-20), 19.8 (CH2, C-7); EIMS m/z 360 [M]+ (48), 342 (3), 332 (2), 264 (4), 236 (11), 204 (13), 176 (12), 131 (25), 94 (100), 79 (14), 55 (14); anal. C 66.79%, H 6.48%, calcd for C20H24O6, C 66.65%, H 6.71%.
Acetylation of Compound 20 to Give Compound 21
Treatment of 20 (0.05 mmol) with Ac2O-pyridine (1:2, 400 μL) for 24 h at room temperature followed by standard workup and purification by FC (95:5 CH2Cl2-acetone as eluent) yielded quantitatively the acetyl derivative 21: amorphous, white solid; [α]D20 −9.1 (c 0.033, CHCl3); Rf (95:5 CH2Cl2-acetone) 0.27; IR (KBr) νmax 3146, 2950, 1776, 1738, 1505, 1377, 1244, 1229, 1179, 1155, 1026, 875, 805, 602 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.46 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.8 Hz, H-16), 7.43 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.40 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.8 Hz, H-14), 5.36 (1H, dd, J12β,11α = 10.5 Hz, J12β,11β = 7.0 Hz, H-12β), 5.24 (1H, quint, J2α,1α = J2α,1β = J2α,3α = J2α,3β = 3.1 Hz, H-2α), 4.32 (1H, dd, J19a,6β = 2.0 Hz, J19a,19b = 9.4 Hz, pro-S H-19a), 4.23 (1H, d, J19b,19a = 9.4 Hz, pro-R H-19b), 2.65 (1H, dd, J8β,7α = 12.0 Hz, J8β,7β = 4.0 Hz, H-8β), 2.32 (1H, dd, J4β,3α = 12.2 Hz, J4β,3β = 6.3 Hz, H-4β), 2.24 (1H, dddd, J3β,1β = 2.1 Hz, J3β,2α = 3.1 Hz, J3β,3α = 14.8 Hz, J3β,4β = 6.3 Hz, H-3β), 2.09 (3H, s, 2β-OAc), 2.05 (1H, m, H-1β), 2.01 (1H, m, H-10β), 2.00 (1H, m, H-6α), 1.91 (1H, dd, J11β,11α = 14.0 Hz, J11β,12β = 7.0 Hz, H-11β), 1.87 (2H, m, H-7α and H-7β), 1.84 (1H, dd, J11α,11β = 14.0 Hz, J11α,12β = 10.5 Hz, H-11α), 1.65 (1H, ddd, J3α,2α = 3.1 Hz, J3α,3β = 14.8 Hz, J3α,4β = 12.2 Hz, H-3α), 1.62 (1H, ddd, J1α,1β = 14.4 Hz, J1α,2α = 3.1 Hz, J1α,10β = 12.6 Hz, H-1α), 1.31 (1H, dddd, J6β,6α = 13.6 Hz, J6β,7α = 13.3 Hz, J6β,7β = 3.7 Hz, J6β,19a = 2.0 Hz, H-6β), 0.82 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 177.2 (C, C-18), 173.1 (C, C-17), 169.9 (C, 2-OCOCH3), 144.0 (CH, C-15), 139.7 (CH, C-16), 124.1 (C, C-13), 108.5 (CH, C-14), 69.8 (CH, C-12), 69.3 (CH2, C-19), 67.6 (CH, C-2), 47.4 (CH, C-8), 45.6 (CH, C-4), 44.6 (CH, C-10), 43.2 (CH2, C-11), 42.4 (C, C-5), 36.3 (C, C-9), 34.6 (CH2, C-6), 28.6 (CH2, C-3), 25.8 (CH2, C-1), 21.3 (CH3, 2-OCOCH3), 19.8 (CH3, C-20), 18.7 (CH2, C-7); EIMS m/z 402 [M]+ (44), 342 (15), 218 (32), 204 (16), 176 (20), 145 (20), 131 (33), 94 (100), 91 (27), 81 (14), 55 (11); anal. C 65.41%, H 6.48%, calcd for C22H26O7, C 65.66%, H 6.51%.
