Abstract
Conquering cardiovascular diseases is one of the most important problems in human health. To overcome cardiovascular diseases, animal models have played important roles. Although the prevalence of genetically modified animals, particularly mice and rats, has contributed greatly to biomedical research, not all human diseases can be investigated in this way. In the study of cardiovascular diseases, mice and rats are inappropriate because of marked differences in lipoprotein metabolism, pathophysiological findings of atherosclerosis, and cardiac function. On the other hand, since lipoprotein metabolism and atherosclerotic lesions in rabbits closely resemble those in humans, several useful animal models for these diseases have been developed in rabbits. One of the most famous of these is the Watanabe heritable hyperlipidemic (WHHL) rabbit, which develops hypercholesterolemia and atherosclerosis spontaneously due to genetic and functional deficiencies of the low-density lipoprotein (LDL) receptor. The WHHL rabbit has been improved to develop myocardial infarction, and the new strain was designated the myocardial infarction-prone WHHL (WHHLMI) rabbit. This review summarizes the importance of selecting animal species for translational research in biomedical science, the development of WHHL and WHHLMI rabbits, their application to the development of hypocholesterolemic and/or antiatherosclerotic drugs, and future prospects regarding WHHL and WHHLMI rabbits.
1. Introduction
According to WHO, the major cause of death within member nations is cardiovascular diseases which account for about 30% of all deaths [1]. This report has indicated that cardiovascular diseases are one of the most important classes of diseases to be overcome. As main risk factors for cardiovascular diseases, hypercholesterolemia, hypertension, disorders in glucose metabolism, smoking, aging, male gender, and social stress are listed. Particularly, control of serum lipid levels is thought to be most important for the prevention of cardiovascular diseases. Currently, in the Japanese population, the upper limits of the normal ranges for serum total cholesterol and LDL cholesterol levels are 220 mg/dL and 140 mg/dL, respectively, and the lower limit of the normal range of HDL cholesterol is defined as 40 mg/dL [2]. According to studies conducted during the 1980s, the incidence of cardiovascular events increases as the serum cholesterol level increases and decreases with hypocholesterolemic treatments [3]. One potent hypocholesterolemic compound is statin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, a rate-limiting enzyme in cholesterol synthesis. The first statin (compactin) was initially developed by a Japanese pharmaceutical company, Sankyo Co. Ltd. [4], and this accelerated the development of cholesterol lowering drugs. The hypocholesterolemic effect of compactin was initially examined with rats. However, the anticipated cholesterol-lowering effect was not observed [5], and the development of this compound was ceased. On the other hand, since compactin showed a potent inhibitory effect on cholesterol synthesis in vitro and in chickens, researchers had been looking for other mammalian species applicable for the assessment of this agent. They found a report of a mutant rabbit strain showing hyperlipidemia, written in a Japanese university's bulletin [6]. This rabbit strain contributed greatly to the development of this compound. The strain was the Watanabe heritable hyperlipidemic (WHHL) rabbit. This was in 1979. Currently, there are seven statins in widespread clinical use. It is estimated that statins are prescribed to more than 40 million patients worldwide and statin therapy has decreased mortality from cardiovascular diseases by 20–50% [7]. Thus statins became essential agents for the treatment of hypercholesterolemia and cardiovascular diseases. These results demonstrate the importance of selecting animal species and/or animal models for translational research to develop therapeutic agents.
This review raises the importance of selecting animal species and/or animal models for translational research by describing the history of the WHHL rabbit and its contribution to studies of hypercholesterolemia and atherosclerosis.
2. The Development of the WHHL Rabbit and Its Characteristics
The history and characteristics of the WHHL rabbit were described in a previous article [8]. In 1973, Dr. Yoshio Watanabe (1927–2008) found one male Japanese white rabbit showing hyperlipidemia. From this mutant, he established a strain, the WHHL rabbit, after seven years of selective breeding. At first, this strain was designated the hyperlipidemic rabbit (HLR) [9]. He submitted a study on this strain to an international journal and renamed it the Watanabe heritable hyperlipidemic (WHHL) rabbit [10], according to a suggestion by the editor.
