Abstract
Objectives
Inflammation has been associated with a wide range of chronic degenerative diseases, but the developmental factors contributing to the regulation of inflammation are poorly understood. This study investigates the within-individual association between antibody response to vaccination in adolescence and C-reactive protein (CRP) concentration in young adulthood.
Methods
In 1998-99, at age 14-15 years, a subset of participants (N=96) in the Cebu Longitudinal Health and Nutrition Survey were administered a typhoid vaccine, and baseline and follow up blood samples were drawn to assess the strength of the antibody response to vaccination. In 2005, at age 20-21 years, blood samples were drawn from the full cohort for measurement of CRP. N=74 individuals had complete data at both time points. Bivariate associations and multivariate logistic regression models were evaluated to test the hypothesis that vaccine responsiveness in adolescence was significantly associated with CRP level in young adulthood.
Results
There was a strong and statistically significant association between antibody response to vaccination in adolescence and CRP in young adulthood. Median CRP was more than four times higher among non-responders than responders, and non-responders were 2.3 to 3.6 times more likely to have CRP in the top tertile of the sample distribution.
Conclusions
This study provides evidence for a prospective, within-individual link between more effective antibody-mediated immune defenses and lower levels of inflammation. In the context of prior research in this population, these results suggest that early environments are important determinants of multiple aspects of an individual’s immuno-phenotype.
Keywords: Inflammation, ecological immunology, human growth and development, infectious disease
Inflammation has historically been studied as a central component of innate immune defenses against infection, while recent research has documented a likely role in chronic degenerative processes, including cardiovascular disease (CVD), type 2 diabetes (Pradhan and others 2001), the metabolic syndrome (Ridker and others 2003), late-life disability (Kuo and others 2006) and mortality (Jenny and others 2007). C-reactive protein (CRP) is an acute phase protein produced by hepatocytes in response to pro-inflammatory cytokines such as IL-6, and it is involved in activating complement, promoting phagocytic activity, and opsonizing bacteria, fungi, and parasites (Ballou and Kushner 1992; Black and others 2004). Innate immune defenses like inflammation are rapid and non-specific, and thus provide an important first line of defense against a wide range of pathogens.
However, this role in anti-pathogen defenses has been overshadowed by intense clinical and epidemiological interest in the measurement of CRP to assess levels of chronic, low-grade inflammation (Pearson and others 2003). The vast majority of research on CRP has been conducted in relatively affluent, industrialized settings where rates of overweight and obesity are high, and levels of infectious disease exposure are low. Additional insight into the factors contributing to the regulation of inflammation may be gained by a comparative, developmental perspective, and in this paper we report on the association between antibody response to vaccination in adolescence and CRP concentration in young adulthood in an ongoing cohort study in the Philippines.
Previously, we have shown that prenatal under-nutrition and microbial exposures in infancy are significant predictors of CRP in young adulthood in the Philippines (McDade and others 2010). Lower birth weight predicted higher CRP in young adulthood, while three different measures of microbial exposure in infancy were negatively associated with CRP in young adulthood. Similarly, we have reported that prenatal under-nutrition and infectious morbidity in infancy are associated with vaccine responsiveness in Filipino adolescents (McDade and others 2001). Individuals born small-for-gestational age were significantly less likely to mount an adequate antibody response to vaccination, while those who experienced high levels of infectious diarrhea in infancy were more likely to respond to the vaccine. Both sets of findings are consistent with prior research on the “hygiene” and “old friends” hypotheses, which propose that microbes have been a normative and ubiquitous component of the human evolutionary environment, and that microbial exposures early in infancy are critical to guiding the development of several immune processes, including the regulation of inflammation (Rook 2009; Yazdanbakhsh and others 2002). These findings are also consistent with a much broader literature documenting the impact of prenatal undernutrition on multiple physiological systems (Barker 1994; Gluckman and others 2007).
Concordance across the vaccine and CRP studies suggests that microbial and nutritional exposures early in life may initiate a more fundamental shift in the development and regulation of multiple aspects of immune function. In this study we test the hypothesis that positive antibody response to vaccination in adolescence is associated with CRP measured seven years later in young adulthood. Results may have implications for two issues related to the developmental ecology of human immune function. First, a weak or non-existent association would suggest that links among early environments, antibody-mediated immunity, and inflammation are relatively independent, whereas a strong within-individual association across time would provide additional evidence for the importance of early environments in shaping an individual’s immuno-phenotype.
