Abstract
Tetragonisca angustula and Tetragonisca fiebrigi have recently been listed as valid species. This study aimed to cytogenetically investigate both species, emphasizing the new registry of B chromosomes in the tribe Meliponini. We analyzed colonies of T. angustula and T. fiebrigi collected at Tangará da Serra, Mato Grosso, Brazil, through conventional Giemsa staining, C-banding, and base-specific fluorochrome staining (CMA3/DAPI). T. angustula showed 2n = 34 chromosomes in females and n = 17 in males, with karyotype formula 2K = 34AM. T. fiebrigi showed numeric variation, with chromosome number varying from 2n = 34 to 2n = 36 in females and from n = 17 to n = 18 in males, with karyotype formula 2K = 32AM+2AMc and 2K = 32AM+2AMc + 1 or 2 B-chromosomes. The B chromosomes are heterochromatic. In T. fiebrigi, the CMA3/DAPI staining revealed four chromosomes with a CMA3 positive band. All individuals from the same colony showed the same number of B chromosomes. T. angustula and T. fiebrigi showed karyotype divergence, principally due to the presence of B chromosomes, which are found only in T. fiebrigi. Our data corroborate the status of valid species for both T. angustula and T. fiebrigi, as recently proposed.
Keywords: stingless bees, cytogenetics, interspecific differentiation, B chromosomes
Until recently, the genus Tetragonisca comprised two species, Tetragonisca weyrauchi and Tetragonisca angustula, the latter with two subspecies: Tetragonisca angustula angustula and Tetragonisca angustula fiebrigi. Moure et al. (2007) considered both subspecies as distinct species (Tetragonisca angustula and Tetragonisca fiebrigi), based on their morphological characteristics and sympatric distribution.
Tetragonisca angustula (Latreille, 1811) has a wide geographical distribution in the Americas, from Southern Mexico to the southernmost state of Rio Grande do Sul in Brazil (Schwarz, 1938; Moure et al., 2007). This species, characterized by the black mesopleura, has been reported in all regions of Brazil (Nogueira-Neto, 1970; Moure et al., 2007). Tetragonisca fiebrigi (Schwarz, 1938) differs morphologically from T. angustula in the ferruginous coloration of the mesopleura and lateral areas of the propodeum. This species ranges from the state of Rio Grande do Sul to the state of Mato Grosso, according to Schwarz (1938). But this distribution range has expanded to the northwestern border with Uruguay (Schwarz, 1938) and to the state of Goiás (Oliveira et al., 2004).
The validity of infraspecific taxa has been particularly debated over the last 50 years, with statements of some authors favorable to it (Wilson and Brown, 1953; Smith et al., 1997; Isaac et al., 2004) and others against it (Mayr 1982; Burbrink et al., 2000; Ward, 2007). The need to use non-morphological characters for the study of subspecies was raised by Mayr (1982), referring to ecological and behavioral characteristics. On the other hand, the ambiguity of some morphological characters in species recognition, such as color pattern, has been demonstrated with molecular data (Burbrink et al., 2000).
The occurrence of B chromosomes is regarded as one of the sources of numerical variation in the karyotype of some species. B chromosomes are rare in Meliponini bees, and have been reported to date in Partamona helleri (Costa et al., 1992; Brito et al. 1997; Tosta et al., 2004; Martins et al., 2009) and Melipona quinquefasciata (Marla P. Rocha, personal communication).
This study was aimed at obtaining new cytogenetic information for Tetragonisca angustula and Tetragonisca fiebrigi, with emphasis on the new occurrence of B chromosomes in the tribe Meliponini.
We analyzed 20 individuals per colony, in 11 colonies of Tetragonisca fiebrigi and three colonies of Tetragonisca angustula, collected in Tangará da Serra, in the state of Mato Grosso, Brazil (14°04′ S, 57°03′ W).
