Abstract
Acute necrotizing encephalopathy and acute disseminated encephalomyelitis are two rare types of acute post-infectious encephalopathy in children. Acute necrotizing encephalopathy is characterized by multiple symmetrical lesions in the thalami, putamen, cerebral and cerebellar white matter and brainstem. Acute disseminated encephalomyelitis is an immune-mediated demyelinating CNS disorder, which predominantly affects the white matter. Diffusion MRI is sensitive to measuring water diffusion in the central nervous system in human and animal models. Recent studies have demonstrated that by an analytical approach to directional diffusivity derived parameters, the axial diffusivity and the radial diffusivity, one can assess the extent of axonal or myelin injury in the CNS white matter. We applied directional diffusivity to acute necrotizing encephalopathy, acute disseminated encephalomyelitis and control cases correlating with neuropathology findings. In acute necrotizing encephalopathy, axonal injury without demyelination, noted on biopsied brain tissue, was suggested by a decreased apparent diffusion coefficient, unchanged fractional anisotropy and decreased axial and radial diffusivity. Whereas in acute disseminated encephalomyelitis, an increased apparent diffusion coefficient, decreased fractional anisotropy, unchanged axial diffusivity and markedly increased radial diffusivity compatible with active inflammatory demyelination, were noted consistent with tissue biopsy neuropathology. In conclusion, diffusion tensor parameters can potentially depict more microstructural changes than conventional MRI in post-infectious encephalopathy in children.
INTRODUCTION
Acute necrotizing encephalopathy of childhood is a rare type of fulminant encephalopathy in which we see multiple, symmetric brain lesions affecting the bilateral thalami, putamen, cerebral and cerebellar white matter and brainstem. Acute necrotizing encephalopathy affects infants and young children predominantly in Far East countries, rarely in North America and Europe. Acute disseminated encephalomyelitis is an immune-mediated inflammatory CNS disorder characterized by widespread demyelination in the cerebral white matter and spinal cord. It can occur at any age, but it is more common in pediatric patients than in adults. Both diseases are commonly preceded by a viral infection. Recently developed diffusion tensor imaging, which enables assessment of the magnitude and directionality of water diffusion in tissue, has shown promise not only to detect lesions in central nervous system disorders in human and animal models, but also allows for MRI specific analysis of the underlying brain architecture. Since diffusion tensor imaging is a physiologically specific imaging technique that is sensitive to myelination and axonal integrity, it is potentially more sensitive for the detection of abnormal white matter than conventional MR. Previous studies suggest a new approach, separating directional diffusivities derived from diffusion tensor imaging, to components describing water movement along (axial diffusivity, λ∥) and across (radial diffusivity, λ⊥) white matter tracts, can be used for assessing the extent of axonal or myelin injury in the CNS white matter of mice.[1] We applied diffusion tensor imaging to one child with acute necrotizing encephalopathy and one with acute disseminated encephalomyelitis and compared the directional diffusivity changes with that in control cases, with correlation to neuropathology.
Case Reports
Patient with acute necrotizing encephalopathy
Patient one is a ten year-old boy with a history of insulin dependent diabetes mellitus. He had diarrhea, vomiting and fever for twelve hours followed by unresponsiveness. He was found to have fixed dilated pupils and decerebrate posturing with intact brain stem reflexes at the time of admission. Patient's finger-stick glucose was 160–200 mg/dl initially and became 395 mg/dl after unconsciousness. He was initially treated with an insulin infusion for possible diabetic ketoacidosis. His arterial blood gas did not show acidosis, although he had trace ketones in his urine. He had normal complete blood count, liver function tests and ammonia. His electrolytes, including sodium level, on admission were completely normal. The cerebrospinal fluid showed no pleocytosis, negative oligoclonal bands, normal immunoglobulin G index, but protein was high at 55 mg/dl. The cerebrospinal fluid, serum and nasopharyngeal viral, including influenza virus and bacterial studies were non contributory. The inherited metabolic diseases work up was negative. A muscle biopsy and DNA testing for point mutations and deletions were negative for mitochondrial disease. His MRI done after two days of hospitalization showed an extensive abnormal hyperintense signal on T2 and fluid- attenuated inversion recovery within the central deep brain structures including the midbrain, bilateral basal ganglia, and thalami. The abnormal signal extended into the medial temporal lobes, medial parietal lobes and the inferior posterior margin of the frontal lobes bilaterally. These areas demonstrated diffusion restriction with decreased signal on apparent diffusion coefficient sequence. (Figure 1.) Accordingly, the radiological features are compatible with the diagnosis of acute necrotizing encephalopathy. The fixed and dilated pupils may be related to the infarction of the mid brain affecting oculomotor nucleus or medial temporal lobes with uncal inflammation causing compression on the 3rd nerves. Ceftriaxone, Vancomycin and Acyclovir were added later for a possible infectious etiology. He was treated with intravenous steroids, followed by intravenous immunoglobulin and plasmapheresis. The patient never recovered from his neurological status and survived in a persistent vegetative state.