Oxidation of Compound 20 to Give Ketone 22
Pyridinium chlorochromate (PCC) (45 mg, 0.209 mmol) was added in one portion to a solution of 20 (45 mg, 0.083 mmol) in dry CH2Cl2 (1 mL). The resulting suspension was stirred under Ar at room temperature for 17 h. Then the mixture was filtered through a short pad of celite and the solvent was removed under reduced pressure. The residue was purified by FC (90:10 CH2Cl2-acetone as eluent) to give pure 22 (40 mg, 89%): amorphous, white solid; [α]D20 +28.4 (c 0.208, CHCl3); Rf (90:10 CH2Cl2-acetone) 0.35; IR (KBr) νmax 3145, 2924, 1773, 1742, 1718, 1505, 1384, 1217, 1154, 1025, 998, 875, 807, 734, 602 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.44 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 1.9 Hz, H-16), 7.42 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.38 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 1.0 Hz, H-14), 5.36 (1H, dd, J12β,11α = 11.1 Hz, J12β,11β = 6.4 Hz, H-12β), 4.37 (1H, dd, J19a,6β = 2.1 Hz, J19a,19b = 9.8 Hz, pro-S H-19a), 4.28 (1H, d, J19b,19a = 9.8 Hz, pro-R H-19b), 2.79 (1H, m, H-3β), 2.63 (1H, dd, J8β,7α = 11.9 Hz, J8β,7β = 3.7 Hz, H-8β), 2.57 (2H, m, H-3α and H-4β), 2.42 (2H, m, H-1α and H-1β), 2.16 (2H, m, H-6α and H-10β), 2.11 (1H, m, H-7β), 2.06 (1H, dd, J11β,11α = 13.9 Hz, J11β,12β = 6.4 Hz, H-11β), 1.86 (1H, dddd, J7α,6α = 3.4 Hz, J7α,6β = 13.7 Hz, J7α,7β = 14.8 Hz, J7α,8β = 11.9 Hz, H-7α), 1.85 (1H, dd, J11α,11β = 13.9 Hz, J11α,12β = 11.1 Hz, H-11α), 1.41 (1H, tdd, J6β,6α = J6β,7α = 13.7 Hz, J6β,7β = 3.8 Hz, J6β,19a = 2.1 Hz, H-6β), 0.88 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 205.8 (C, C-2), 176.1 (C, C-18), 172.7 (C, C-17), 144.0 (CH, C-15), 139.6 (CH, C-16), 123.8 (C, C-13), 108.3 (CH, C-14), 70.0 (CH2, C-19), 69.9 (CH, C-12), 49.7 (CH, C-10), 48.0 (CH, C-4), 46.8 (CH, C-8), 42.9 (CH2, C-11), 42.1 (C, C-5), 38.6 (CH2, C-3), 37.4 (CH2, C-1), 36.7 (C, C-9), 35.9 (CH2, C-6), 19.9 (CH3, C-20), 18.6 (CH2, C-7); EIMS m/z 358 [M]+ (53), 343 (2), 330 (1), 262 (3), 247 (9), 220 (15), 192 (13), 176 (7), 147 (6), 133 (7), 94 (100), 91 (14), 79 (12), 55 (8); anal. C 67.31%, H 6.02%, calcd for C20H22O6, C 67.02%, H 6.19%.
Sodium Borohydride Reduction of Compound 22: Mixture of Epimers 20 and 23
To a solution of 22 (30 mg, 0.084 mmol) in THF (800 μL), NaBH4 (1.2 mg, 0.0315 mmol) was added while keeping the temperature below 0° C. The mixture was stirred at 0° C for 15 min. Then, the reaction was quenched by adding a few drops of 0.1 N HCl. The solvent was removed under reduced pressure and the solid residue was recovered with CH2Cl2 and purified by FC (80:20 CH2Cl2-acetone as eluent). A mixture of the C-2 epimers 20 and 23 (single TLC spot) was obtained (30 mg, 100 %). The 1H NMR spectrum (400 MHz, CDCl3) showed that the product of reaction was a 10:1 mixture of two compounds, in which 23 was the major constituent: δ 7.45 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 1.9 Hz, H-16), 7.43 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.40 