Abstract
In the past years aldose reductase (AKR1B1; AR) is thought to be involved in the pathogenesis of secondary diabetic complications such as retinopathy, neuropathy, nephropathy and cataractogenesis. Subsequently, a number of AR inhibitors have been developed and tested for diabetic complications. Although, these inhibitors have found to be safe for human use, they have not been successful at the clinical studies because of limited efficacy. Recently, the potential physiological role of AR has been reassessed from a different point of view. Diverse groups suggested that AR besides reducing glucose, also efficiently reduces oxidative stress-generated lipid peroxidation-derived aldehydes and their glutathione conjugates. Since lipid aldehydes alter cellular signals by regulating the activation of transcription factors such as NF-kB and AP1, inhibition of AR could inhibit such events. Indeed, a wide array of recent experimental evidence indicates that the inhibition of AR prevents oxidative stress-induced activation of NF-kB and AP1 signals that lead to cell death or growth. Further, AR inhibitors have been shown to prevent inflammatory complications such as sepsis, asthma, colon cancer and uveitis in rodent animal models. The new experimental in-vitro and in-vivo data has provided a basis for investigating the clinical efficacy of AR inhibitors in preventing other inflammatory complications than diabetes. This review describes how the recent studies have identified novel plethoric physiological and pathophysiological significance of AR in mediating inflammatory complications, and how the discovery of such new insights for this old enzyme could have considerable importance in envisioning potential new therapeutic strategies for the prevention or treatment of inflammatory diseases.
Keywords: Aldose reductase, inflammation, oxidative stress, sepsis, cancer, uveitis, diabetes
Introduction
Aldose reductase (AKR1B1; AR) is a monomeric NADPH-dependent cytosolic enzyme that belongs to a superfamily of aldo-keto reductases (AKR). The story of AR was started 5 decades back when it was first identified as a protein with glucose reducing activity in 1956 by Hers et al. (1). Subsequently, van Heningern (2) reported that high levels of sorbitol and galactitol accumulated in the diabetic and galactosemic rat ocular lens is due to increased AR activity. Following this observation, Kinoshita et al (3–5) have shown that inhibition of this enzyme with flavinoids and pharmacological inhibitors such as sorbinil and tolrestat prevented the cataractogenesis in diabetic rats. Further, several series of studies showed that the pathophysiological conditions attributed to hyperglycemia are believed to be caused by the accumulation of sorbitol in tissues via polyol pathway hyper activity (Figure-1; 5–8). Sorbitol being impermeable through the biological membranes accumulates inside the tissues leading to osmotic stress. This results in ionic imbalance and protein insolubilization leading to secondary diabetic complications such as diabetic cataractogenesis, retinopathy, nephropathy, and neuropathy. Extensive investigations have been performed in the identification and development of potential AR inhibitors that could suppress sorbitol accumulation and prevent secondary diabetic complications. Several in vitro and experimental animal models indicate that drugs with varying AR inhibiting efficacy show significant protection against diabetic complications (please see ref 9–11 for recent reviews). The involvement of AR in diabetes is further supported by the demonstration that its overexpression in transgenic animals enhances hyperglycemic injury to specific target-organs (12, 13). Such mice develop cataracts more rapidly during hyperglycemia as compared to non-transgenic litter mates. In addition, polymorphism of the AR gene is a genetic risk marker for diabetic nephropathy reported to date (14), and AR gene expression is increased in peripheral blood mononuclear cells obtained from IDDM patients with nephropathy (15). Together, these observations provide compelling evidence pointing to a significant role of AR in mediating hyperglycemic injury. However, in clinical trials some of the AR inhibitors have yielded uncertain results in part due to lack of efficacy, skin allergic reactions and liver toxicities. Despite three decades of intense investigations, including some clinical studies, the details of AR-mediated hyperglycemic injury remain unclear. In particular, the mechanisms which control and regulate the expression of AR gene and the catalytic activity of AR protein remain poorly understood. Recent studies from the last decade or so suggested that reducing glucose may not be the major physiological function of AR. This is supported by the studies which show the prevention of diabetic cataracts by using anti-oxidants without affecting sorbitol levels (16–18). Further, reports have also suggested that the increased activity of AR under hyperglycemia leads to the depletion of cellular NADPH, which compromises anti-oxidative defenses, since NADPH is an essential cofactor for reduction of oxidized glutathione (GSSG) by glutathione reductase (11).
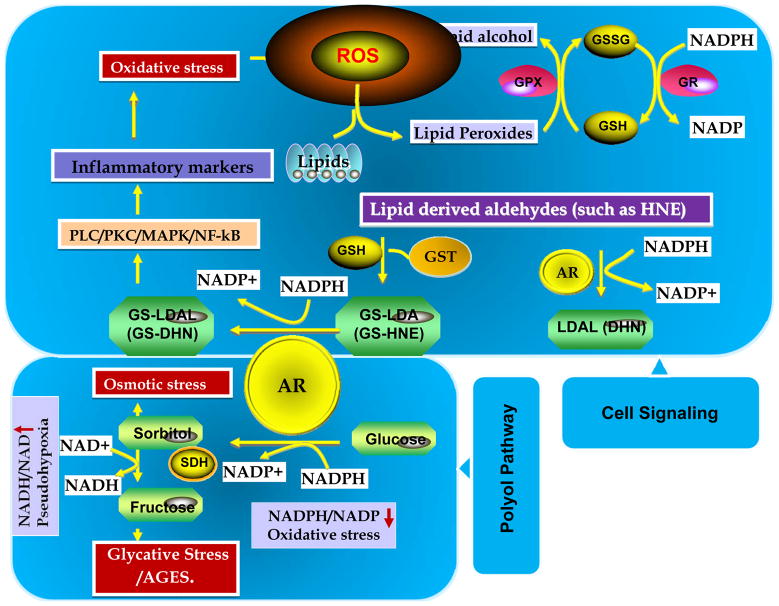
Figure-1. Aldose reductase regulates polyol pathway of glucose metabolism and lipid aldehyde mediated cell signaling.
During hyperglycemia AR reduces glucose to sorbitol using NADPH as a co-factor and later sorbitol dehydrogenase (SDH) reduces sorbitol to fructose using NAD as a co-factor. AR causes oxidative stress by decreasing the ratio of NADP/NADPH and also competing with glutathione reductase (GR) for NADPH. Increased accumulation of sorbitol could cause osmotic stress. Metabolites of fructose increase AGE formation and cause glycative stress. Further, during oxidative stress conditions, AR catalyzed lipid aldehyde reaction products mediate cellular signals via activation of redox sensitive transcription factors.
The isolated, homogenous enzyme has a poor affinity for glucose (Km of 50 to 100 mM), and its kinetic and structural properties are unlike those of another glucose-metabolizing enzymes. In the mid 90’s, the structural studies and X-ray analyses of AR crystals indicated that the active site of AR lacked the ionic residues characteristic of polyol-binding proteins but revealed highly plastic and hydrophobic residues at its active site (19). These studies thus indicate that the high hydrophobicity of the substrate-binding domain essentially precludes efficient carbohydrate reduction, and suggests that hydrophobic aldehydes are likely to be the preferred substrates. From there on, several studies were directed to identify potential physiological substrates of AR.