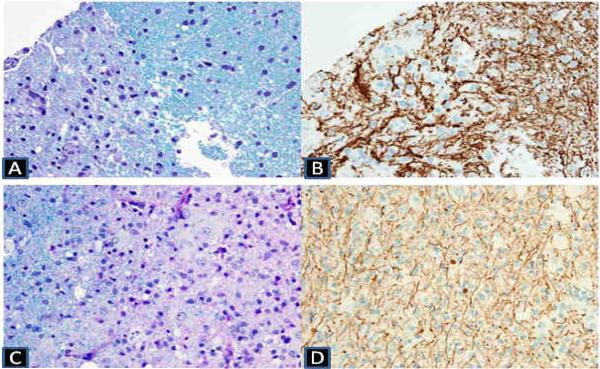
Figure 1.
A–B (upper row) Luxol fast blue / periodic acid Schiff stain shows myelin pallor and immunohistochemical neurofilament stain on the upper row shows evidence of damage and spheroid formation of the axons in the infracted area of the ANE patient.
C–D (lower row) Luxol fast blue / periodic acid-Schiff stain (lower left) shows myelin loss in the region of white matter pallor and Immunohistochemical neurofilament stain shows relative preservation of axons compared to myelin loss with scattered axonal swellings in the ADEM patient.
Patient with acute disseminated encephalomyelitis
Patient two is a seventeen-year-old girl with no significant past history who, about five weeks prior to admission, was noted to have a gradually progressive dysconjugate gaze, worsening confusion, dysarthria, and gait instability. On the day following admission, she was not able to follow commands, was combative and not oriented to person, time and place. She also had several brief generalized tonic clonic seizures. She had very limited expressive speech, had mild right hemiparesis, was ataxic and hyperreflexic. She deteriorated quickly during the hospitalization and became unresponsive and hypertonic. Her MRI study demonstrated numerous T1 hypointensity and T2 hyperintensity lesions scattered throughout the periventricular and subcortical white matter of both hemispheres, the corpus callosum, the cerebral peduncles, the midbrain and the pons. Various enhancements were demonstrated on some lesions. These widespread bilateral white matter lesions were most consistent with an acute disseminated encephalomyelitis (Figure 1) A cerebrospinal fluid exam was notable for a normal glucose and protein level, slightly elevated nucleated cell count (9 cells/mcl), an elevated level of myelin basic protein (29.6 ng/ml with normal being less than 1.4 ng/ml) and five oligoclonal bands (0 in serum). After high dose steroids, plasmapheresis and intravenous immunoglobulin treatment, the patient's motor function gradually recovered. She was subsequently transferred to neurorehabilitation. On follow up MRI at 2 years, her previous T1 hypointese lesions disappeared, but T2 hyperintese lesions persisted. No new lesions were noted on follow up scans. She recovered with mild right hemiparesis without other motor deficit, but had significant deficits in concentration, attention, executive functions and memory.
There is no known history of autoimmune diseases, acute necrotizing encephalopathy, or other parainfectious or demyelinating diseases in the family in both cases.
Control cases
Eight age-matched control subjects: 5 for acute necrotizing encephalopathy patients and 3 for acute disseminated encephalomyelitis patients; were selected retrospectively from headache patients whose image data was normal with low suspicion for cerebral white matter disease. The use of diffusion tensor imaging data has been approved by the Washington University medical school institutional review board.
Neuropathology
In the acute necrotizing encephalopathy patient, brain biopsy was done in the right parietal area on the tenth day from clinical onset. Histological sections of the brain biopsy material consisting of neocortex and white matter showed multiple foci of infarction, as evidenced by bland necrosis, vascular proliferation, macrophage infiltration/proliferation, and eosinophilic neuronal necrosis. There was no evidence of significant lymphocytic infiltrates, infectious organisms, or vasculitis. Sections treated histochemically with Luxol fast blue/periodic acid Schiff stain showed no evidence of a demyelinating process. Immunohistochemically stained sections showed reactivity for neurofilament protein that highlighted axonal processes. In areas of infarction, the axons showed evidence of damage and spheroid formation and were focally splayed by infiltrating macrophages (Fig 1.).