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 1.0 Hz, H-14), 5.34 (1H, dd, J12β,11α = 10.8 Hz, J12β,11β = 6.7 Hz, H-12β), 4.35 (1H, dd, J19a,6β = 2.1 Hz, J19a,19b = 9.4 Hz, pro-S H-19a), 4.18 (1H, d, J19b,19a = 9.4 Hz, pro-R H-19b), 3.76 (1H, br t, J = 11 Hz, H-2β), 2.57 (1H, dd, J8β,7α = 12.0 Hz, J8β,7β = 3.8 Hz, H-8β), 2.27 (1H, dddd, J3β,1β = 2.0 Hz, J3β,2β = 4.1 Hz, J3β,3α = 13.0 Hz, J3β,4β = 6.4 Hz, H-3β), 2.20 (1H, dd, J4β,3α = 11.7 Hz, J4β,3β = 6.4 Hz, H-4β), 2.07 (1H, dd, J11β,11α = 13.9 Hz, J11β,12β = 6.7 Hz, H-11β), 2.03 (2H, m, H-6α and H-7β), 1.95 (1H, dddd, J1β,1α = 12.9 Hz, J1β,2β = 4.5 Hz, J1β,3β = 2.0 Hz, J1β,10β = 2.7 Hz, H-1β), 1.88 (1H, dd, J11α,11β = 13.9 Hz, J11α,12β = 10.8 Hz, H-11α), 1.84 (1H, m, H-7α), 1.64 (1H, dd, J10β,1α = 12.9 Hz, J10β,1β = 2.7 Hz, H-10β), 1.48 (1H, td, J3α,2β = J3α,4β = 11.6 Hz, J3α,3β = 13.0 Hz, H-3α), 1.40 (1H, td, J1α,1β = J1α,10β = 12.9 Hz, J1α,2β = 10.2 Hz, H-1α), 1.19 (1H, dddd, J6β,6α = 13.2 Hz, J6β,7α = 14.0 Hz, J6β,7β = 4.7 Hz, J6β,19a = 2.1 Hz, H-6β), 0.83 (3H, s, Me-20).
Acetylation of the Mixture of 20 and 23 to Give Compounds 21 and 24
Treatment of the mixture described above with Ac2O-pyridine (1:2, 500 μL) for 24 h at room temperature, followed by standard workup and purification by FC (95:5 CH2Cl2-acetone as eluent), yielded the acetyl derivatives 21 (3 mg, 8%) and 24 (30 mg, 90%).
Compound 24
colorless quadrangular plates (EtOAc-petroleum ether); mp 217–219 °C; [α]D20 +8.2 (c 0.182, CHCl3); Rf (95:5 CH2Cl2-acetone) 0.29; IR (KBr) νmax 3143, 3115, 2921, 1781, 1756, 1706, 1502, 1372, 1264, 1227, 1174, 1152, 1054, 1031, 1016, 876, 825, 609 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.45 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.8 Hz, H-16), 7.42 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.39 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.8 Hz, H-14), 5.35 (1H, dd, J12β,11α = 10.8 Hz, J12β,11β = 6.7 Hz, H-12β), 4.79 (1H, tt, J2β,1α = J2β,3α = 10.3 Hz, J2β,1β = J2β,3β = 4.3 Hz, H-2β), 4.35 (1H, dd, J19a,6β = 2.0 Hz, J19a,19b = 9.5 Hz, pro-S H-19a), 4.20 (1H, d, J19b,19a = 9.5 Hz, pro-R H-19b), 2.59 (1H, dd, J8β,7α = 11.9 Hz, J8β,7β = 3.8 Hz, H-8β), 2.27 (1H, dddd, J3β,1β = 1.9 Hz, J3β,2β = 4.3 Hz, J3β,3α = 12.1 Hz, J3β,4β = 6.4 Hz, H-3β), 2.24 (1H, dd, J4β,3α = 12.1 Hz, J4β,3β = 6.4 Hz, H-4β), 2.07 (1H, dd, J11β,11α = 13.9 Hz, J11β,12β = 6.7 Hz, H-11β), 2.04 (2H, m, H-6α and H-7β), 2.04 (3H, s, 2α-OAc), 1.99 (1H, dddd, J1β,1α = 12.9 Hz, J1β,2β = 4.3 Hz, J1β,3β = 1.9 Hz, J1β,10β = 2.7 Hz, H-1β), 1.85 (1H, dd, J11α,11β = 13.9 Hz, J11α,12β = 10.8 Hz, H-11α), 1.84 (1H, dddd, J7α,6α = 3.3 Hz, J7α,6β = 13.3 Hz, J7α,7β = 15.1 Hz, J7α,8β = 11.9 Hz, H-7α), 1.72 (1H, dd, J10β,1α = 12.9 Hz, J10β,1β = 2.7 Hz, H-10 β), 1.58 (1H, td, J3α,2β = 10.3 Hz, J3α,3β = J3α,4β = 12.1 Hz, H-3α), 1.46 (1H, td, J1α,1β = J1α,10β = 12.9 Hz, J1α,2β = 10.3 Hz, H-1α), 1.21 (1H, dddd, J6β,6α = 13.5 Hz, J6β,7α = 13.3 Hz, J6β,7β = 3.7 Hz, J6β,19a = 2.0 Hz, H-6β), 0.83 