The most obvious endogenous source of hydrophobic aldehydes is lipid peroxidation, which generates high concentrations of long-chain aldehydes. Since several of these are unsaturated (such as 4-hydroxynonenal (HNE) the most abundant), they display high toxicity, due to their ability to bind cellular glutathione (GSH). In the early 2000’s, simultaneous reports from two groups indicated that AR efficiently catalyzes lipid peroxidation –derived aldehydes (LDAs) such as HNE (20, 21). Later our studies show that recombinant human AR catalyzes the reduction of a large series of saturated and unsaturated aldehydes with 1000-fold higher efficiency than glucose (22–25). Further, AR is particularly efficient in catalyzing medium- to long-chain (C-6 to C-18) aldehydes generally generated during lipid peroxidation (Figure-1). In addition to aldehydes, this enzyme exhibited a higher efficiency in catalyzing the reduction of the GSH conjugates of unsaturated aldehydes than that of their parent free aldehydes (23–25). Thus our studies show that AR is an important metabolic route for the detoxification of lipid-derived aldehydes. This conclusion is supported by the observations that (a) homogenous AR catalyzes the reduction of HNE and its conjugate GS-HNE (to DHN and GS-DHN, respectively) with an affinity which is 4 orders of magnitude higher than that for glucose (20); (b) the generation of GS-DHN in perfused rat hearts (26), lens (27) and erythrocytes (28) exposed to HNE is prevented by AR inhibition; (c) inhibition of AR exacerbates the toxicity of HNE to the ocular lens, isolated cardiac myocytes and vascular smooth muscle cells in culture; and (d) exposure of VSMC to HNE leads to marked upregulation of AR (29). Taken together, these observations provide firm support to the idea that metabolism of LDAs is a significant in vivo role of AR (Figure-2). Since LDAs are known to alter the cellular function by regulating the oxidative stress signals mediated by NF-kB and AP1 (30–32), it was hypothesized that AR regulates cellular function by altering the oxidative stress signals. This hypothesis is supported by the studies that indicate the inhibition of AR prevents HNE-, growth factors- and cytokines – induced cytotoxicity in cultured cells (33–37). Most importantly, our studies indicating inhibition of AR prevents endotoxin, allergen, cytokine and growth factor-induced activation of NF-kB signals made a solid foundation in recapitulating the novel role of AR in the pathophysiology of various disease processes (figure-2). Very recent and currently ongoing studies indicate that the inhibition of AR prevents various inflammatory diseases such as sepsis, asthma and colon cancer in experimental animals. In this review, we recapitulate the novel role of AR in the pathophysiology of inflammatory diseases.
Figure-2. Aldose reductase catalyzes a wide array of substrates and regulates cellular signals initiated by various oxidants.
The polyol pathway hypothesis of diabetic complications
The polyol pathway enzyme AR has been implicated in the development of secondary diabetic complications in general and diabetic nephropathy, in particular. The polyol pathway consists of two enzymes (Figure-1): AR, which catalyzes the NADPH-mediated reduction of glucose to sorbitol, and sorbitol dehydrogenase (SDH), which catalyzes the conversion of sorbitol to fructose, utilizing NAD. The net result of the polyol pathway is the formation of fructose from glucose and the transfer of reducing equivalents from NADPH to NAD. Under normoglycemic conditions the polyol pathway affects < 3% of glucose flux. However, under hyperglycemia this pathway can account for 25 to 30 % of the total glucose metabolism (11). It has been suggested that the hyperglycemia-induced increase in the polyol pathway’s activity (and the attendant metabolic changes) are the primary cause of hyperglycemic injury. In agreement with a critical role of AR in mediating hyperglycemic injury, it was demonstrated that synthetic inhibitors of AR prevent, delay and in some cases even reverse tissue injury due to several secondary diabetic complications, and AR inhibitors decrease elevated urinary albumin excretion. Although overwhelming evidence derived from inhibitor studies, transgenic animals and genetic susceptibility analysis suggest a critical role of AR in diabetic nephropathy, the lack of a clear mechanistic understanding made the data remain only correlative. Based on a high accumulation of polyol in diabetic and galactocemic lens, it was initially suggested that the AR perturbs cell structure, function and ion balance by inducing osmotic stress due to membrane-impermeable polyols. However, later experiments demonstrated that even under extreme and prolonged hyperglycemia the sorbitol concentrations are not osmotically relevant. Although several other explanations have been put forward to account for its injurious effects (e.g. depletion of myoinositol and NADPH, as well as generation of pseudo-hypoxia), AR’s role in mediating hyperglycemic injury remains obscure. However, even from the beginning, several features of AR were disconcerting. The isolated, homogenous enzyme has a poor affinity for glucose, (Km of 50 to 100 mM), and its kinetic and structural properties are unlike those of other glucose-metabolizing enzymes. The high hydrophobicity of the substrate-binding domain of AR essentially precludes efficient carbohydrate reduction, and suggests that hydrophobic aldehydes are likely to be the preferred substrates. Indeed, we and others have shown that AR efficiently catalyzes lipid aldehydes and their GSH-conjugates (20–27). Specifically, we have also found that the AR catalytic site has more affinity towards GS-aldehyde conjugates than parent aldehydes alone. By using site directed mutagenesis experiments, we have found that the AR active site has residues responsible for binding to glutathione moiety (24). Indeed, we have recently solved the crystal structure of GSH-analogue bound to AR, and our structural studies confirmed that AR has a specific GS-aldehydes binding site in its active site (38). Taken together, these observations provide firm support to the idea that metabolism of LDAs is a significant in vivo role of AR. Based on this identification, it was concluded that upregulation of AR during diabetes may be a response to oxidative stress, which appears to be an important feature of long-term diabetes, given the increased generation of ROS and increased accumulation of lipid peroxidation products in tissues from diabetic animals and humans. The significance of AR in diabetes is also strengthened by identification of genetic polymorphisms associated with the human AR gene. Ko et al identified that (A-C)n dinucleotide repeat domain at the 5′ end of the AR gene is associated with early-onset diabetic retinopathy in NIDDM patients (39). Later, several studies indicated AR Z-2 (A-C)n microsatellite polymorphism in the AR gene is associated with several secondary diabetic complications, including retinopathy, neuropathy and nephropathy (40–46). Thus the studies performed in different population groups have established the association of AR gene polymorphism and diabetic complications.
Significance of AR in oxidative stress signaling
It is well established that ROS generated in response to cytokines, growth factors, lipopolysaccharide (LPS) and hyperglycemia cause lipid peroxidation and form LDAs. The LDAs and their GSH conjugates are excellent AR substrates. Because ROS are essential mediators of intracellular signaling under a variety of conditions, some of the mitogenic and cytotoxic effects of ROS may be mediated by LDAs and their GSH conjugates. Indeed, at low levels HNE is a potent smooth muscle cell mitogen, and at high concentrations it induces apoptosis in several cell types (47–50). Moreover, inhibition of HNE metabolism by inhibiting AR prevents the growth of vascular lesions (29). To build on these observations, our lab undertook a systematic study to delineate the role of AR in mediating the cytotoxic signals of cytokines. These studies indicate that reduction of LDAs and their GSH conjugates is essential for transducing the cytotoxic signals (47). Increased oxidative stress and lipid peroxidation are key features of inflammation-induced cytotoxicity and activation of redox sensitive transcription factors such as NF-kB and AP1, which stimulate the expression of genes that transcribe inflammatory cytokines and chemokines. Uncontrolled and excessive production of inflammatory mediators causes cytotoxicity in an autocrine and paracrine manner. The ROS-sensitive transcription factor NF-kB is a critical mediator of oxidative stress-induced inflammation initiated by bacterial infections, xenobiotics, environmental pollutants and auto-immune diseases (51,52). When inactive, it is sequestered in the cytosol as a complex with its inhibitor, IkB; stimulation of protein kinases such as PKC, MAPK, and IKK results in the activation of NF-kB via phosphorylation of IkB (51.52). Several studies have shown that ROS activate NF-kB, but the mechanisms are not clearly understood. The other major redox-sensitive transcription factor, AP1, is formed by homo- or heterodimerization of members of the Jun and Fos families of proteins; ROS can regulate AP-1 activity via several mechanisms. AP-1 can be regulated via the c-JunN-terminal kinase (JNK) cascade; JNKs arepart of the mitogen-activated protein kinase (MAPK) superfamily of serine/threonine kinases that also includes the extracellular signal-regulated kinases ERK1/2 and p38MAPK (53–55). All MAPKs are activated via a cascade of phosphorylation reactions. We have recently shown that inhibition or ablation of AR attenuates the phosphorylation of p38 and JNK in endothelial cells and macrophages (56,57).