Metaphasic chromosomes were obtained from cerebral ganglia of prepupae following the method described by Imai et al. (1988). The slides were then submitted to conventional Giemsa staining, C-banding (Pompolo and Takahashi, 1990), and base-specific fluorochrome staining (CMA3/DAPI) (Schweizer, 1976). The slides were analyzed and the best metaphases were photographed using an Olympus photomicroscope. Karyotypes were arranged in decreasing order of length of euchromatic arms. Chromosome nomenclature followed Imai (1991), with pseudoacrocentric chromosomes showing an extraordinarily elongated heterochromatic short arm (AM), acrocentric with totally heterochromatic arms (Ah), and pseudoacrocentric with pericentromeric heterochromatin (AMc).
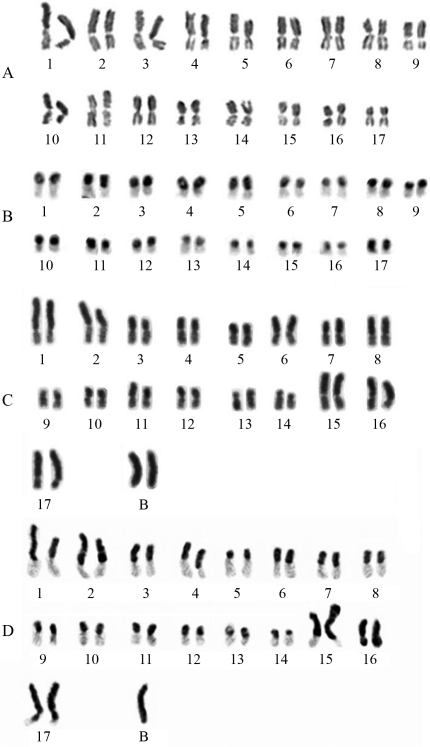
We found chromosome numbers 2n = 34 in females and n = 17 in males of Tetragonisca angustula species. C-banding revealed heterochromatin in one arm of all chromosomes, and karyotypic formula 2K = 34AM (Figure 1 A, B). CMA3/DAPI showed no evident differential staining in chromosomes of this species (Figure 2 A, B).
Figure 1.
Female karyograms. A) T. angustula submitted to conventional Giemsa staining. B) T. angustula submitted to C-banding. C) T. fiebrigi submitted to Giemsa staining showing two B-chromosomes. D) T. fiebrigi submitted to C-band staining showing one B chromosome.
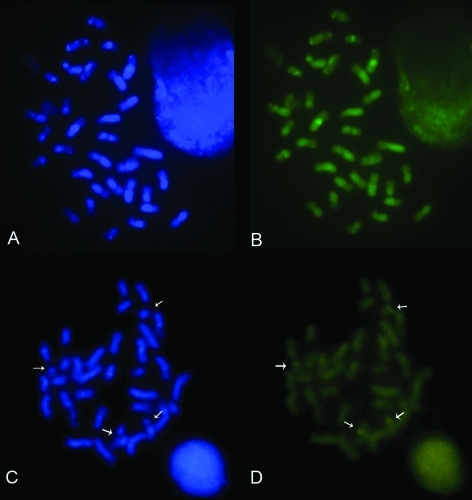
Figure 2.
Female metaphases of A and B) T. angustula submitted to DAPI and CMA3 staining, respectively, and C and D) T. fiebrigi submitted to DAPI and CMA3 staining, respectively. Arrows in T. fiebrigi metaphases indicate positive CMA3 bands.
Tetragonisca fiebrigi showed variation in diploid chromosome number with 2n = 34 (one colony), 2n = 35 (eight colonies), and 2n = 36 (two colonies) (Figure 1 C, D). In three of the colonies in which we found females with 2n = 35, we also found males with n = 18.
The differences in diploid number observed in these colonies (2n = 34 to 2n = 36) were due to the presence of up to two B chromosomes per individual. The length of the B chromosome (about 5 μm) was similar to that of the regular complement, and was observed in all individuals of the same colony.