Second, results may shed light on the adaptive significance of these processes. Robust antibody-mediated immune defenses are critical for resistance against infectious disease, but the interpretation of inflammation is more problematic. Innate immune defenses like inflammation are also critical for resisting infection, but poorly regulated, chronically activated inflammatory processes increase risk for a wide range of chronic degenerative diseases. By investigating two aspects of immunity across time we may gain insight into whether higher levels of chronic inflammation represent potentially pathological consequences of suboptimal early environments, or adaptive trade-offs in allocations of effort to subsystems of immune defenses.
Methods
Study participants and protocol
Participants were recruited from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), an ongoing population-based study of maternal and child health in the Philippines that began in 1983 with the recruitment of 3,327 pregnant women representative of the childbearing population in Cebu City (Cebu_Study_Team 1991). The women and their children have been followed through multiple rounds of data collection since 1983, including the most recent survey conducted in 2005.
In 1998-99, 2,089 CLHNS participants—14 or 15 years of age at the time—were contacted for follow-up data collection, and a subsample of 96 individuals was included in a vaccine sub-study based on the following criteria: full term birth ( ≥37 weeks), currently healthy, and small-for-gestational age (SGA: defined as <10th percentile of birthweight for gestational age) versus appropriate-for-gestational age (AGA: ≥10th percentile). Upon enrollment in the vaccine study, approximately 5mL of EDTA plasma were collected and immediately frozen, followed by vaccination against typhoid fever with a 25μg dose of purified Vi cell surface polysaccharide extracted from Salmonella typhi, delivered in 500μL sterile solution via intramuscular injection (Pasteur Merieux, Lyon, France). Follow-up blood was drawn two weeks later. Additional details of this study have been published previously (McDade and others 2001).
In 2005, 1,885 participants were contacted for follow-up, 1,486 of whom provided complete anthropometric and interview data, as well as a blood sample for the analysis of CRP (McDade and others 2009). Participants provided information on household demographics and economic resources, environmental quality, and health behaviors in face-to-face interviews conducted in their homes. Waist circumference was measured during the in-home interview using standard procedures (Lohman and others 1988).
Of the 96 participants in the vaccine sub-study, 75 also provided complete data for the 2005 survey. One woman who was pregnant at the time of blood collection in 2005 was removed from the analyses, yielding a final sample size of N=74. Compared to individuals in the original cohort as assessed when the study began in 1983, the individuals in this sample did not differ in terms of maternal education, household assets, or household income. They did, however, have lower average birth weights (2,774 g vs. 2,995 g, p<0.001) than other participants in the cohort. This difference is to be expected since the vaccine sub study over-sampled for individuals born SGA. The 21 individuals lost to follow-up between 1998-99 and 2005 did not differ in these attributes from the 75 individuals remaining from the sub-study.
All data were collected under conditions of informed consent using protocols approved by the institutional review board of the University of North Carolina, Chapel Hill.
Measurement of vaccine response and CRP
Anti-typhoid IgG antibody titers were analyzed in samples from 1998-99 as a functional measure of antibody responsiveness to vaccination. Samples were serially diluted 1:2 to a final concentration of 1:1280, and antibody titer was determined using an enzyme immunoassay procedure (McDade and others 2001). The highest dilution at which anti-typhoid antibodies were still detectable was defined as the endpoint concentration for that sample. Baseline and two week follow-up samples for each individual were analyzed on the same assay plate to maximize comparability.
In 2005, blood samples were collected into EDTA-coated vacutainer tubes in the participants’ homes in the morning after an overnight fast. Blood samples were kept in coolers on ice packs for no more than 2 hours and were then centrifuged to separate plasma prior to freezing at −70°C. Samples were express shipped to Northwestern University on dry ice and stored frozen at −80°C until analysis. CRP concentrations were determined using a high sensitivity immunoturbidimetric method (Synchron LX20, lower detection limit: 0.1 mg/L).