Lipid peroxidation has been suggested to be a major contributor to the pathophysiology of inflammation (58). At low concentrations lipid peroxidation products such as HNE can stimulate proliferation of VSMC and apoptosis in VEC and at high concentrations HNE is genotoxic and mutagenic (47, 59, 60). Thus, as discussed above, the enzymes that can detoxify HNE may be involved in mediating oxidative stress-induced signals, including those of cytokine and LPS that activate transcription factors. The enzymes that regulate the concentration of HNE and its metabolites are known to modify the activities of multiple cytoskeletal proteins, MAPKs and transcription factors (61). Several reports suggest that PKC activation by HNE and its metabolites cause inflammation (62–65). Increased generation of ROS by growth factors, cytokines, chemokines and LPS may be an essential step for cell growth because over-expression of antioxidants such as catalase and super oxide dismutase (SOD) or treatment with N-acetylcysteine are known to diminish growth factor and cytokine-stimulated cell growth (66–68). It has been demonstrated that through ROS, growth factors stimulate redox-sensitive transcription factors such as NF-κB, AP1, CREB and AFT-2 (Figure-3; 69). Of these, NF-κB is the major transcription factor activated by oxidative stress (70). Since LPS signals are propagated by autocrine and paracrine effects to generate excessive amounts of cytokines and growth factors during the inflammatory response, antibodies against cytokines such as IL-18, TNF-α and IL-6 had been shown to attenuate the progression of LPS-induced cytotoxicity (71–73).
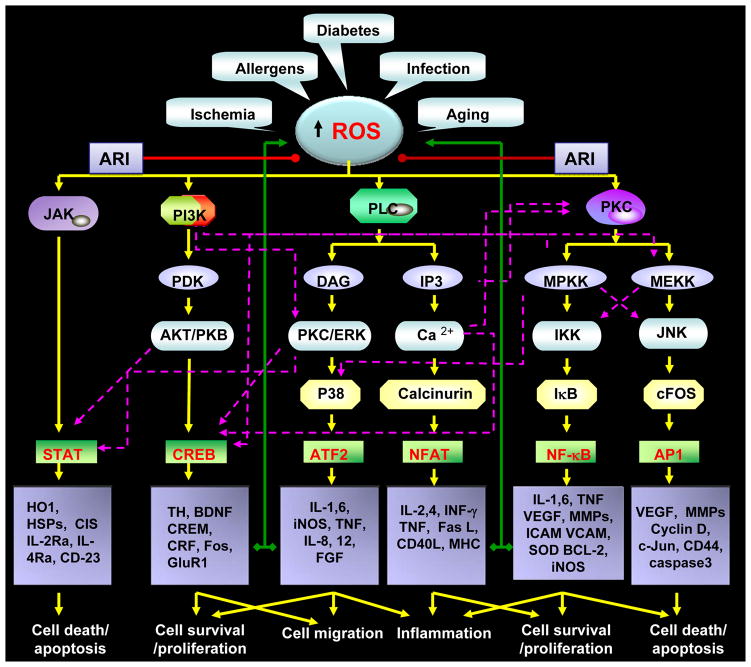
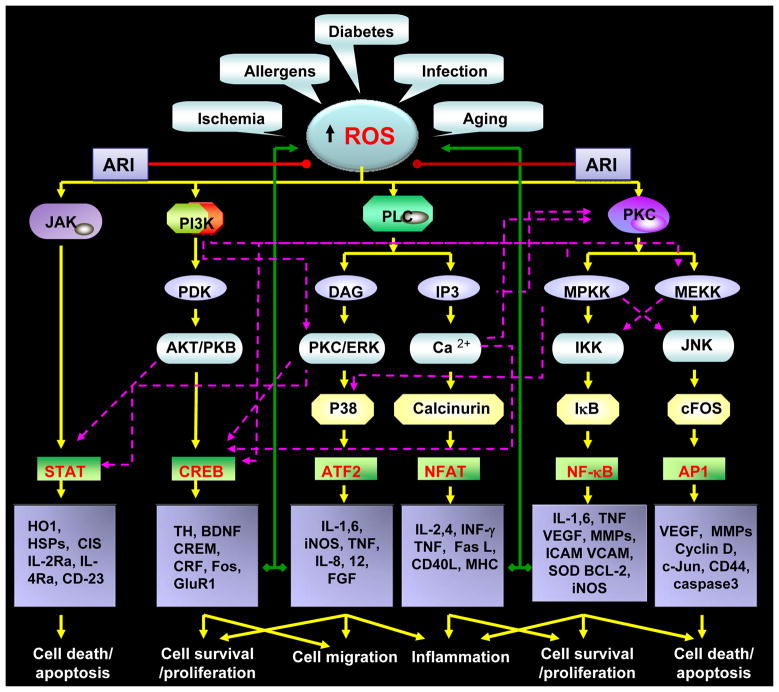
Figure-3. Aldose reductase prevents inflammatory complications by preventing the oxidative stress –induced inflammatory signals downstream to reactive oxygen species.
Various disease conditions such as sepsis, diabetes and infections and by allergens could cause oxidative stress by generating the reactive oxygen species. The increased ROS levels are well known to mediate inflammatory signaling by activating various protein kinases such as JNK, PI3K, PKC and PLC etc which activate redox sensitive transcription factors such as STAT, CREB, NF-kB, AP-1, NFAT and ATF2 via a series of signaling events transduced by other kinases such as MAPK, ERK and JAK. The activation of transcription factors lead to the transcriptional activation of inflammatory cytokines, chemokines and growth factors which by autocrine and paracrine manner could amplify inflammatory complications. Aldose reductase inhibitors (ARI) could prevent the inflammatory signaling by preventing pathways downstream to ROS as well as autocrine/paracrine mediated formation of ROS by inflammatory proteins.