The strain has 300–700 mg/dL of total cholesterol and 300–400 mg/dL of triglyceride in plasma. There were atherosclerotic lesions in the aorta and xanthoma in the digital joints. The serum glucose level and blood pressure were in normal ranges. In WHHL rabbits, the function of low-density lipoprotein (LDL) receptors on the cell membrane was almost deficient and the clearance of LDL from the circulation delayed [11]. Such symptoms closely resemble human familial hypercholesterolemia (FH), which develops spontaneously, and thus the WHHL rabbit is recognized as the first animal model of this disease. Later, the Nobel Prize winners Goldstein and Brown used WHHL rabbits to verify their hypothesis of an LDL receptor pathway for the metabolism of lipoproteins and clarified human lipoprotein metabolism [12–15]. Their studies revealed that lipoprotein metabolism in the WHHL rabbit closely resembles human FH. Consequently, WHHL rabbits were used as an animal model for the development of cholesterol-lowering agents.
One of the most important features of an animal model for hyperlipidemia is the occurrence of myocardial infarction, the final event of human hypercholesterolemia. The development of severe atherosclerotic lesions in the coronary arteries is a prerequisite for the occurrence of myocardial infarction, but the incidence of coronary atherosclerosis in the WHHL rabbit was initially very low. To establish a new strain which develops coronary atherosclerosis, serial selective breeding was conducted and in 1985, the coronary atherosclerosis-prone WHHL rabbit was developed [16]. Further, a strain with severe coronary atherosclerosis was developed in 1992 [17]. Despite such long-term efforts, the incidence of myocardial infarction remained very low. After a further seven years of selective breeding with improved criteria, such as the use of descendents of rabbits with macrophage-rich coronary lesions, a new strain of WHHL rabbits was established; the myocardial infarction-prone WHHL (WHHLMI) rabbit that spontaneously develops myocardial infarction by progression of coronary atherosclerosis followed by occlusion of the coronary arteries [18]. The characteristics of WHHLMI rabbits are described in a previous review [19]. During their establishment, marked differences in the composition of atherosclerotic plaques were found between the aorta and coronary arteries [20], and the WHHLMI rabbit became an animal model with which to examine the inhibitory effects of drugs on coronary atherosclerosis. These studies suggested genetic factors other than hypercholesterolemia to be important to myocardial infarction and coronary atherosclerosis.
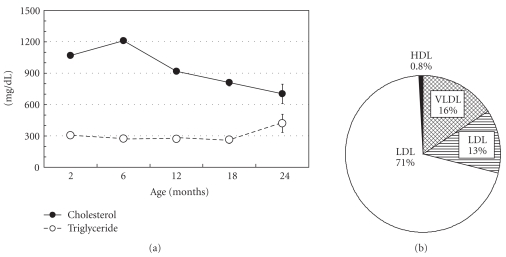
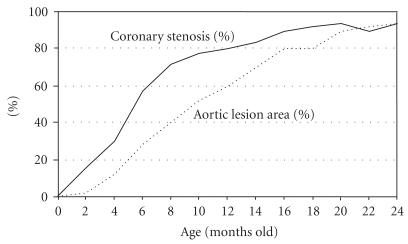
Figure 1 shows the changes in serum lipid levels with aging and the distribution of cholesterol in lipoproteins among WHHLMI rabbits [8]. Serum cholesterol levels are 900–1,400 mg/dL at weaning (3 months old) and at 6 months old, and then decrease gradually (700–1,200 mg/dL at 12 months old, 600–1,100 mg/dL at 18 months old, and 500–1,000 mg/dL at 24 months old). Serum triglyceride levels are 150–500 mg/dL and the change with aging is small. The HMG Co-A reductase activity (cholesterol biosynthesis) in WHHLMI rabbits does not decrease with aging and the precise mechanism of the age-related decrease in cholesterol is still unknown [21]. About 70% of cholesterol occurs in the LDL fraction, 16% in the very low-density lipoprotein (VLDL) fraction, 13% in the intermediate density lipoprotein (IDL) fraction, and 0.8% in the high density lipoprotein (HDL) fraction. Figure 2 shows the extent of atherosclerotic lesions in the coronary arteries and aorta of WHHLMI rabbits [8]. The main coronary artery is the left circumflex artery and the atherosclerotic lesion is more progressed compared to that in the left anterior descending artery and the right coronary artery. Therefore, the degree of coronary atherosclerosis (cross-sectional narrowing) has been evaluated using the left circumflex artery. The degree of aortic atherosclerosis was shown as the ratio of the surface lesion area to the lumen surface area of the aorta. Atherosclerotic lesions develop from 2 months old. At age 12 months, coronary cross-sectional narrowing was about 80% and about 60% of the aortic lumen surface was covered by atherosclerotic lesions. At 18 months old, coronary cross-sectional narrowing and aortic lesion increased to 90% and 80%, respectively [22].