Data Analysis
Vaccine response in 1998-99 was the primary independent variable of interest. Prior research evaluating the efficacy of the anti-typhoid vaccine has defined a 4-fold or greater increase in antibody titer as a positive response to the vaccine challenge (Tacket and others 1988). For this analysis, we defined a 2-fold increase in antibody titer from baseline as a mild response, and a 4-fold or greater increase as a robust response to vaccination. Individuals with no difference in titers between baseline and two weeks were considered non-responders.
We tested the hypothesis that vaccine responsiveness in adolescence was associated with inflammation in adulthood in two ways. First, we inspected the bivariate associations between vaccine response in 1998-99 and CRP concentration in 2005. Second, we determined the odds of elevated CRP in relation to vaccine response in a bivariate logistic regression model. Lastly, we implemented two multivariate logistic regression models to evaluate whether the association between vaccine response and CRP was independent of characteristics in adulthood known to influence CRP, including waist circumference, smoking, household pathogenicity, and recent symptoms of infectious disease. We hypothesized that associations would strengthen after controlling for these potentially confounding factors. No women in the sample were taking oral contraceptives at the time of blood collection. Vaccine response was modeled using indicator variables, with non-responders as the comparison group.
Following prior cross-sectional analyses of the predictors of CRP in this sample (McDade and others 2009) we constructed a household pathogen exposure variable based on five measures, each scored on a three point scale (0=low exposure, 1=moderate, 2=high) and assessed at the time of CRP measurement: cleanliness of the food preparation area, means of garbage disposal, presence of excrement near the house, level of garbage and excrement present in the neighborhood surrounding the household. To assess infectious morbidity at the time of blood collection, we asked participants if they were currently experiencing any symptoms of infection. Symptoms included runny nose, cough, fever, diarrhea, sore throat, as well as the more general categories of “flu,” “cold,” and “sinusitis”. Responses were used to construct a single variable indicating the presence of any infectious symptoms at the time of blood collection. Information on symptoms of infectious disease and smoking behavior was collected during the in-home interview preceding blood collection.
Logistic regression models predicted the odds of having CRP concentration in the top tertile of the sample distribution (≥0.7 mg/L). Individuals in the top tertile of the CRP distribution have been shown to be at increased risk for cardiovascular disease, and this approach has been used previously in CLHNS (McDade and others 2010) as well as several other population-based studies of CRP (Danesh and others 2000).
Studies using a single CRP measure as an indicator of chronic, low grade inflammation must acknowledge that the acute phase response to infection leads to short-term spikes in CRP concentration that may obscure assessment of chronic CRP production. We pursued three strategies to address this issue. First, individuals with CRP > 10 mg/L were removed from the analyses based on recommendations issued by a recent joint scientific statement from the American Heart Association and the Centers for Disease Control and Prevention suggesting that this level of CRP is presumed to be the result of acute inflammatory processes (Pearson and others 2003). Second, we controlled for the presence of current or recent symptoms of infectious disease by including this variable as a covariate in our analyses. And third, we considered a final model that eliminated all individuals with CRP > 10 mg/L and/or symptoms of infectious disease. All statistical analyses were conducted with Stata for Windows, version 10 (StataCorp, College Station, TX).
Results
Basic descriptive statistics for vaccine responders and non-responders are presented in Table 1. Age, proportion of females, household income, education level, and presence of infectious disease symptoms at the time of blood collection did not differ significantly across the three groups (p>0.05). There was a marginally significant difference in 2005 waist circumference across the groups (p=0.06), with mild responders having waist circumferences approximately 5 cm larger than the other groups.
Table 1.