The association of ROS and AR is supported by the observation that inhibitors of AR attenuate glucose-induced oxidative stress and superoxide production in retinal pericytes, bovine aortic endothelial cells, and rabbit aortas (74–76). The strongest evidence that AR is involved in mediating growth comes from our studies showing that inhibition of AR prevents proliferation of cultured VSMCs in response to FGF, high glucose and thrombin (29,34,77). The observation that the AR inhibitor, epalrestat prevents initial thickening in the coronary arteries of galactose-fed beagle dogs provides additional support for the role of AR in abnormal VSMCs growth (78, 79). We recently demonstrated that AR plays a pivotal role in the proliferation of VSMCs, apoptosis of VECs and restenosis of rat carotid arteries after balloon injury (33, 57, 77). Besides a significant decrease in neointima formation in balloon-injured rat carotid arteries, inhibition of AR diminished in situ activation of NF-κB during restenosis as well as in cultured VSMCs (35, 77,80, 81). The inhibition of AR has been shown to regulate cell growth and death by modulating the cell cycle events at the G1/S transition in VSMC as well as in colon cancer cells (82, 83). Our studies also indicated that AR mediates high glucose-induced VSMC growth by regulating the high glucose triggered release of TNF-α, a major proinflammatory cytokine, in VSMC via activation of PKC and TACE (84, 85). Our recent observations show that AR mediates the mitogenic and cytotoxic signals of cytokines and growth factors. We have further shown that inhibition or ablation of AR attenuates TNF-α– and growth factor-induced IκB-α phosphorylation, degradation and activation of NF-κB, and PKC, proliferation of VSMC and apoptosis of VEC, HLEC and macrophages (24, 57). These findings are consistent with our hypothesis that AR, via modulation of NF-κB, is involved in the regulation of many genes during inflammation induced by cytokines (TNF, IL-1, IL-8, IL-6), cell adhesion proteins such as ICAM-1, MHC genes, enzymes such as NOS, Cox and Mn-SOD, and endotoxins such as LPS (Figure-3). In addition, AR inhibition has also been shown to regulate the expression of glucose transporter proteins in macrophages. Reddy et al (86) have shown that AR inhibitors prevent the LPS-induced activation of transcription factor CREB via regulating the ROS/cAMP/PKA pathway. Ramasamy et al (87) have also shown that ROS activated JAK2 and STAT5 pathway in ischemic hearts was inhibited by AR inhibition, indicating that AR also mediates the JAK/STAT signaling pathway. These studies indicate that AR inhibition works downstream of ROS formation and also blocks ROS generation by an autocrine and paracrine manner. Since various oxidant stimuli such as LPS, cytokines, growth factors and high glucose are known to cause oxidative stress and increase the synthesis of inflammatory cytokines and chemokines, AR-catalyzed reaction products should play an important role in eliciting oxidative stress-induced cytotoxicity and inflammation. This is further substantiated by our demonstration that HNE, GS-HNE and GS-DHN promote cultured VSMC growth (88). AR inhibition or ablation of AR by siRNA prevents the HNE and GS-HNE-induced growth, but has no effect on the GS-DHN-induced VSMC proliferation. These studies indicate that it is the reduced form of lipid-aldehyde glutathione conjugates (GS-DHN) that are involved in the oxidative stress-induced LDA-mediated signaling. Further, studies are required to investigate how GS-DHN activates gene transcription. Nevertheless, our observations have opened a wide area of research into the role of lipid peroxide and lipid aldehyde formation in oxidative stress signaling.
Significance of AR in inflammatory complications
Inflammation is a complex system of a host systemic and local response to injury and infection. Inflammation contributes to almost all disease processes, including immunological and vascular pathology, sepsis, and chemical and metabolic injury. In inflammation, the regulation of the immune response by macrophages plays a central role which triggers gene induction of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and biosynthesis of prostaglandins(PGE2). These and other cytokines act in an autocrine or paracrine manner to induce and amplify the host cell response and defense systems that help to eliminate the infection. However, uncontrolled and excessive cytokine expression can induce acute or chronic inflammatory processes. Recent studies indicate that inhibition of AR prevents cytokine-, growth factor- and high glucose-induced apoptosis of the human lens epithelial cells (HLEC), VECs, macrophages, and proliferation of VSMCs and colon cancer cells. As shown in the Figure 4, the ROS generated during growth factor and cytokine signaling induce lipid peroxidation, which in turn leads to the generation of a wide range of cytotoxic aldehydes such as HNE. These aldehydes react readily with reduced glutathione (GSH) to form glutathionyl aldehydes such as GS-HNE. We have found that AR efficiently reduces GS-HNE to GS-DHN and that the GS-DHN formed in turn activates PLC via an unidentified mechanism. This leads to the activation of signaling cascades that involve the activation of such transcription factors as NF-κB and AP-1 via PKC/PI3K/MAPK/IKK. NF-κB and AP1 stimulate transcription of various inflammatory cytokines (TNF-α, Interleukins), chemokines (MCP-1, MIP-1), and inflammatory mediators such as cycloxygenase-2 (Cox2) and inducible nitric oxide synthase (iNOS). Uncontrolled productions of these inflammatory markers cause cytotoxicity leading to tissue damage and dysfunction leading to pathologies such as cancer and inflammatory response syndrome. We have shown that AR inhibitors such as sorbinil, tolrestat, zopolrestat and fidarestat, as well as ablation of AR by siRNA, effectively block the ROS-induced formation of GS-DHN and down-regulate stress signals that activate NF-κB and AP-1 in vascular cells and macrophages (88). Further, AR inhibitors also prevented the activation of caspase-3 and degradation of nucleosomal histones by high glucose or TNF-α in HLECs and by LPS in macrophages (35,56). These results raised the interesting and significant question of how AR regulates the signaling events initiated by cytokines and growth factors, and how inhibition of AR prevents cytokine and growth factor signaling. Understanding this role of AR should provide pharmacological tools for eventual therapeutic interventions to control cell proliferation, apoptosis, tissue repair, and to prevent the cytotoxicity of cytokines and chemokines, which are increased during oxidative stress. More importantly, these studies will provide a mechanistic link between oxidative stress and inflammation. We have extended our investigations from cultured cells to various animal models of inflammatory diseases such as colon cancer, sepsis, asthma and uveitis and have shown that inhibition of AR prevents these inflammatory disorders. These studies backed by strong evidence obtained using cellular as well as animal models suggest that AR plays a pivotal role in the pathophysiology of inflammation.
Figure-4. Aldose reductase mediates growth factor-induced inflammatory signals.
Growth factor stimulated oxidative stress generates lipid peroxidation-derived lipid aldehydes such as highly toxic HNE. HNE being highly electrophillic conjugates with cellular GSH to form GS-HNE. AR catalyzes the reduction of GS-HNE to GS-DHN. The GS-DHN has been shown to mediate oxidative stress signals upstream to PLC/PKC leading to activation of transcription factors such as NF-kB and AP-1 which transcribes inflammatory genes. The inflammatory proteins propagate the carcinogenic signals and cause tissue damage, dysfunction leading to uncontrolled tumor growth.
Aldose reductase in Sepsis
During sepsis and related systemic inflammatory response syndrome (SIRS), the chemical or biological (bacterial and viral) agents cause severe toxicity by increasing oxidative stress (89,90). The acute uncontrolled inflammatory response can lead to extensive tissue injury and multiple organ failure. Studies from our lab have indicated that the toxic effects of uncontrolled inflammation can be effectively prevented or significantly ameliorated by inhibiting AR either by pharmacological AR inhibiting drugs or by genetic ablation of AR message (88). Despite mechanistic ambiguities, our demonstration that AR mediates cytokine-induced activation of NF-κB suggested to us an entirely new modality for prevention and treatment of the acute inflammatory episodes. Therefore, to examine the role of AR in a cellular model of inflammation, we investigated how AR mediates the lipopolysaccharide (LPS)-induced release of inflammatory mediators in RAW264.7 murine macrophages and peritoneal macrophages (88, 91). Pharmacological inhibition or siRNA ablation of AR prevented the biosynthesis of cytokines in LPS-activated RAW264.7 cells. Inhibition or ablation of AR significantly attenuated LPS-induced activation of PKC and PLC, nuclear translocation of NF-κB, and phosphorylation and proteolytic degradation of IκB-α in macrophages, suggesting that inhibition of AR prevents key steps in the development of inflammation. Given our results with macrophages in culture, we tested whether inhibition of AR would also prevent acute inflammatory events in vivo. For this, we chose to study endotoxin-induced sepsis complications in mice (92). Our results show that as in LPS-treated macrophages administration of AR inhibitor in the mice prevented the serum, liver, spleen and heart inflammatory cytokines in response to LPS challenge (88). Treatment with AR inhibitor blunted the activation of PKC, JNK, and p38-MAPK, as well as phosphorylation of IκB-α, IKK, and PLC. These changes were associated with decreased myocardial NF-κB and AP-1 activity, PGE-2 production, induction of Cox-2, and inducible NO synthase. Further, our studies demonstrate that inhibition of AR prevented the LPS-induced functional recovery in myocardial fractional shortening in vivo and preserved contractile function of isolated perfused hearts, indicating that AR inhibition prevents LPS-induced cardiomyopathy (92). Most importantly, inhibition of AR increased survival in mice injected with lethal doses of LPS. Similarly, AR inhibition also prevented the inflammatory cytokine levels in a cecum ligation and puncture (CLP) model of polymicrobial sepsis, which closely mimics the sepsis syndrome in humans (93). These observations provided a promising demonstration of the potentially high therapeutic efficacy of AR inhibitors in treating sepsis and other acute inflammatory syndromes.