Figure 1.
Changes in the serum lipid levels of WHHLMI rabbits with age (a), and the distribution of cholesterol in lipoproteins (b). Data are represented as the mean ± standard error of the mean. The serum cholesterol levels at 12 months old were about 900 mg/dL. Excess LDL cholesterol is atherogenic and HDL has antiatherogenic function. In WHHL rabbits, LDL is accumulated in the plasma and HDL-cholesterol is low, less than 20 mg/dL. The serum cholesterol levels decrease gradually with aging.
Figure 2.
Development of atherosclerotic lesions in WHHLMI rabbits with age. The solid line denotes the degree of coronary atherosclerosis shown as coronary cross-sectional narrowing; lesion areas/area surrounded by the internal elastic lamina ×100 (%). The dotted line denotes the degree of aortic atherosclerosis; sum of the surface areas of the lesion/total surface area of the aortic lumen ×100 (%). Modified from Shiomi and Ito [8].
Prior to the development of the WHHLMI strain, WHHL rabbits were used to investigate mechanisms of the development of atherosclerosis, and many aspects have been clarified: accumulation of oxidized LDL in the atherosclerotic lesions [23, 24]; antiatherosclerotic effects of antioxidants (inhibition of oxidized-LDL formation) [25, 26]; the expression of monocyte adhesion molecules on arterial endothelial cells at the initiation of atherosclerosis [27]; scavenging of oxidized LDL at the lesions by macrophages through the scavenger receptors, VLDL receptors, and remnant receptors; accumulation of form cells derived from macrophages in arterial intima followed by further development of atherosclerotic lesions [28–32].
3. Species Differences in Lipid Metabolism and Atherosclerosis
As mentioned, lipoprotein metabolism in rabbits closely resembles that in humans. However, representative laboratory animals such as mice and rats have very different lipoprotein metabolism from that in humans (Table 1). Some examples of major species differences in lipid metabolism are the following. (1) In mice and rats, apoB editing enzyme is observed in the intestine and in the liver, but in humans and rabbits, this enzyme is expressed only in the intestine [33]. In humans and rabbits, apoB-48 is a major apolipoprotein of chylomicron and chylomicron remnants, which carry exogenous lipids derived from foods and apoB100 is a major apolipoprotein of VLDL, IDL, and LDL, which are endogenous lipoproteins derived from liver. In mice and rats, however, endogenous lipoproteins as well as exogenous lipoproteins also contain apoB-48, because of the expression of apoB editing enzyme in the liver [34]. Since the metabolic clearance of lipoproteins containing apoB-48 is very rapid, apoB-48 containing VLDL particles disappear rapidly from the circulation in mice and rats. As a result, the LDL lipid levels in mice and rats are very low compared with those in humans. (2) Hepatic lipase is circulating in the blood stream in mice thus different from humans in degradation of neutral lipids and transportation of free fatty acids into the tissues [35]. (3) In mice and rats, there is no cholesterol-ester transfer protein (CETP) activity in plasma, which transfers cholesterol from HDL to VLDL, IDL, and LDL [36], although CETP plays an important role in humans and rabbits. As a result, in mice and rats, the proportion of cholesterol in the HDL fraction is high compared with other lipoprotein fractions. Therefore, lipoprotein profiles of mice and rats are markedly different from that of humans, even in knockout mice lacking apoE or the LDL-receptors [8]. (4) Competitive inhibitors of a rate-limiting enzyme for cholesterol synthesis, statins, showed potent hypocholesterolemic effects in WHHL rabbits [37–45] but not in mice and rats [5]. In humans, statins are the most effective hypocholesterolemic drugs. These results demonstrate how it is important to choose appropriate species in translational research. (5) C-reactive protein (CRP), a major inflammatory marker in humans and rabbits, which increases in patients with acute coronary syndrome [46], is not responsive to inflammation in mice and rats, due to a lack of complement activation [47]. The major inflammatory marker of mice is serum amyloid P component (SAP), instead of CRP. (6) The types of myocardial fibers in mice are also different from those of humans and rabbits [48]. (7) Moreover, the ECG waveforms in mice and rats are clearly different from those of humans, but rabbit ECG shows similar waveforms to humans [49, 50]. As such, mice and rats have greatly different sets of factors for lipoprotein metabolism and cardiovascular diseases. Therefore, to employ mice and rats for studies on cardiovascular diseases and lipid metabolism, great care is required with analyses and/or the interpretation of the results obtained from experiments.
Table 1.