Descriptive statistics for the study sample, and by vaccine response status. All values are from the 2005 survey, with the exception of birth weight. Mean (SD) values are presented for continuous variables (median, 25th and 75th %ile values for CRP).
| Non- responders |
Mild responders |
Robust responders |
Total | |
|---|---|---|---|---|
| N=18 | N=19 | N=37 | N=74 | |
| Age (years) | 20.8 (0.4) | 20.9 (0.5) | 21.0 (0.3) | 20.9 (0.4) |
| Female (%) | 44.4 | 50.0 | 51.4 | 49.3 |
| Education (years) | 11.3 (3.2) | 11.2 (2.2) | 10.6 (3.2) | 10.9 (2.9) |
| Weekly household income (pesos) |
491.2 (226.4) | 445.1 (310.1) | 505.6 (506.2) | 487.1 (404.4) |
| Waist circumference (cm) | 67.4 (5.9) | 72.5 (11.7) | 67.0 (6.8) | 68.5 (8.3) |
| Symptoms of infection at time of blood collection (%) |
16.7 | 16.7 | 13.5 | 15.1 |
| CRP (mg/L) | 0.8 (0.1, 1.0) | 0.2 (0.1, 0.6) | 0.1 (0.1, 0.7) | 0.3 (0.1, 0.8) |
Median CRP concentration in 2005 was substantially higher among individuals who did not respond to the typhoid vaccination in 1998-99: For non-responders, median CRP was 0.8 mg/L, compared to 0.2 mg/L and 0.1 mg/L for mild responders and robust responders, respectively. Similarly, 52.9% of non-responders had high CRP in 2005 (>0.7 mg/L), compared to 16.7% of mild responders and 25.0% of robust responders status (Pearson X2=6.26, p<0.05).
Logistic regression results controlling for contemporaneous factors known to influence CRP production reveal a similar pattern of results. In a simple bivariate model, individuals who mounted a robust antibody response to vaccination in 1998-99 were significantly less likely to have elevated CRP in 2005 than individuals who did not respond to vaccination (Table 2, model 1). Mild responders were similarly less likely to have elevated CRP, although this association was of marginal statistical significance. These associations strengthened when gender, waist circumference, household pathogenicity, smoking, and symptoms of infectious disease were included in the model (Table 2, model 2). The association between robust vaccine response in adolescence and low likelihood of elevated CRP in young adulthood strengthened further when individuals with symptoms of infection at the time of blood collection were removed from the model (Table 2, model 3).
Table 2.
Results of maximum likelihood logistic regression models predicting elevated CRP in 2005 in relation to vaccine response in 1998-99. All models exclude individuals with CRP > 10 mg/L. Model 3 also excludes individuals with symptoms of infectious disease at the time of blood collection.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| N=74 | N=74 | N=65 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Mild responder1 | 0.24+ | 0.06, 1.02 | 0.16* | 0.03, 0.88 | 0.19+ | 0.03, 1.15 |
| Robust responder1 | 0.28* | 0.08, 0.93 | 0.26* | 0.07, 0.94 | 0.14* | 0.03, 0.66 |
| Female | 0.37 | 0.10, 1.30 | 0.53 | 0.12, 2.27 | ||
| Waist circumference (cm) | 1.05 | 0.98, 1.12 | 1.13* | 1.02, 1.25 | ||
| Pathogenicity scale (0-2) | 1.34 | 0.27, 6.64 | 1.13 | 0.19, 6.75 | ||
| Current smoker (0, 1) | 0.64 | 0.13, 3.03 | 0.69 | 0.12, 3.93 | ||
| Infectious symptoms (0, 1) | 2.42 | 0.50, 11.72 | ||||
p<0.10,
p<0.05,
p<0.01
Non-responder is the omitted reference group.
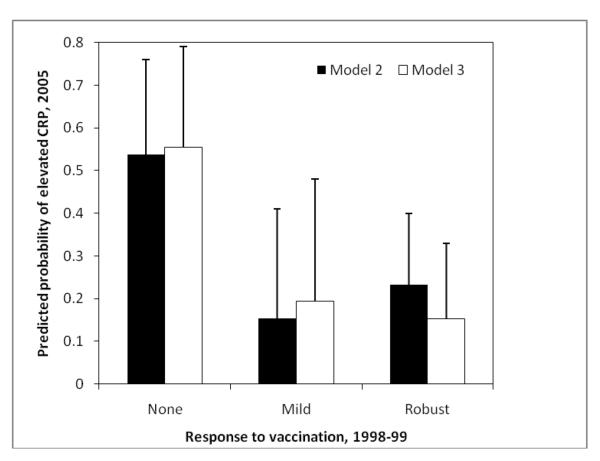
To facilitate interpretation, we calculated the predicted probability of elevated CRP in 2005 as a function of response to vaccination in 1998-99 (Figure 1). Probabilities are based on coefficients from models 2 and 3 (Table 2), and represent the independent association between vaccine response in adolescence and CRP in young adulthood, controlling for other variables in the logistic regression models. We present probabilities from models 2 and 3 to evaluate whether results are sensitive to how we deal with the presence of infectious symptoms at the time of blood collection In model 2, the predicted probability of elevated CRP in 2005 is 0.54 for non-responders, compared to 0.15 and 0.23 for mild and robust responders, respectively. In model 3, which excludes individuals with symptoms of infectious disease at the time of blood collection, the probability of elevated CRP is 0.56 for non-responders, compared to 0.19 and 0.15 for mild and robust responders.