C-banding revealed that most chromosomes of T. fiebrigi were pseudoacrocentric with karyotype formulae 2K = 32AM+2AMc, 2K = 32AM+2AMc+1B, and 2K = 32AM+2AMc+2B. B chromosomes were entirely heterochromatic – Ah (Figure 1D). Four CMA3 positive bands were also observed in two chromosome pairs, one in the interstitial region, and one in the terminal region (Figure 2 C, D).
Although B chromosomes are frequently found in natural populations of animals and plants, they have been poorly studied in Hymenoptera. In the stingless bee Partamona helleri, B chromosomes have been reported to vary from none to four minute chromosomes per specimen (Costa et al., 1992; Brito et al., 1997; Tosta et al., 2004). Recently, Martins et al. (2009) described the occurrence of a new B chromosome in this species, larger in size than those previously reported. In Melipona quinquefasciata, up to four B chromosomes have been observed (Marla P. Rocha, personal communication).
In the ant Camponotus sp, the occurrence of up to three small B chromosomes has been reported (Mariano et al., 2001). In the wasp Nasonia vitripennis, a small submetacentric and heterochromatic B chromosome called “psr chromosome” was reported to be involved in the elimination of the paternal chromosome set in fertilized eggs, converting them into haploid eggs (Nur et al., 1988). The wasp Trypoxylon albitarse showed a constant distribution pattern of B chromosomes among individuals, with one and two B chromosomes in males and females, respectively (Rocha-Sanchez and Pompolo, 2004). In this case, the authors suggested that the B chromosome behaves as a chromosome of the regular complement during meiosis, undergoing balanced segregation at the end of cell division. A similar mechanism could explain the constant distribution of B chromosomes in T. fiebrigi.
In the colonies of T. angustula and T. fiebrigi we studied, karyotypes were distinct and conserved within each species, and no hybrid karyotypes were detected. This differentiation suggests reproductive isolation, which is reinforced by the presence of B chromosomes and specific CMA3 bands only in T. fiebrigi species, allowing the use of these features as cytological markers to karyotipically distinguish the two species.
Our cytogenetic data corroborate the specific status of Tetragonisca angustula and Tetragonisca fiebrigi proposed by Moure et al. (2007). The numerical constancy of colonies with B chromosomes, however, demands more investigation to elucidate if the predominance of B chromosomes in the progeny of T. fiebrigi has been favored by events such as meiotic drive.
Acknowledgments
We thank UNEMAT, FAPEMAT, and CNPq for technological and financial support and Dr. Favísia de Oliveira Freitas for the identification of the specimens.
Footnotes
Associate Editor: Yatiyo Yonenaga-Yassuda
References
- Brito RM, Costa MA, Pompolo SG. Characterization and distribution of supernumerary chromosomes in 23 colonies of Partamona helleri (Hymenoptera, Apidae, Meliponinae) Rev Bras Genet. 1997;20:185–188. [Google Scholar]
- Burbrink FT, Lawson R, Slowinski JB. Mitochondrial DNA phylogeography of the polytypic North American rat snake (Elaphe obsoleta): A critique of the subspecies concept. Evolution. 2000;54:2107–2118. doi: 10.1554/0014-3820(2000)054[2107:MDPOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Costa MA, Pompolo SG, Campos LAO. Supernumerary chromosomes in Partamona cupira (Hymenoptera, Apidae, Meliponinae) Rev Bras Genet. 1992;15:801–806. [Google Scholar]