Comparison of lipid metabolism, atherosclerosis, and cardiac functions between genetically modified mice and WHHLMI rabbits.
| Genetically modified mice | WHHLMI rabbits | |
|---|---|---|
| Lipid metabolism | ||
| Major lipoprotein in the blood | X (Chylomicron, VLDL) | O (LDL) |
| Structural protein in theendogenous lipoprotein | X (apoB48) | O (apoB100) |
| Expression of apoB editing enzyme | X (The small intestine, liver) | O (The small intestine) |
| CETP activity in the blood | X (No) | O (Exists) |
| Hepatic lipase | X (Released to circulation) | O (Bound to vessel membrane) |
| Atherosclerosis | ||
| The coronary lesion | X (Resistant) | Δ (Spontaneously develops) |
| Composition of the lesions | X (Over accumulation of macrophages) | O (Various lesions) |
| VLDL receptor | X (no expression) | O (expression) |
| Heart | ||
| Electrocardiogram | ||
| Limb lead | X (Largely different waveforms) | O (Similar to humans) |
| Chest lead | X (Difficult to monitor) | O (Similar to humans) |
| Myocardial ion channel | X (Ito and IK,slow) | O (Ikr and IKs) |
| Myocardial fibers | X (α-myosin heavy chain) | O (β-myosin heavy chain) |
| Others | ||
| Inflammatory markers | X (SAP) | O (CRP) |
| The hypocholesterolemic effect of statins | X (Resistant) | O (Effective) |
O: similar to humans; Δ: partly similar to humans; X: largely different from humans.
4. Translational Research on the Development of the Lipid-Lowering Agents
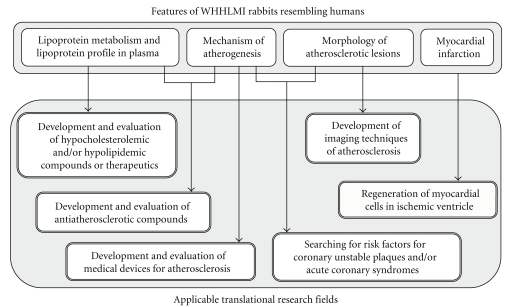
Figure 3 shows features of WHHLMI rabbits which resemble humans and applicable translational research fields. Since the WHHL rabbit is close to humans in lipoprotein metabolism, it was used for the development of various lipid-lowering agents and atherosclerosis-suppressing agents [8]. The hypolipidemic effects of various drugs have been investigated with WHHL rabbits (Table 2): cholesterol synthesis inhibitors, such as HMG-CoA reductase inhibitors and squalene synthetase inhibitors; inhibitors of microsomal triglyceride transfer protein, which works in the assembly of VLDL particles in liver; anionic exchange resins, which block the enterohepatic circulation of bile acids; omega-3 fatty acids, which are a component of fish oil; fibrates, which lower serum triglyceride levels. In studies with a cholesterol synthesis inhibitor, statin, serum total cholesterol levels of WHHL rabbits were decreased dose-dependently by 10–30% compared with the control group [37, 39]. The mechanisms for the reduction in serum cholesterol levels by statins are an increase in expression of mRNA of LDL receptors in the liver [39] and, decrease in the excretion of VLDL cholesterol from the liver in cases of high-dose treatment [38]. The agents that inhibit squalene synthetase, another rate-limiting enzyme in cholesterol synthesis, also decreased the serum cholesterol level by similar mechanisms [51]. Since a small amount of LDL receptor protein can be processed from a precursor to a mature form in WHHL fibroblasts [52], inhibition of cholesterol synthesis in the liver is expected to cause LDL receptors to accumulate on the surface of hepatocytes. Anion exchange resins absorb bile acids at the duodenum and block the enterohepatic circulation [53]. As a result, cholesterol is utilized in the hepatocytes for the synthesis of bile acids, and then the hepatocytes, which was exhausted the cholesterol pool, increase the number of LDL receptor molecules to acquire external cholesterol [39]. Therefore, the combination of an inhibitor for cholesterol synthesis and an anion exchange resin can decrease the serum cholesterol level markedly, and this was proved using WHHL rabbits [40]. Since microsomal triacylglycerol transfer protein (MTP) inhibitors are also effective in WHHL rabbits [54], they may have potential benefit for human FH. The successful treatment in WHHL rabbits means that patients with FH, excluding the LDL-receptor negative type, can be treated with these agents.
Figure 3.
Features of the WHHLMI rabbit resembling humans and applicable translational research fields.