Figure 1.
Predicted probability of elevated CRP in 2005 in relation to vaccine response in 1998-99. Probabilities are based on regression coefficients from model 2 and model 3 in Table 2. Results for model 2 exclude individuals with CRP>10 mg/L at the time of blood collection, while results for model 3 also excluded individuals with symptoms of infection at the time of blood collection.
Results for both models lead to the same conclusion: Mild and robust responses to vaccination in adolescence are comparable in their associations with low probabilities of elevated CRP in young adulthood. In contrast, individuals who did not mount an antibody response to vaccination in 1998-99 were 2.3 to 3.6 times more likely to have elevated CRP in 2005.
Discussion
We find that antibody responsiveness to vaccination in adolescence significantly predicts lower CRP in young adulthood. Prior research with this population has demonstrated that prenatal under-nutrition and postnatal infectious exposures are associated both with vaccine responsiveness as well as CRP concentration (McDade and others 2001; McDade and others 2010). Results from this analysis bring these observations together and demonstrate strong, prospective, within-individual associations between two distinct components of immune defenses. Collectively, these studies suggest that early environments are important determinants of multiple aspects of an individual’s immuno-phenotype.
Vaccination protocols are often used as a model for studying the real-world process of pathogen exposure and immune response, but with the advantage of delivering a controlled dose of antigen that is comparable across individuals. Activating aspects of anti-pathogen defenses allows for a more dynamic assessment of immune processes than is possible with static, baseline measures of activity. While prior research has focused primarily on antibody response to vaccination as a measure of immunocompetence, recent research suggests that vaccination represents a useful model for studying the dynamics of inflammation (Posthouwer and others 2004).
Since many vaccines are mild, pro-inflammatory stressors, assessments of cytokine and acute phase protein production in the days following vaccination may reveal individual differences in the regulation of inflammation. Concentrations of CRP and pro- and anti-inflammatory cytokines increase substantially during the week following vaccine administration, with peak levels often found on day 2 or 3 (Posthouwer and others 2004; Tsai and others 2005). Using this model, a combined vaccine for diphtheria, tetanus, poliomyelitis, and typhoid has been associated with increased production of IL-6 and IL-10 in young adults compared to older adults (El Yousfi and others 2005). Similarly, influenza vaccination resulted in a larger, prolonged inflammatory response among older adults reporting mild depressive symptoms compared to those reporting no depressive symptoms (Glaser and others 2003). These studies reveal significant differences in the regulation of inflammation that can be traced to attributes of individuals, and that may have implications for subsequent risk for diseases with an inflammatory component.
To the best of our knowledge, our study is the first to combine a short-term assessment of antibody response to vaccination with a long term assessment of chronic, low-grade inflammation. The association is strong: Median CRP is more than four times higher among non-responders than responders, and non-responders are 2.3 to 3.6 times more likely to have CRP in the top tertile of the sample distribution. In multivariate models, vaccine response status was the strongest predictor of CRP, and associations strengthened further following adjustment for potentially confounding variables. It should be emphasized that even though vaccine responsiveness was measured prior to CRP concentration, results do not necessarily imply a temporally ordered, directly causal relationship.