Aldose reductase in Asthma pathogenesis
Asthma is one of the most common chronic respiratory diseases, with more than 100 million sufferers worldwide (94). This inflammatory disorder is caused by a hypersensitive immune system that results from a number of triggers, such as dust, pollen, viruses and changes in the weather. While it is not clear how asthma is initiated in the setting of chronic inflammation, accumulating evidence strongly support the association of airway inflammation to asthma (95). Furthermore, the increase in inflammation in the bronchial epithelium leads to eosinophil infiltration, an increase in mucus production, and most importantly, upregulation of cytokines such as TNF-α, IL-4, IL-5, IL-6, and IL-13, chemokines such as MCP-1, and MIP-1, adhesion molecules such as ICAM-1, and E and P-Selectins (95). Thus, exposure of nearby cells to inflammatory cytokines and chemokines can trigger various autocrine/paracrine effects, leading to Th2 immune response and inflammatory cell accumulation. Our studies indicate that inhibition of AR prevents expression of inflammatory markers in human small airway epithelial cells, indicating AR inhibition could prevent asthma (96–98). Indeed, we examined the efficacy of AR inhibitors in prevention of allergen-induced airway inflammation in mouse models of asthma. We found that AR inhibition prevents ragweed pollen extract and ovalbumin–induced allergic responses, airway inflammation and hyper-responsiveness in mouse models of asthma. Our studies also indicate that AR inhibitors significantly prevent the number of eosinophils and mucin levels in BAL fluid of allergens-treated mice. These results indicate that AR inhibition could significantly prevent the patho-physiological effects of allergens-induced respiratory complications.
Aldose reductase in Uveitis
Uveitis is a systemic inflammatory response syndrome characterized by excessive production of inflammatory cytokines generated in response to bacterial infections (99). Our investigations indicating that AR plays an obligatory role in mediating bacterial endotoxin-stimulated inflammatory signaling suggest that inhibition of AR may be an useful approach for attenuating maladaptive host responses and for treating acute ocular inflammation due to uveitis. To determine whether inhibition of AR prevents ocular inflammation in vivo, we examined the effects of AR inhibitor on NF-κB signaling pathways and ocular inflammation in a rat model of LPS-induced uveitis (100). Inhibition of AR prevents inflammatory marker levels in the aqueous humor of uveitis rat eyes. AR inhibition also suppressed the inflammatory cells infiltration and protein concentration in the aqueous humor of uveitis rat eyes. Similarly, the rise of inflammatory cytokines such as TNF-α, NO and PGE2 levels in the aqueous humor of uveitis rat eyes was significantly attenuated by AR inhibition. Similarly, the increased expression of TNF-α, iNOS and Cox-2 proteins in the ciliary body, corneal epithelium and retinal wall was significantly prevented by AR inhibition. In addition to pharmacological inhibitors of AR, natural compounds such as benfotiamine and guggulsterone which prevent the expression of AR and the activation of NF-kB also ameliorate endotoxin-induced uveitis in rats (101–103). Thus, based upon these results, AR inhibitors could be used therapeutically to treat patients with uveitis and its associated complications that have the potential of stimulating the inflammatory signals.
Aldose reductase in Cancer
Colon cancer is the third most common form of cancer and the second leading cause of cancer-related deaths in Western countries, including the United States (104). Epidemiological and experimental studies indicate that colon cancer is usually mediated by dietary and environmental factors and is more pronounced in genetically predisposed subjects (104, 105). Recent studies indicate that inflammation plays a major role in the colon carcinogenesis (106). Results from our investigations have established the role of AR in the carcinogenic signaling induced by growth factors and cytokines, and provided new insights into the physiological role of this enzyme in colon cancer cell mitogenicity as well as inflammation associated with colon carcinogenesis. Our recent studies indicate that ROS-induced signaling that activates NF-κB and transcribes genes responsible for tumor progression is prevented by AR inhibition in human colon cancer cells (37, 107, 108). Similarly, inhibition of AR also prevented the tumor growth in nude mice bearing human adenocarcinoma (SW480 cell) xenografts. Further, we have identified that reduced lipid aldehyde glutathione conjugate catalyzed by AR is a novel signaling intermediate in the transduction of reactive oxygen species-initiated cell signals leading to mitogenicity in colon cancer cells. We have found that AR knockout mice are resistant to chemically induced colon cancer in azoxymethane-induced mouse model (109). Thus, our results suggest that AR inhibitors could be used therapeutically to prevent colon cancer and its associated complications.
Expert opinion
Our demonstration that AR also efficiently reduces lipid aldehydes and their conjugates with GSH has opened new dimensions in understanding the detoxification of reactive aldehydes generated during lipid peroxidation. Using kinetic, structural, and physiological studies, we have investigated the mechanisms by which AR selectively recognizes and catalyzes the reduction of LDAs and their GSH conjugates. We have also shown that AR activity can be regulated by lipid aldehydes, as well as by nitric oxide. To our surprise, we have found that AR-catalyzed lipid aldehyde products are obligatory mediators of cytokine-, chemokine-, growth factor- and LPS-induced cellular cytotoxicity as measured by decreased cell growth or apoptosis. Recent studies demonstrate that AR plays a pivotal role in inflammation (110, 111). Understanding this role of AR has provided pharmacological tools for eventual therapeutic interventions to control cell proliferation, apoptosis, tissue repair, and to prevent the cytotoxicity of cytokines, which are increased during infections and inflammation. More importantly, these studies provided a mechanistic link with oxidative stress-induced toxicity, especially in inflammatory pathologies where oxidative stress is known to cause toxicity through the expression of proinflammatory cytokines and chemokines. Thus, based upon these results, AR inhibitors could be used therapeutically to treat patients with inflammatory diseases such as asthma, colon cancer, uveitis, sepsis, burn and other injuries such as those caused by viruses and bioterrorism that have the potential of stimulating the immune system and generating large amounts of inflammatory cytokines and chemokines. These AR inhibitors could also be used to prevent inflammation mediated by cytokines and chemokines, irrespective of the source.
Outlook
Inflammatory complications, including sepsis, cancer and asthma remain huge clinical problems worldwide despite improved health care and specific treatment approaches. Accordingly, there is an ongoing need for development of new therapeutic strategies in the treatment of such diseases. Elucidation of cytokine signaling is critical for understanding multiple diseases, including infection, atherosclerosis and cancer, and for developing therapeutic interventions for minimizing their inflammatory components. Hence, investigating the mechanisms that normalize inflammatory signals has intense importance for understanding and managing a wide array of disease processes. As described in the present review, extensive research during recent years has identified that AR plays a major role in mediation of oxidative stress-induced inflammatory signals via PLC/PKC/IKK/MAPK/NF-kB/AP-1. Inhibition of AR prevents inflammatory diseases such as uveitis, sepsis, colon cancer, atherosclerosis and asthma in experimental animal models. We expect that these results will shed new light on the fundamental mechanisms regulating inflammation as well as lay down the foundation for future studies to devise strategies for clinical implications. Accordingly, potential strategies that prevent AR and retard the progression of inflammatory complications still need to be evaluated. The challenge for future research will be to unravel these complex interactions that AR mediates between cellular metabolism, inflammation and cancer. A better understanding of the signaling pathways engaged by AR catalyzed lipid peroxidation products and their glutathione conjugates will help in understanding the causes of tissue and organ dysfunction. The future search for new potential pathways and the development of rational therapeutic options for better management of inflammatory complications may be facilitated by using newly developed tools like microRNA technology, nanoparticle based drug delivery and microarrays to identify the signaling pathways mediated by AR.