Table 2.
Drug development using WHHL/WHHLMI rabbits.
| Lipid-lowering effect | Lipid-lowering effect | ||
|---|---|---|---|
| Aorta | Coronary arteries | ||
| Cholesterol synthesis inhibitors | |||
| Statins | O | X, O | O |
| Squalene synthesis inhibitor | O | O | O |
| Anion exchanger | O | O | |
| Statins + Anion exchanger | O | O | O |
| MTP inhibitor | O | ||
| ACAT inhibitor | X, O | X, O | X, O |
| Antioxidants | |||
| Probucol | O | O | |
| Vitamin E | X | X, O | |
| Colony stimulating factor | |||
| MCSF | X, O | O | |
| GMCSF | X, O | O | |
| Apo E | X, O | O | |
| Fibrate | X | ||
| Fish oils, ommega-3 fatty acids | X, O | X, O | |
| Thiazolidinedione | X | Δ | Δ |
| Thiazolidinedione + statin | O | O | O |
| Antihypertensive | |||
| ACE inhibitor | X | O | |
| AT-II receptor antagonists | X | O | |
| Calcium antagonists | X | X | |
| Beta-blockers | X | X | |
| Gene therapy | O | ||
O: effective; Δ: partly effective; X: no effect.
Modified from Shiomi and Ito [8].
5. Translational Research on Antiatherosclerotic Effects
The purpose of lowering serum cholesterol levels is to inhibit atherogenesis and to circumvent the cardiovascular and cerebrovascular events. The WHHL rabbit contributed to prove the effects of cholesterol-lowering therapies on delaying the progression of atherosclerosis. Statin treatment resulted in a decrease in serum total cholesterol levels by 20–30%, and the cross-sectional narrowing of the coronary arteries was significantly decreased [41–45].
In several clinical studies, the incidence of cardiovascular events was significantly reduced in the statin-treated groups despite little or no improvement in coronary stenosis on evaluation by coronary angiography [55]. The WHHL rabbit contributed to the clarification of this paradoxical mechanism [42–45]. On the administration of statin to 10-month old WHHL rabbits for one year, in which coronary atherosclerosis had already developed to a mature stage, statin treatment showed not only the prevention of further progression of the coronary atherosclerotic lesions, but also various stabilizing effects on coronary plaques, such as reductions in the contents of macrophages and extra cellular lipids in lesions, and increase in the contents of collagen fibers and preservation of the smooth muscle cells in lesions. Thus it was clarified that, statin administration makes atherosclerotic lesions more stable, that is, less likely to rupture. With this study, it was confirmed that the stabilization of atherosclerotic lesions is important for the prevention of coronary events. Nowadays, more than 40 million patients worldwide are prescribed statins. Another type of cholesterol synthesis inhibitor, squalene synthesis inhibitors, that act downstream of the cholesterol synthesis pathway, also showed similar hypocholesterolemic and atheroma-stabilizing effects in WHHLMI rabbits [56].
Using WHHLMI rabbits, antiatherosclerotic effects have also been evaluated with other compounds such as omega-3 fatty acids, which decrease serum triglyceride levels by changing the composition of fatty acids [57–61]; antioxidants, such as probucol, vitamin C, and vitamin E [62–65]; agents that regulate the function of macrophages [66, 67]; drugs that inhibit the rennin-angiotensin pathway [68–71]. Interestingly, antiatherosclerotic effects of antihypertensive agents were unequal in WHHL or WHHLMI rabbits. Angiotensin converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARBs) showed antiatherogenic effects [69–72], but calcium antagonists and beta-blockers were not effective [73, 74]. Systolic blood pressure in WHHL and WHHLMI rabbits is 100–120 mmHg, which is slightly higher than normal [75]. This may be why calcium antagonists and beta-blockers did not show distinct antiatherosclerotic effects. In contrast, antihypertensive effects of ACE inhibitors and ARBs are mediated by suppressing the effects of angiotensin II. Angiotensin-II stimulates atherogenesis by impairing the function of arterial endothelial cells, proliferation of arterial smooth muscle cells, and inflammation [76]. These pleiotropic effects of angiotensin-II are considered to be mediated by reactive oxygen species. Thus, the WHHL rabbit is indispensable for studies on the antiatherosclerotic effects of the various compounds.