- Imai HT, Taylor RW, Crozier RH. Modes of spontaneous chromosomal mutation and karyotypic evolution in ants with reference to the minimun interacion hypothesis. Jpn J Genet. 1988;63:159–185. doi: 10.1266/jjg.63.159. [DOI] [PubMed] [Google Scholar]
- Imai HT. Mutability of constitutive heterochromatin (c-bands) during eukaryotic chromosomal evolution and their cytological meaning. Jpn J Genet. 1991;66:635–66. doi: 10.1266/jjg.66.635. [DOI] [PubMed] [Google Scholar]
- Isaac NJB, Mallet J, Mace GM. Taxonomic inflation: Its influence on macroecology and conservation. Trends Ecol Evol. 2004;19:464–470. doi: 10.1016/j.tree.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Mariano CFS, Pompolo SG, Delabie JHC, Campos LAO. Estudos cariotípicos de algumas espécies neotropicais de Camponotus Mayr (Hymenoptera, Formicidae) Rev Bras Entomol. 2001;45:267–274. [Google Scholar]
- Martins CCC, Duarte OMP, Waldschmidt AM, Alves RMO, Costa MA. New occurrence of B chromosomes in Partamona helleri (Friese, 1900) (Hymenoptera, Meliponini) Genet Mol Biol. 2009;32:782–785. doi: 10.1590/S1415-47572009005000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E. Of what use are subspecies? Auk. 1982;99:593–595. [Google Scholar]
- Moure JS, Urban D, Melo GAR. Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region. Sociedade Brasileira de Entomologia, Curitiba. 2007. p. 1058.
- Nogueira-Neto P. A Criação de Abelhas Indígenas Sem Ferrão. 2nd edition. 1970. p. 365. Tecnapis, São Paulo.
- Nur U, Werren JH, Eickbush D, Burke W, Eickbush T. A “selfish” B chromosome that enhances its transmission by eliminating the paternal chromosomes. Science. 1988;240:512–514. doi: 10.1126/science.3358129. [DOI] [PubMed] [Google Scholar]
- Oliveira RC, Nunes FMF, Campos APS, Vasconcelos SM, Roubik D, Goulart LR, Kerr WE. Genetic divergence in Tetragonisca angustula Latreille, 1811 (Hymenoptera, Meliponinae, Trigonini) based on RAPD markers. Genet Mol Biol. 2004;27:181–186. [Google Scholar]
- Pompolo SG, Takahashi CS. Chromosome numbers and C banding in two species of the genus Polistes (Hymenoptera, Polistinae, Polistinii) Insectes Soc. 1990;37:251–257. [Google Scholar]
- Rocha-Sanchez SMS, Pompolo SG. Imitate to integrate: Reviewing the pathway for B chromosome integration in Trypoxylon (Trypargilum) albitarse (Hymenoptera, Sphecidae) Cytogenet Genome Res. 2004;106:398–401. doi: 10.1159/000079318. [DOI] [PubMed] [Google Scholar]
- Schwarz HF. The stingless bees (Meliponidae) of British Guianaand some related forms. Bull Am Mus Nat Hist. 1938;74:437–508. [Google Scholar]
- Schweizer D. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 1976;58:307–324. doi: 10.1007/BF00292840. [DOI] [PubMed] [Google Scholar]
- Smith H, Chiszar D, Montanucci R. Subspecies and classification. Herpetol Rev. 1997;28:13–16. [Google Scholar]
- Tosta VC, Fernandes-Salomão M, Tavares MG, Pompolo SG, Barros RG, Campos LAO. A RAPD marker associated with B chromosome in Partamona helleri (Hymenoptera, Apidae) Cytogenet Genome Res. 2004;106:279–283. doi: 10.1159/000079299. [DOI] [PubMed] [Google Scholar]
- Ward PS. Edward O. Wilson and his contributions to ant systematics. In: Snelling RR, Fisher BL, Ward PS, editors. Advances in Ant Systematics (Hymenoptera, Formicidae): Homage to E O Wilson - 50 Years of Contributions. Memoirs of the American Entomological Institute; Gainesville: 2007. pp. 3–7. [Google Scholar]
- Wilson EO, Brown WL., Jr The subspecies concept and its taxonomic application. Syst Zool. 1953;2:97–111. [Google Scholar]