Acknowledgments
The research work in the author’s lab was supported by National Institutes of Health (NIH) Grants EY015891 and GM071036.
Abbreviations
- AR
Aldose reductase
- AP1
activator protein1
- Cox
cycloxygenase
- DAG
diaceylglycerol
- DHN
1,4-dihydroxy-2-nonene
- FGF
fibroblast growth factor
- GSH
glutathione
- GSNO
nitrosoglutathione
- GSSG
oxidized glutathione
- HNE
4-hydroxynonenal
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- LDAs
lipid-derived aldehydes
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NAD
nicotinamide adenine dinucleotide
- NADPH
reduced NAD phosphate
- NF-κB
nuclear factor-κ-binding protein
- NO
nitric oxide
- PKC
protein kinase C
- PLC
phospholiase C
- ROS
reactive oxygen species
- VEC
vascular endothelial cells
- VSMC
vascular smooth muscle cells
References
- 1.Hers HG. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochim Biophys Acta. 1956;22:202–3. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- 2.van Heyningen R. Formation of polyols by the lens of the rat with ‘sugar’ cataract. Nature. 1959;468:194–5. [Google Scholar]
- 3.Kinoshita JH, Dvornik D, Kraml M, Gabbay KH. The effect of an aldose reductase inhibitor on the galactose-exposed rabbit lens. Biochim Biophys Acta. 1968;158:472–5. doi: 10.1016/0304-4165(68)90305-x. [DOI] [PubMed] [Google Scholar]
- 4.Varma SD, Kinoshita JH. Inhibition of lens aldose reductase by flavonoids–their possible role in the prevention of diabetic cataracts. Biochem Pharmacol. 1976;25:2505–13. doi: 10.1016/0006-2952(76)90457-3. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita JH, Fukushi S, Kador P, Merola LO. Aldose reductase in diabetic complications of the eye. Metabolism. 1979;28:462–9. doi: 10.1016/0026-0495(79)90057-x. [DOI] [PubMed] [Google Scholar]
- 6.van Heyningen R. Sugar alcohols in the pathogenesis of galactose and diabetic cataracts. Birth Defects Orig Artic Ser. 1976;12:295–303. [PubMed] [Google Scholar]
- 7.Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973;288:831–6. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M, Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- 9.Oates PJ. Aldose reductase inhibitors and diabetic kidney disease. Curr Opin Investig Drugs. 2010;11:402–17. [PubMed] [Google Scholar]
- 10.Schemmel KE, Padiyara RS, D’Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications. 2010;24:354–60. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–92. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka T, Nishimura C, Yamashita K, Itakura M, Yamada T, Fujimoto J, Kokai Y. Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia. 1995;38:255–61. doi: 10.1007/BF00400627. [DOI] [PubMed] [Google Scholar]
- 13.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A. 1995;92:2780–4. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah VO, Scavini M, Nikolic J, Sun Y, Vai S, Griffith JK, Dorin RI, Stidley C, Yacoub M, Vander Jagt DL, Eaton RP, Zager PG. Z-2 microsatellite allele is linked to increased expression of the aldose reductase gene in diabetic nephropathy. J Clin Endocrinol Metab. 1998;83:2886–91. doi: 10.1210/jcem.83.8.5028. [DOI] [PubMed] [Google Scholar]
- 15.Hodgkinson AD, Søndergaard KL, Yang B, Cross DF, Millward BA, Demaine AG. Aldose reductase expression is induced by hyperglycemia in diabetic nephropathy. Kidney Int. 2001;60:211–8. doi: 10.1046/j.1523-1755.2001.00788.x. [DOI] [PubMed] [Google Scholar]
- 16.Ansari NH, Bhatnagar A, Fulep E, Khanna P, Srivastava SK. Trolox protects hyperglycemia-induced cataractogenesis in cultured rat lens. Res Commun Chem Pathol Pharmacol. 1994;84:93–104. [PubMed] [Google Scholar]
- 17.Ansari NH, Srivastava SK. Allopurinol promotes and butylated hydroxy toluene prevents sugar-induced cataractogenesis. Biochem Biophys Res Commun. 1990;168:939–43. doi: 10.1016/0006-291x(90)91119-d. [DOI] [PubMed] [Google Scholar]
- 18.Bhatnagar A, Ansari NH, Zacarias A, Srivastava SK. Digital image analysis of cultured rat lens during oxidative stress-induced cataractogenesis. Exp Eye Res. 1993;57:385–91. doi: 10.1006/exer.1993.1139. [DOI] [PubMed] [Google Scholar]
- 19.Borhani DW, Harter TM, Petrash JM. The crystal structure of the aldose reductase. NADPH binary complex. J Biol Chem. 1992;267:24841–7. doi: 10.2210/pdb1abn/pdb. [DOI] [PubMed] [Google Scholar]
- 20.Vander Jagt DL, Kolb NS, Vander Jagt TJ, Chino J, Martinez FJ, Hunsaker LA, Royer RE. Substrate specificity of human aldose reductase: identification of 4-hydroxynonenal as an endogenous substrate. Biochim Biophys Acta. 1995;1249:117–26. doi: 10.1016/0167-4838(95)00021-l. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun. 1995;217:741–6. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- 23.Dixit BL, Balendiran GK, Watowich SJ, Srivastava S, Ramana KV, Petrash JM, Bhatnagar A, Srivastava SK. Kinetic and structural characterization of the glutathione-binding site of aldose reductase. J Biol Chem. 2000;275:21587–95. doi: 10.1074/jbc.M909235199. [DOI] [PubMed] [Google Scholar]
- 24.Ramana KV, Dixit BL, Srivastava S, Balendiran GK, Srivastava SK, Bhatnagar A. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39:12172–80. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- 25.Ramana KV, Dixit BL, Srivastava S, Bhatnagar A, Balendiran GK, Watowich SJ, Petrash JM, Srivastava SK. Characterization of the glutathione binding site of aldose reductase. Chem Biol Interact. 2001;130–132:537–48. doi: 10.1016/s0009-2797(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhary S, Srivastava S, Xiao T, Andley UP, Srivastava SK, Ansari NH. Metabolism of lipid derived aldehyde, 4-hydroxynonenal in human lens epithelial cells and rat lens. Invest Ophthalmol Vis Sci. 2003;44:2675–82. doi: 10.1167/iovs.02-0965. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava SK. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic Biol Med. 2000;29:642–51. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 29.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest. 1999;103:1007–13. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forman HJ. Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction. Ann N Y Acad Sci. 2010;1203:35–44. doi: 10.1111/j.1749-6632.2010.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida K. Lipid peroxidation and redox-sensitive signaling pathways. Curr Atheroscler Rep. 2007;9:216–21. doi: 10.1007/s11883-007-0022-7. [DOI] [PubMed] [Google Scholar]
- 32.Kutuk O, Basaga H. Apoptosis signaling by 4-hydroxynonenal: a role for JNK-c-Jun/AP-1 pathway. Redox Rep. 2007;12:30–4. doi: 10.1179/135100007X162329. [DOI] [PubMed] [Google Scholar]
- 33.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–70. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 34.Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, Bhatnagar A. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1745–52. doi: 10.1161/01.atv.20.7.1745. [DOI] [PubMed] [Google Scholar]
- 35.Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–7. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- 36.Ramana KV, Bhatnagar A, Srivastava SK. Inhibition of aldose reductase attenuates TNF-alpha-induced expression of adhesion molecules in endothelial cells. FASEB J. 2004;18:1209–18. doi: 10.1096/fj.04-1650com. [DOI] [PubMed] [Google Scholar]