6. Imaging Technology for Evaluation of Atherosclerotic Lesions
Although it is important to evaluate drug efficacy in clinical use, it is difficult to evaluate atheroma-stabilizing effects of drugs in clinical practice. With coronary angiography, it is possible to see the degree of stenosis but difficult to evaluate the severity of lesions, if the lesions are spread and extended in the coronary arteries, or if the coronary arteries are expanded due to the outward remodeling of the vessels. Furthermore, it is very important to develop noninvasive technologies and equipment to detect dangerous lesions, that is, vulnerable plaques that are prone to rupture, not only for the diagnosis but for the prevention of cardiovascular events. As vulnerable plaques that cause cardiovascular events, soft-type plaques rich in macrophages and large lipid droplets covered with a thin fibrous cap are important. To detect such soft-type plaques, computed tomography (CT) [77], positron emission tomography (PET) [77], CT plus PET [78], magnetic resonance (MRI) [78, 79], and intravascular ultrasound (IVUS) [80] have been applied to WHHLMI rabbits. One successful example was evaluation of the antiatherosclerotic effect of probucol, a potent antioxidant, in WHHLMI rabbits by imaging with CT plus PET [81]. Ogawa et al. demonstrated clearly that imaging with CT plus PET is powerful technology to detect antiatherosclerotic effects of compounds. Once imaging technologies for the evaluation of atherosclerotic lesions are established, they can be used not only for the assessment of drug effects, but also for the detection of dangerous coronary lesions that could lead to cardiovascular events such as acute coronary syndromes and consequently the prevention of ischemic heart diseases.
7. Perspectives
To overcome cardiovascular diseases, many research issues remain unresolved, despite diligent studies for the development of diagnostic methods and lipid-lowering agents. Particularly important is clarifying the mechanism of the disruption of coronary lesions (arterial plaque rupture and the following formation of a thrombus), which depress the trigger for the onset of acute coronary syndromes, and establishment of treatments. Still no suitable animal model, which is compatible with the study of human acute coronary syndromes, has been developed. To develop a suitable animal model for human acute coronary syndromes, trial studies/experiments such as the enhancement of vulnerable coronary lesions, and application of physical pressure to coronary lesions, are currently underway with WHHLMI rabbits. To destabilize coronary lesions, serial selective breeding with new criteria such as the formation of vulnerable plaques is also ongoing, in parallel with the development of genetically modified WHHLMI rabbits overexpressing matrix metalloproteinases (MMPs), and so forth. The established strain would be a subject of analyses for the identification of the genes/loci responsible for the phenotype established. In the near future, with advances in gene-targeting technologies by using ES or iPS cells capable of germ-line transmission, in combination with the nuclear transfer technique, more precise manipulation of the rabbit genome may also be available. Since the lesion composition and severity of coronary lesions differ even in WHHLMI rabbits, despite no difference in the serum cholesterol levels, it will be important to explore marker proteins and/or risk factors affecting coronary lesions. Once markers and risk factors relating to vulnerable coronary atheromas are found, the mechanism of cardiovascular events may be clarified. Such findings would contribute to the development of new clinical diagnostics and thence to the prevention of cardiovascular events.
In conclusion, selecting appropriate animal model is important in translational research. WHHL and WHHLMI rabbits have contributed to development of hypocholesterolemic and antiatherosclerotic compounds and medical devices, such as imaging technologies for atherosclerosis, and diagnostic techniques for acute coronary syndromes, in addition to elucidation of the mechanisms of atherogenesis and coronary plaque rupture. These studies are helpful for progression of therapeutics.
Acknowledgments
This work was supported in part by a Grant-in-Research on Biological Resource and Animal Models for Drug Development from the Ministry of Health and Labor in Japan and a research grant from the Ministry of Education, Culture, Science and Technology of Japan. The authors thank Sankyo Co., Ltd., Tokyo, Japan; Takeda Pharmaceutical Co., Ltd., Osaka, Japan; Daiich-Sankyo Co., Ltd., Tokyo, Japan; Shionogi & Co. Ltd, Osaka, Japan; Taisho Pharmaceutical Co. Ltd., Tokyo, Japan; Otsuka Pharmaceutical Co. Ltd., Tokushima, Japan, Banyu Pharmaceutical Co. Ltd., Tokyo, Japan; Nippon Shinyaku Co. Ltd., Osaka, Japan for their support in the maintenance of the WHHL or WHHLMI rabbit strain from 1980 to 2010.