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 176.4 (C, C-18), 173.0 (C, C-17), 170.2 (C, 2-OCOCH3), 143.9 (CH, C-15), 139.6 (CH, C-16), 124.0 (C, C-13), 108.4 (CH, C-14), 70.6 (CH, C-2), 69.9 (CH, C-12), 69.5 (CH2, C-19), 49.7 (CH, C-10), 48.0 (CH, C-4), 47.2 (CH, C-8), 43.3 (CH2, C-11), 42.3 (C, C-5), 36.7 (C, C-9), 34.6 (CH2, C-6), 29.9 (CH2, C-3), 27.7 (CH2, C-1), 21.1 (CH3, 2-OCOCH3), 20.1 (CH3, C-20), 18.7 (CH2, C-7); EIMS m/z 402 [M]+ (42), 342 (17), 264 (26), 218 (18), 204 (15), 176 (18), 145 (18), 131 (29), 94 (100), 91 (23), 81 (14), 55 (13); anal. C 65.71%, H 6.43%, calcd for C22H26O7, C 65.66%, H 6.51%.
Biological Assays
The new neoclerodane derivatives were assayed for affinity at human opioid receptors, except for 6, 11 and 13 which were not tested due to the scarcity of the sample available. Cell culture and opioid binding assays proceeded as described elsewhere2 using [3H]DAMGO, [3H]DADLE, and [3H]U69,593 as radioligands. Recombinant CHO cells (hMOR-CHO, hDOR-CHO, and hKOR-CHO) were produced by stable transfection with the respective human opioid receptor cDNA and provided by Dr. Larry Toll (SRI International, CA).
Acknowledgments
This work was supported in part by funds from the Spanish ‘Comisión Interministerial de Ciencia y Tecnología’ (CICYT), grant No. CTQ2006-15279-C02-02, and from the Italian ‘Università degli Studi di Palermo’, grant ‘Ex 60%-2005’, and R01DA018151 (to TEP) from the National Institute on Drug Abuse. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. Portions of this work were also supported by the Intramural Research Program, National Institute on Drug Abuse (NIDA), NIH, DHHS (RBR and CMD).
References and Notes
- 1.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 2.Fontana G, Savona G, Rodriguez B, Dersch CM, Rothman RB, Prisinzano TE. Tetrahedron. 2008;64:10041–10048. doi: 10.1016/j.tet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana G, Savona G, Rodríguez B. J Nat Prod. 2006;69:1734–1738. doi: 10.1021/np068036d. [DOI] [PubMed] [Google Scholar]
- 4.Fontana G, Savona G, Rodríguez B. Magn Reson Chem. 2006;44:962–965. doi: 10.1002/mrc.1869. [DOI] [PubMed] [Google Scholar]
- 5.Munro TA, Rizzacasa MA, Roth BL, Toth BA, Yan F. J Med Chem. 2005;48:345–348. doi: 10.1021/jm049438q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Béguin C, Richards MR, Wang Y, Chen Y, Liu-Chen L-Y, Ma Z, Lee DYW, Carlezon WA, Jr, Cohen BM. Bioorg Med Chem Lett. 2005;15:2761–2765. doi: 10.1016/j.bmcl.2005.03.113. [DOI] [PubMed] [Google Scholar]
- 7.Lee DYW, Karnati VVR, He M, Liu-Chen L-Y, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C, Carlezon WA, Jr, Cohen B. Bioorg Med Chem Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Fernández MC, Esquivel B, Cárdenas J, Sánchez AA, Toscano RA, Rodríguez-Hahn L. Tetrahedron. 1991;47:7199–7208. [Google Scholar]
- 9.Compound 6 is sparingly soluble in many solvents. However, it can be extracted from the reaction mixture with EtOAc, in which this compound forms a stable opaque dispersion.