- 37.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–13. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 38.Singh R, White MA, Ramana KV, Petrash JM, Watowich SJ, Bhatnagar A, Srivastava SK. Structure of a glutathione conjugate bound to the active site of aldose reductase. Proteins. 2006;64:101–10. doi: 10.1002/prot.20988. [DOI] [PubMed] [Google Scholar]
- 39.Ko BC, Lam KS, Wat NM, Chung SS. An (A-C)n dinucleotide repeat polymorphic marker at the 5′ end of the aldose reductase gene is associated with early-onset diabetic retinopathy in NIDDM patients. Diabetes. 1995 Jul;44(7):727–32. doi: 10.2337/diabetes.44.7.727. [DOI] [PubMed] [Google Scholar]
- 40.Heesom AE, Millward A, Demaine AG. Susceptibility to diabetic neuropathy in patients with insulin dependent diabetes mellitus is associated with a polymorphism at the 5′ end of the aldose reductase gene. J Neurol Neurosurg Psychiatry. 1998;64:213–6. doi: 10.1136/jnnp.64.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao YL, Donaghue K, Chan A, Knight J, Silink M. An aldose reductase intragenic polymorphism associated with diabetic retinopathy. Diabetes Res Clin Pract. 1999;46:155–60. doi: 10.1016/s0168-8227(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 42.Moczulski DK, Scott L, Antonellis A, Rogus JJ, Rich SS, Warram JH, Krolewski AS. Aldose reductase gene polymorphisms and susceptibility to diabetic nephropathy in Type 1 diabetes mellitus. Diabet Med. 2000;17:111–8. doi: 10.1046/j.1464-5491.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 43.Neamat-Allah M, Feeney SA, Savage DA, Maxwell AP, Hanson RL, Knowler WC, El Nahas AM, Plater ME, Shaw J, Boulton AJ, Duff GW, Cox A. Analysis of the association between diabetic nephropathy and polymorphisms in the aldose reductase gene in Type 1 and Type 2 diabetes mellitus. Diabet Med. 2001;18:906–14. doi: 10.1046/j.0742-3071.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Xie P, Huang J, Gu Y, Zeng W, Song H. Polymorphisms and functions of the aldose reductase gene 5′ regulatory region in Chinese patients with type 2 diabetes mellitus. Chin Med J (Engl) 2002;115:209–13. [PubMed] [Google Scholar]
- 45.Sivenius K, Pihlajamäki J, Partanen J, Niskanen L, Laakso M, Uusitupa M. Aldose reductase gene polymorphisms and peripheral nerve function in patients with type 2 diabetes. Diabetes Care. 2004;27:2021–6. doi: 10.2337/diacare.27.8.2021. [DOI] [PubMed] [Google Scholar]
- 46.Richeti F, Noronha RM, Waetge RT, de Vasconcellos JP, de Souza OF, Kneipp B, Assis N, Rocha MN, Calliari LE, Longui CA, Monte O, de Melo MB. Evaluation of AC(n) and C(-106)T polymorphisms of the aldose reductase gene in Brazilian patients with DM1 and susceptibility to diabetic retinopathy. Mol Vis. 2007;13:740–5. [PMC free article] [PubMed] [Google Scholar]
- 47.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–60. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch Biochem Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10:R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerbone A, Toaldo C, Laurora S, Briatore F, Pizzimenti S, Dianzani MU, Ferretti C, Barrera G. 4-Hydroxynonenal and PPARgamma ligands affect proliferation, differentiation, and apoptosis in colon cancer cells. Free Radic Biol Med. 2007;42:1661–70. doi: 10.1016/j.freeradbiomed.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava SK, Ramana KV. Focus on molecules: nuclear factor-kappaB. Exp Eye Res. 2009;88:2–3. doi: 10.1016/j.exer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattie MD, McElwee MK, Freedman JH. Mechanism of copper-activated transcription: activation of AP-1, and the JNK/SAPK and p38 signal transduction pathways. J Mol Biol. 2008;383:1008–18. doi: 10.1016/j.jmb.2008.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanos T, Marinissen MJ, Leskow FC, Hochbaum D, Martinetto H, Gutkind JS, Coso OA. Phosphorylation of c-Fos by members of the p38 MAPK family. Role in the AP-1 response to UV light. J Biol Chem. 2005;280:18842–52. doi: 10.1074/jbc.M500620200. [DOI] [PubMed] [Google Scholar]
- 55.Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS, Park NG, Nakajima H, Magae J, Chang YC. Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis. 2007;28:1104–10. doi: 10.1093/carcin/bgl217. [DOI] [PubMed] [Google Scholar]
- 56.Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radic Biol Med. 2007;42:1290–302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramana KV, Bhatnagar A, Srivastava SK. Aldose reductase regulates TNF-alpha-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett. 2004;570:189–94. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 58.Uchida K. Lipid peroxidation and redox-sensitive signaling pathways. Curr Atheroscler Rep. 2007;9:216–21. doi: 10.1007/s11883-007-0022-7. [DOI] [PubMed] [Google Scholar]
- 59.Yadav UC, Ramana KV, Awasthi YC, Srivastava SK. Glutathione level regulates HNE-induced genotoxicity in human erythroleukemia cells. Toxicol Appl Pharmacol. 2008;227:257–64. doi: 10.1016/j.taap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knoll N, Ruhe C, Veeriah S, Sauer J, Glei M, Gallagher EP, Pool-Zobel BL. Genotoxicity of 4-hydroxy-2-nonenal in human colon tumor cells is associated with cellular levels of glutathione and the modulation of glutathione S-transferase A4 expression by butyrate. Toxicol Sci. 2005;86:27–35. doi: 10.1093/toxsci/kfi171. [DOI] [PubMed] [Google Scholar]
- 61.Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- 62.Rinaldi M, Barrera G, Aquino A, Spinsanti P, Pizzimenti S, Farace MG, Dianzani MU, Fazio VM. 4-Hydroxynonenal-induced MEL cell differentiation involves PKC activity translocation. Biochem Biophys Res Commun. 2000;272:75–80. doi: 10.1006/bbrc.2000.2691. [DOI] [PubMed] [Google Scholar]
- 63.Nitti M, Domenicotti C, d’Abramo C, Assereto S, Cottalasso D, Melloni E, Poli G, Biasi F, Marinari UM, Pronzato MA. Activation of PKC-beta isoforms mediates HNE-induced MCP-1 release by macrophages. Biochem Biophys Res Commun. 2002;294:547–52. doi: 10.1016/S0006-291X(02)00512-0. [DOI] [PubMed] [Google Scholar]
- 64.Castello L, Marengo B, Nitti M, Froio T, Domenicotti C, Biasi F, Leonarduzzi G, Pronzato MA, Marinari UM, Poli G, Chiarpotto E. 4-Hydroxynonenal signalling to apoptosis in isolated rat hepatocytes: the role of PKC-delta. Biochim Biophys Acta. 2005;1737:83–93. doi: 10.1016/j.bbalip.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruunsgaard H, Poulsen HE, Pedersen BK, Nyyssonen K, Kaikkonen J, Salonen JT. Long-term combined supplementations with alpha-tocopherol and vitamin C have no detectable anti-inflammatory effects in healthy men. J Nutr. 2003;133:1170–73. doi: 10.1093/jn/133.4.1170. [DOI] [PubMed] [Google Scholar]
- 67.Bai J, Cederbaum AI. Overexpression of catalase in the mitochondrial or cytosolic compartment increases sensitivity of HepG2 cells to tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2000;275:19241–49. doi: 10.1074/jbc.M000438200. [DOI] [PubMed] [Google Scholar]
- 68.Lin SJ, Shyue SK, Hung YY, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-alpha in human endothelial cells through the JNK/p38 pathways. Arterioscler Thromb Vasc Biol. 2005;25:334–40. doi: 10.1161/01.ATV.0000152114.00114.d8. [DOI] [PubMed] [Google Scholar]
- 69.Naito Y, Takano H, Yoshikawa T. Oxidative stress-related molecules as a therapeutic target for inflammatory and allergic diseases. Curr Drug Targets Inflamm Allergy. 2005;4:511–15. doi: 10.2174/1568010054526269. [DOI] [PubMed] [Google Scholar]