With respect to insights into the developmental ecology of human immune function, there are at least three potential, not mutually exclusive, interpretations of these findings. First, in conjunction with our prior research in this setting, the pattern of results is consistent with the idea that vaccine responsiveness and the regulation of inflammation represent two dimensions of an underlying immuno-phenotype that can be traced back—at least in part—to environments early in life. Infancy is a critical period of immunological development, and microbial exposures may comprise a normative set of inputs that promote investment in specific immune defenses such as antibody responses to pathogen exposures, as well as the development of tightly regulated inflammatory processes that respond acutely and efficiently to pathogenic challenge (McDade and others 2005; Rook 2009; Yazdanbakhsh and others 2002). Similarly, nutritional deprivation during critical periods in infancy may impede the development of effective specific immune defenses as well as pathways relevant to the regulation of inflammation, regardless of the level of microbial exposure (McDade 2006; Moore and others 2004).
Under this scenario, the strong relationship between antibody responsiveness and CRP production represents the developmental impact of microbial and nutritional exposures in infancy on multiple dimensions of immune function later in life. Low levels of microbial inputs and/or inadequate nutrition lead to poorly trained specific immune defenses and poorly regulated inflammatory processes, contributing to a potentially pathological pro-inflammatory immuno-phenotype in adulthood that is more likely to produce CRP in response to inflammatory stimuli, or less likely to downregulate CRP production following activation. Conversely, adequate microbial and nutritional exposures promote the development of a more robust, tightly regulated, and integrated network of specific and non-specific immune defenses against infectious disease. A testable assumption under this scenario is that vaccine responsiveness and CRP production are each shaped by early environments, and that the relationship across these outcomes would be even stronger in this study if both had been measured simultaneously.
Alternatively, early environments may have a direct impact on specific immune defenses only, with secondary consequences for inflammation. The absence of microbial inputs and/or inadequate nutrition impairs the development of specific immune defenses during critical periods in infancy, resulting in compromised antibody-mediated immunity in adolescence and adulthood. Higher levels of inflammation in adulthood could manifest as a second-tier, compensatory response to pathogenic challenges that are not effectively contained by specific immune defenses. Phrased another way, robust specific immune defenses make inflammatory responses unnecessary. In this scenario, higher CRP represents an adaptive response to concurrent pathogen exposure, in contrast to the first scenario, where higher CRP is interpreted as evidence of dysregulation in inflammatory pathways. While our analyses attempt to isolate the effects of infectious disease on CRP production, it is possible that subclinical infectious processes may contribute to small elevations in CRP concentration (Roberts and others 2010). Direct, serological tests of pathogen exposure could be used to evaluate this pathway. This pathway could also be evaluated indirectly by investigating the extent to which vaccine responsiveness accounts for the association between early environments and CRP production. Unfortunately, the sample size available for this study is too small for a formal mediation analysis.
Lastly, the negative association between vaccine response and CRP may indicate a trade-off in allocation of resources across different subsystems of immune defenses in response to local disease and nutritional ecologies. Life history theory predicts such trade-offs as key mechanisms of developmental plasticity (Kuzawa 2007; McDade 2003; Muehlenbein and Bribiescas 2005; Stearns 1992). For example, under conditions of prenatal energetic stress, individuals might invest fewer resources in tissues and regulatory processes related to specific immune defenses, and more in the development of innate, non-specific immune defenses. This investment strategy could be advantageous if the costs of building and operating innate immune defenses were lower than specific immune defenses, if under-nutrition early in life was a relatively accurate predictor of marginal nutritional environments later in life, and if innate immune defenses provided an adequate level of protection against infectious diseases, despite deficits in specific immunity. While theoretically plausible, the notoriously high energetic costs of aspects of inflammation (e.g., fever) are difficult to reconcile with this hypothesis. However, a more detailed evaluation of the direct costs and benefits of various immune pathways is required to test whether adaptive trade-offs in the development of immune defenses may be in play.
These scenarios suggest a number of testable hypotheses for future work. Concurrent measures of multiple aspects of immune function and follow-up measures of morbidity and mortality will be important for evaluating the developmental ecology of human immune function and its adaptive significance. Small sample size is a substantial limitation of this study, although the number of participants is comparable to—and in some cases exceeds—prior vaccine studies that include only short-term follow up assessments. Our use of a single measure of CRP to represent chronic inflammation is also a limitation, and we pursue multiple strategies for identifying acute inflammatory responses that may reduce our ability to detect associations with chronic inflammation. Despite the small sample size and single CRP measure, we find strong associations between CRP in young adulthood and vaccine responsiveness in adolescence. These results highlight the value of an ecological, developmental approach to research on human immune function, and suggest productive directions for future research on the impact of environments in infancy on the regulation of inflammation in adulthood.