In the acute disseminated encephalomyelitis patient, a brain biopsy was performed in the right temporal white matter showing numerous foamy macrophages and reactive astrocytes. The vessels showed perivascular inflammation with lymphocytes and macrophages. Luxol fast blue/periodic acid Schiff staining showed nearly complete loss of myelin. Immunohistochemical stains were performed: CD68 highlighted abundant parenchymal and perivascular macrophages, as well as activated microglia in the adjacent cortex; CD20 highlighted a few perivascular B lymphocytes; CD3 showed scattered perivascular and parenchymal T lymphocytes, and; stains for neurofilament highlighted relative preservation of axonal processes, although axonal spheroids are also present, consistent with some axonopathy (Fig 1.).
Image Acquisition and Data Analysis
Imaging was performed on a 3 Tesla Siemens TIM Trio (Erlangen, Germany) in St. Louis Children's Hospital with quantum gradients using 8 channel head coils. Routine MRI examination includes T1weighted images and/or magnetization prepared rapid gradient echo, T2Weighted imaging, apparent diffusion coefficient, diffusion weighted imaging, and diffusion tensor imaging. A single shot spin-echo echo-planar imaging was used for diffusion tensor imaging acquisition with the following parameters: 60 slices without gaps, field of view = 190 mm, phase field of view = 100 %, thickness = 2 mm, base resolution = 96, phase resolution = 100 (2 × 2 × 2 mm3 voxels), phase partial Fourier = 6/8, repetition time (TR) = 9900 msec, echo time (TE) = 102 msec, average = 1, b-value = 1400 sec/mm2, directions = 25, bandwidth = 1080 Hz, echo planner imaging factor = 96, echo spacing = 1 msec.
Image processing was performed with the previous published method.[2] Multiple parameters derived from the diffusion tensor: the apparent diffusion coefficient; the fractional anisotropy; the axial diffusivity (λ∥= λ1), and; the radial diffusivity (λ⊥=λ2+λ3/2) were calculated off-line (Fig 2). Image J was used to draw regions of interest on the biopsy sites of the two cases. The region of interest maps were then applied to the same regions of age-matched control cases. Student t-tests were used for computing the statistical significance of differences in apparent diffusion coefficient, fractional anisotropy, the axial diffusivity and the radial diffusivity between patients and control cases.
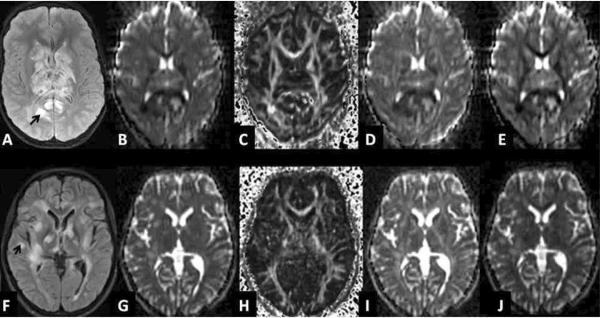
Figure 2.
A–E (upper row) shows representative MR images from the acute necrotizing encephalopathy case: A fluid attenuation inversion recovery, B apparent diffusion coefficient, C fractional anisotropy, D axial diffusivity, E radial diffusivity. F–J (lower row) shows representative MR images from the acute disseminated encephalomyelitis case: F fluid attenuation inversion recovery, G apparent diffusion coefficient, H fractional anisotropy, I axial diffusivity, J radial diffusivity.
Arrows indicate the biopsied sites.
Results
The difference in water diffusion, in acute necrotizing encephalopathy and acute disseminated encephalomyelitis, was seen by apparent diffusion coefficient measures. Water apparent diffusion parallels to fiber tracts (axial diffusivity) - indicative of axonal damage, orthogonal to them (radial diffusivity) - indicative of demyelination, and fractional anostropy- a normalized expression of the difference in axial and radial diffusivities, were measured in both cases and compared with the controls.