- 70.Sen CK, Roy S. Relief from a heavy heart: redox-sensitive NF-kappaB as a therapeutic target in managing cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2005;289:17–19. doi: 10.1152/ajpheart.00250.2005. [DOI] [PubMed] [Google Scholar]
- 71.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol. 2001;167:7060–68. doi: 10.4049/jimmunol.167.12.7060. [DOI] [PubMed] [Google Scholar]
- 72.Call GB, Husein OF, McIlmoil CJ, Adams A, Heckmann RA, Judd AM. Bovine adrenal cells secrete interleukin-6 and tumor necrosis factor in vitro. Gen Comp Endocrinol. 2000;118:249–61. doi: 10.1006/gcen.2000.7458. [DOI] [PubMed] [Google Scholar]
- 73.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–70. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miwa K, Nakamura J, Hamada Y, Naruse K, Nakashima E, Kato K, Kasuya Y, Yasuda Y, Kamiya H, Hotta N. The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res Clin Pract. 2003;60:1–9. doi: 10.1016/s0168-8227(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 75.Murata M, Ohta N, Sakurai S, Alam S, Tsai J, Kador PF, Sato S. The role of aldose reductase in sugar cataract formation: aldose reductase plays a key role in lens epithelial cell death (apoptosis) Chem Biol Interact. 2001;130–132:617–25. doi: 10.1016/s0009-2797(00)00289-1. [DOI] [PubMed] [Google Scholar]
- 76.Tesfamariam B, Palacino JJ, Weisbrod RM, Cohen RA. Aldose reductase inhibition restores endothelial cell function in diabetic rabbit aorta. J Cardiovasc Pharmacol. 1993;21:205–11. doi: 10.1097/00005344-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Srivastava S, Ramana KV, Tammali R, Srivastava SK, Bhatnagar A. Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells. Diabetes. 2006;55:901–10. doi: 10.2337/diabetes.55.04.06.db05-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasuya Y, Ito M, Nakamura J, Hamada Y, Nakayama M, Chaya S, Komori T, Naruse K, Nakashima E, Kato K, Koh N, Hotta N. An aldose reductase inhibitor prevents the intimal thickening in coronary arteries of galactose-fed beagle dogs. Diabetologia. 1999;42:1404–09. doi: 10.1007/s001250051310. [DOI] [PubMed] [Google Scholar]
- 79.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–29. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- 80.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–29. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 81.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–20. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 82.Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase regulates vascular smooth muscle cell proliferation by modulating G1/S phase transition of cell cycle. Endocrinology. 2010;151:2140–50. doi: 10.1210/en.2010-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–24. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramana KV, Tammali R, Reddy AB, Bhatnagar A, Srivastava SK. Aldose reductase-regulated tumor necrosis factor-alpha production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology. 2007;148:4371–84. doi: 10.1210/en.2007-0512. [DOI] [PubMed] [Google Scholar]
- 85.Reddy AB, Ramana KV, Srivastava S, Bhatnagar A, Srivastava SK. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology. 2009;150:63–74. doi: 10.1210/en.2008-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy AB, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents lipopolysaccharide-induced glucose uptake and glucose transporter 3 expression in RAW264.7 macrophages. Int J Biochem Cell Biol. 2010;42:1039–45. doi: 10.1016/j.biocel.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang YC, Shaw S, Kaneko M, Redd H, Marrero MB, Ramasamy R. Aldose reductase pathway mediates JAK-STAT signaling: a novel axis in myocardial ischemic injury. FASEB J. 2005;19:795–7. doi: 10.1096/fj.04-2780fje. [DOI] [PubMed] [Google Scholar]
- 88.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–29. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 89.Crimi E, Sica V, Slutsky AS, Zhang H, Williams-Ignarro S, Ignarro LJ, Napoli C. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic Res. 2006;40:665–72. doi: 10.1080/10715760600669612. [DOI] [PubMed] [Google Scholar]
- 90.Sakaguchi S, Furusawa S. Oxidative stress and septic shock: metabolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS Immunol Med Microbiol. 2006;47:167–77. doi: 10.1111/j.1574-695X.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 91.Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–22. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–46. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 93.Reddy AB, Srivastava SK, Ramana KV. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine. 2009;48:170–6. doi: 10.1016/j.cyto.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore WC, Pascual RM. Update in asthma 2009. Am J Respir Crit Care Med. 2010;181:1181–7. doi: 10.1164/rccm.201003-0321UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy DM, O’Byrne PM. Recent advances in the pathophysiology of asthma. Chest. 2010;137:1417–26. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 96.Yadav UC, Naura AS, Aguilera-Aguirre L, Ramana KV, Boldogh I, Sur S, Boulares HA, Srivastava SK. Aldose reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009;183:4723–32. doi: 10.4049/jimmunol.0901177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4(8):e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srivastava SK, Ramana KV. Aldose reductase inhibition for the treatment of asthma. Expert Rev Clin Immunol. 2010;6:1–4. doi: 10.1586/eci.09.79. [DOI] [PubMed] [Google Scholar]
- 99.Srivastava A, Rajappa M, Kaur J. Uveitis: Mechanisms and recent advances in therapy. Clin Chim Acta. 2010;411:1165–71. doi: 10.1016/j.cca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 100.Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–42. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–82. doi: 10.1167/iovs.08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalariya NM, Shoeb M, Reddy AB, Zhang M, van Kuijk FJ, Ramana KV. Prevention of endotoxin-induced uveitis in rats by plant sterol guggulsterone. Invest Ophthalmol Vis Sci. 2010;51:5105–13. doi: 10.1167/iovs.09-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yadav UC, Srivastava SK, Ramana KV. Understanding the role of aldose reductase in ocular inflammation. Curr Mol Med. 2010;10:540–9. doi: 10.2174/1566524011009060540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, de Braud F, Wils J. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–33. doi: 10.1016/j.critrevonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–37. [PubMed] [Google Scholar]
- 106.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed]
- 107.Tammali R, Ramana KV, Srivastava SK. Aldose reductase regulates TNF-alpha-induced PGE2 production in human colon cancer cells. Cancer Lett. 2007;252:299–306. doi: 10.1016/j.canlet.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–24. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramana KV, Srivastava SK. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int J Biochem Cell Biol. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexiou P, Pegklidou K, Chatzopoulou M, Nicolaou I, Demopoulos VJ. Aldose reductase enzyme and its implication to major health problems of the 21(st) century. Curr Med Chem. 2009;16:734–52. doi: 10.2174/092986709787458362. [DOI] [PubMed] [Google Scholar]