References
- 1.World Health Organization. The World Health Report 2002—Reducing Risks, Promoting Healthy Life: Statistical Annex. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 2.Teramoto T, Sasaki J, Ueshima H, et al. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. Journal of Atherosclerosis and Thrombosis. 2007;14(2):45–50. doi: 10.5551/jat.14.45. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Anand S. Cost of prevention: the case of lipid lowering. Circulation. 1996;93(10):1774–1776. doi: 10.1161/01.cir.93.10.1774. [DOI] [PubMed] [Google Scholar]
- 4.Endo A, Kuroda M, Tsujita Y. ML 236A, ML 236B, and ML 236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. Journal of Antibiotics. 1976;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 5.Tsujita Y, Kuroda M, Shimada Y. CS-514, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase: tissue-selective inhibition of sterol synthesis and hypolipidemic effect on various animal species. Biochimica et Biophysica Acta. 1986;877(1):50–60. doi: 10.1016/0005-2760(86)90117-7. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe Y. Studies on characteristic of spontaneously hyperlipidemic rabbit and development of the strain with such property. Bulletin of Azabu Veterinary College. 1977;2(1):99–124. [Google Scholar]
- 7.Liew TV, Ray KK. Intensive statin therapy in acute coronary syndromes. Current Atherosclerosis Reports. 2008;10(2):158–163. doi: 10.1007/s11883-008-0023-1. [DOI] [PubMed] [Google Scholar]
- 8.Shiomi M, Ito T. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of development: a tribute to the late Dr. Yoshio Watanabe. Atherosclerosis. 2009;207(1):1–7. doi: 10.1016/j.atherosclerosis.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Ito T, Kondo T. Breeding of a rabbit strain of hyperlipidemia and characteristic of these strain. Experimental Animals. 1977;26(1):35–42. [PubMed] [Google Scholar]
- 10.Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Incidence and development of atherosclerosis and xanthoma. Atherosclerosis. 1980;36(2):261–268. doi: 10.1016/0021-9150(80)90234-8. [DOI] [PubMed] [Google Scholar]
- 11.Tanzawa K, Shimada Y, Kuroda M. WHHL-rabbit: a low density lipoprotein receptor-deficient animal model for familial hypercholesterolemia. FEBS Letters. 1980;118(1):81–84. doi: 10.1016/0014-5793(80)81223-3. [DOI] [PubMed] [Google Scholar]
- 12.Havel RJ, Kita T, Kotite L. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis. 1982;2(6):467–474. doi: 10.1161/01.atv.2.6.467. [DOI] [PubMed] [Google Scholar]
- 13.Kita T, Brown M, Bilheimer DW, Goldstein JL. Delayed clearance of very low density and intermediate density lipoprotein with enhanced conversion to low density lipoprotein in WHHL rabbits. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(18):5693–5697. doi: 10.1073/pnas.79.18.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kita T, Goldstein JL, Brown MS. Hepatic uptake chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(11):3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietschy JM, Kita T, Suckling KE. Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. Journal of Lipid Research. 1983;24(4):469–480. [PubMed] [Google Scholar]
- 16.Watanabe Y, Ito T, Shiomi M. The effect of selective breeding on the development of coronary atherosclerosis in WHHL rabbits. An animal model for familial hypercholesterolemia. Atherosclerosis. 1985;56(1):71–79. doi: 10.1016/0021-9150(85)90085-1. [DOI] [PubMed] [Google Scholar]
- 17.Shiomi M, Ito T, Shiraishi M, Watanabe Y. Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis. 1992;96(1):43–52. doi: 10.1016/0021-9150(92)90036-g. [DOI] [PubMed] [Google Scholar]
- 18.Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit) Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(7):1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50. [DOI] [PubMed] [Google Scholar]
- 19.Shiomi M, Fan J. Unstable coronary plaques and cardiac events in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits: questions and quandaries. Current Opinion in Lipidology. 2008;19(6):631–636. doi: 10.1097/MOL.0b013e3283189c18. [DOI] [PubMed] [Google Scholar]
- 20.Shiomi M, Ito T, Tsukada T, Yata T, Ueda M. Cell compositions of coronary and aortic atherosclerotic lesions in WHHL rabbits differ: an immunohistochemical study. Arteriosclerosis and Thrombosis. 1994;14(6):931–937. doi: 10.1161/01.atv.14.6.931. [DOI] [PubMed] [Google Scholar]