In the acute necrotizing encephalopathy case, apparent diffusion coefficient decreased by 71% (p<0.01), the relatively unchanged fractional anisotropy (p=0.30) resulting from axial diffusivity decreased by 72% (p<0.01) and radial diffusivity decreased by 70% (p<0.01) in the right parietal white matter. In the acute disseminated encephalomyelitis case, apparent diffusion coefficient increased by 30% (p<0.01), fractional anisotropy decreased by 63% (p<0.01), axial diffusivity was relatively unchanged (p=0.03) and radial diffusivity increased markedly by 59% ( p<0.01) in the right temporal white matter (Table 1).
Table 1.
This table shows the t-test results of MR diffusion parameters including ADC, FA, axial diffusivity (λ∥), and radial diffusivity (λ⊥) in comparison between ANE/ADEM cases and control cases.
| ANE | ADEM | |||||
|---|---|---|---|---|---|---|
| Patient (n=1) | Control (n=5) | P | Patient (n=1) | Control (n=3) | p | |
| ADC | 0.23 ± 0.06 | 0.78 ± 0.07 | <0.01 | 0.99 ± 0.14 | 0.76 ± 0.10 | <0.01 |
| FA | 0.51 ± 0.13 | 0.50 ± 0.09 | 0.30 | 0.16 ± 0.05 | 0.43 ± 0.13 | <0.01 |
| λ ∥ | 0.35 ± 0.09 | 1.24 ± 0.16 | <0.01 | 1.17 ± 0.19 | 1.15 ± 0.21 | 0.03 |
| λ ⊥ | 0.17 ±0.05 | 0.56 ± 0.09 | <0.01 | 0.91 ± 0.13 | 0.57 ± 0.11 | <0.01 |
DISCUSSION
To our knowledge, this study is the first application of diffusion tensor imaging directional diffusivity in pediatric acute necrotizing encephalopathy and acute disseminated encephalomyelitis patients correlated with tissue pathology findings. Diffusion tensor imaging has been established as an important diagnostic tool in pediatric CNS diseases. [3] Unlike conventional MRI, diffusion tensor imaging provides a unique method of better understanding pathophysiology of microstructural tissue in specific diseases. Recent studies have demonstrated that separating directional diffusivities derived from diffusion tensor imaging to components describing water movement along (axial diffusivity; λ∥= λ1) and across (radial diffusivity; λ⊥=λ2+λ3/2) white matter tracts, can be used for assessing the extent of axonal or myelin injury in the CNS white matter of Shiverer, spinal cord injury, and experimental autoimmne encephalomyelitis mice.[1,4,5] Histological verification has been correlated well with in vivo diffusion tensor imaging measurements.[1] Results from these studies support the hypothesis that decreased axial (λ∥) diffusivity and increased radial (λ⊥) diffusivity can be used as biomarkers of axonal damage and demyelination in mice.[4]
Acute necrotizing encephalopathy, first established as a novel disease in 1995 by Mizuguchi et al, is characterized by acute encephalopathy accompanied by a viral febrile disease, rapid disturbance of consciousness, seizures, elevation of cerebrospinal fluid protein without pleocytosis. Neuroimaging features, on CT or MRI, are multiple, symmetric lesions in thalami, putamen, cerebellum, brainstem tegmentum, and periventricular white matter.[6] The etiology and pathogenesis of acute necrotizing encephalopathy remain unclear. Familial and recurrent cases of acute necrotizing encephalopathy have been described in family members with positive mutation in Ran-binding protein 2 (RANBP2). [7] That raises the suspicion of genetic susceptibility to acute necrotizing encephalopathy, although our patient does not have a family history. It is interesting to note that our patient has insulin dependent diabetes mellitus, which is an autoimmune disorder. Although the direct relationship between acute necrotizing encephalopathy and autoimmune diseases has not been described as such, acute necrotizing encephalopathy has been noted to occur after viral illness, particularly post influenza A infection. Para infectious/ post viral illness such as acute necrotizing encephalopathy may have some association with autoimmune diseases. We also considered the possibility of sodium shifts related to diabetes causing osmotic myelinolysis affecting the basal ganglion. However, our patient maintained normal sodium level during the earlier days of his illness; therefore sodium shift is not responsible for his symptoms or MRI findings.