Acknowledgments
Research support: Funding for this study was provided by the National Institutes of Health (RO1 HL085144; 5 RO1 TW05596); biomarker data collection was supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E).
References cited
- Ballou SP, Kushner I. C-reactive protein and the acute phase response. Advances in Internal Medicine. 1992;37:313–336. [PubMed] [Google Scholar]
- Barker DJ. Mothers, Babies and Diseases in Later Life. BMJ Publishing Group; London: 1994. [Google Scholar]
- Black S, Kushner I, Samols D. C-reactive Protein. Journal of Biological Chemistry. 2004;279(47):48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- Cebu Study Team Underlying and proximate determinants of child health: The Cebu Longitudinal Health and Nutrition Study. Am J Epidemiol. 1991;133:185–201. [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. British Medical Journal. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yousfi M, Mercier S, Breuille D, Denis P, Papet I, Mirand PP, Obled C. The inflammatory response to vaccination is altered in the elderly. Mech Ageing Dev. 2005;126(8):874–81. doi: 10.1016/j.mad.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009–14. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am J Hum Bio. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation biomarkers and near-term death in older men. American Journal of Epidemiology. 2007;165(6):684–695. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- Kuo H-K, Bean JF, Yen C-J, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(4):380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Developmental origins of life history: growth, productivity, and reproduction. Am J Hum Biol. 2007;19(5):654–61. doi: 10.1002/ajhb.20659. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- McDade T, Beck MA, Kuzawa C, Adair L. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. American Journal of Clinical Nutrition. 2001;74(4):543–548. doi: 10.1093/ajcn/74.4.543. [DOI] [PubMed] [Google Scholar]
- McDade T, Rutherford JN, Adair L, Kuzawa C. Population differences in C-reactive protein concentration and associations with adiposity: Comparing young adults in the Philippines and the U.S. American Journal of Clinical Nutrition. 2009;89(4):1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Am J Phys Anthropol Suppl. 2003;37:100–25. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. American Journal of Human Biology. 2006;17:81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McDade TW, Leonard WR, Burhop J, Reyes-Garcia V, Vadez V, Huanca T, Godoy RA. Predictors of C-reactive protein in Tsimane’ 2-15 year-olds in lowland Bolivia. American Journal of Physical Anthropology. 2005;128:906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proceedings of the Royal Society B. 2010;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SE, Jalil F, Ashraf R, Szu SC, Prentice AM, Hanson LA. Birth weigth predicts response to vaccination in adults born in an urban slum in Lahore, Pakistan. American Journal of Clinical Nutrition. 2004;80:453–459. doi: 10.1093/ajcn/80.2.453. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17(5):527–58. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. others. [DOI] [PubMed] [Google Scholar]
- Posthouwer D, Voorbij HA, Grobbee DE, Numans ME, van der Bom JG. Influenza and pneumococcal vaccination as a model to assess C-reactive protein response to mild inflammation. Vaccine. 2004;23(3):362–5. doi: 10.1016/j.vaccine.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of American Medical Association. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-Year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus Antibody Levels, Inflammation, and Mortality Among Elderly Latinos Over 9 Years of Follow-up. Am J Epidemiol. 2010 doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GAW. Review series on helminths, immune modulation and they hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. xii. Oxford University Press; Oxford ; New York: 1992. p. 249. [Google Scholar]
- Tacket CO, Levine MM, Robbins JB. Persistence of antibody titers three years after vaccination with Vi polysaccharide vaccine against typhoid fever. Vaccine. 1988;6:307–308. doi: 10.1016/0264-410x(88)90175-2. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Hanson NQ, Straka RJ, Hoke TR, Ordovas JM, Peacock JM, Arends VL, Arnett DK. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med. 2005;145(6):323–7. doi: 10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Dremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]