The pathology findings from literature of acute necrotizing encephalopathy reveal diffuse cerebral edema, perivascular hemorrhage, and necrosis. Our first case fulfilled the clinicopathological diagnosis as acute necrotizing encephalopathy. In our study, the diffusion tensor imaging showed a significantly reduced apparent diffusion coefficient, as well as axial and radial diffusivity. A decreased apparent diffusion coefficient is commonly seen in many acute disease processes such as acute ischemic stroke. It could be caused by cytotoxic edema from migration of extracellular water into intracellular space, an increase in extracelluar space tortuosity, a decrease in energy dependent intracellular circulation and an increase in water viscocity.[8] Simultaneously decreasing axial and radial diffusivity may reflect acute axonal injury. Relatively unchanged fractional anisotropy is due to simultaneous decreased axial and radial diffusivity. Those diffusion tensor imaging findings are consistent with histological findings of infarcted tissue with axonal damage without demyelination in this patient with acute necrotizing encephalopathy.
Acute disseminated encephalomyelitis is an acute inflammatory demyelinating, usually monophasic, CNS disorder. It is believed to be an autoimmune reaction to myelin which is triggered by a viral infection or vaccination. Clinical symptoms of acute disseminated encephalomyelitis patients include headache, fever, vomiting, and drowsiness. The International Pediatric Multiple Sclerosis Study Group proposes that acute disseminated encephalomyelitis must be multifocal, polysymptomatic and include encephalopathy (as an essential requirement).[9] Histological features include diffuse infiltration of T cells and macrophages associated with demyelination mainly in the white matter. Conventional MR abnormalities are typically large, multiple and asymmetric lesions on T2-weighted and fluid attenuation inversion recovery imaging which involve the subcortical and central white matter of cerebral hemispheres, cerebellum, brainstem, and spinal cord. Balasubramanya et al. used diffusion weighted images to study acute disseminated encephalomyelitis patients and found restricted diffusion (reduced apparent diffusion coefficient) in the acute stage and free diffusion (elevated apparent diffusion coefficient) in the subacute stage.[10] In our case, the diffusion tensor imaging findings in the subacute stage showed a significantly elevated apparent diffusion coefficient and reduced fractional anisotropy. The further directional diffusivity study revealed relatively unchanged axial diffusivity, markedly increased radial diffusivity suggestive of demyelination as seen in the previous animal studies in our laboratory. [5] These findings are also consistent with active inflammatory demyelination with mild axonal damage showed in the neuropathology.
In conclusion, the diffusion tensor imaging findings of affected white matter of acute necrotizing encephalopathy and acute disseminated encephalomyelitis cases are consistent with the neuropathological findings. Diffusion tensor imaging parameters can depict more microstructure change than conventional MRI in postinfectious encephalopathy of children. Directional diffusivity may potentially be useful as an MR biomarker for diagnosis and prognosis among different post-infectious encephalopathy.
Acknowledgements
This work was supported by the American Roentgen Ray Scholar Award, The American Society for Neuroradiology Scholar Award, NMSS PP1361, RG 3670A3/2, and RG 4009-A-13 NIH/NINDS 1P01NS059560-01A1.
Footnotes
Conflicts of interest: TB is a consultant for Biomedical Systems, Inc. She has participated in the Siemens Speakers Bureau and has received manuscript royalties from MRUpdate. No conflict is identified for the work presented in this manuscript. There is no contribution of industry-sponsored research or of corporate participation in preparing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–95. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- [2].Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rollins NK. Clinical application of diffusion tensor imaging and tractography in children. Pediatr Radiol. 2007;37:769–780. doi: 10.1007/s00247-007-0524-z. [DOI] [PubMed] [Google Scholar]
- [4].Kim JH, Loy DN, Liang HF, Trinkaus K, Schmidt RE, Song SK. Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn Reson Med. 2007;58:253–60. doi: 10.1002/mrm.21316. [DOI] [PubMed] [Google Scholar]
- [5].Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- [6].Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- [7].Neilson DE, Adams MD, Orr CM, Schelling DK, Eiben RM, Kerr DS. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet. 2009;84:44–51. doi: 10.1016/j.ajhg.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury - a review. NMR Biomed. 2002;15:561–9. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- [9].Tenembaum S, Chitnis T, Ness J, Hahn JS. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–26. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- [10].Balasubramanya KS, Kovor JME, Jayakumar PN, Ravishankar S, Kamble RB, Panicker J, Nagaraja D. Diffusion-weighted imaging and proton MR spectroscopy in the characterization of acute disseminated encephalomyelitis. Neuroradiology. 2007;49:177–183. doi: 10.1007/s00234-006-0164-2. [DOI] [PubMed] [Google Scholar]