- 21.Shiomi M, Ito T, Fujioka T, Tsujita Y. Age-associated decrease in plasma cholesterol and changes in cholesterol metabolism in homozygous Watanabe heritable hyperlipidemic rabbits. Metabolism. 2000;49(4):552–556. doi: 10.1016/s0026-0495(00)80025-6. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Yamada S, Shiomi M. Progression of coronary atherosclerosis relates to the onset myocardial infarction in an animal model of spontaneous myocardial infarction (WHHLMI rabbits) Experimental Animals. 2004;53(4):339–346. doi: 10.1538/expanim.53.339. [DOI] [PubMed] [Google Scholar]
- 23.Mowri HO, Ohkuma S, Takano T. Monoclonal DLR1a/104G antibody recognizing peroxidized lipoproteins in atherosclerotic lesions. Biochimica et Biophysica Acta. 1988;963(2):208–214. doi: 10.1016/0005-2760(88)90282-2. [DOI] [PubMed] [Google Scholar]
- 24.Boyd HC, Gown AM, Wolfbauer G, Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. American Journal of Pathology. 1989;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- 25.Kita T, Nagano Y, Yokode M, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carew TE, Schwenke DC, Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cybulsky MI, Gimbrone MA., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 28.Buja LM, Kuta T, Goldstein JL. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis. 1983;3(1):87–101. doi: 10.1161/01.atv.3.1.87. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld ME, Tsukada T, Gown AM, Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987;7(1):9–23. doi: 10.1161/01.atv.7.1.9. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld ME, Tsukada T, Chait A, Bierman EL, Gown AM, Ross R. Fatty streak expansion and maturation in Watanabe heritable hyperlipidemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987;7(1):24–34. doi: 10.1161/01.atv.7.1.24. [DOI] [PubMed] [Google Scholar]
- 31.Takano T, Amanuma K, Kimura J, Kanaseki T, Ohkuma S. Involvement of macrophages in accumulation and elimination of cholesterol ester in atherosclerotic aorta. Acta Histochemica et Cytochemica. 1986;19(1):135–143. [Google Scholar]
- 32.Tsukada T, Rosenfeld M, Ross R, Gown AM. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- 33.Kozarsky KF, Bonen DK, Giannoni F, Funahashi T, Wilson JM, Davidson NO. Hepatic expression of the catalytic subunit of the apolipoprotein B mRNA editing enzyme (apobec-1) ameliorates hypercholesterolemia in LDL receptor-deficient rabbits. Human Gene Therapy. 1996;7(8):943–957. doi: 10.1089/hum.1996.7.8-943. [DOI] [PubMed] [Google Scholar]
- 34.Nakamuta M, Taniguchi S, Ishida BY, Kobayashi K, Chan L. Phenotype interaction of apobec-1 and CETP, LDLR, and ApoE gene expression in mice: role of ApoB mRNA editing in lipoprotein phenotype expression. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(5):747–755. doi: 10.1161/01.atv.18.5.747. [DOI] [PubMed] [Google Scholar]
- 35.Perret B, Mabile L, Martinez L, Tercé F, Barbaras R, Collet X. Hepatic lipase: structure/function relationship, synthesis, and regulation. Journal of Lipid Research. 2002;43(8):1163–1169. [PubMed] [Google Scholar]
- 36.Agellon LB, Walsh A, Hayek T, et al. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. The Journal of Biological Chemistry. 1991;266(17):10796–10801. [PubMed] [Google Scholar]
- 37.Watanabe Y, Ito T, Saeki M. Hypolipidemic effects of CS-500 (ML-236B) in WHHL-rabbit, a heritable animal model for hyperlipidemia. Atherosclerosis. 1981;38(1-2):27–31. doi: 10.1016/0021-9150(81)90100-3. [DOI] [PubMed] [Google Scholar]
- 38.Shiomi M, Ito T. Pravastatin sodium, a competitive inhibitor of hepatic 3-hydroxy-3- methylglutaryl coenzyme a reductase, decreases the cholesterol content of newly secreted very-low-density lipoprotein in Watanabe heritable hyperlipidemic rabbits. Metabolism. 1994;43(5):559–564. doi: 10.1016/0026-0495(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda M, Matsumoto A, Itakura H, et al. Effects of pravastatin sodium alone and in combination with cholestyramine on hepatic, intestinal and adrenal low density lipoprotein receptors in homozygous Watanabe heritable hyperlipidemic rabbits. Japanese Journal of Pharmacology. 1992;59(1):65–70. doi: 10.1254/jjp.59.65. [DOI] [PubMed] [Google Scholar]
- 40.Shiomi M, Ito T, Watanabe Y, et al. Suppression of established atherosclerosis and xanthomas in mature WHHL rabbits by keeping their serum cholesterol levels extremely low. Effect of pravastatin sodium in combination with cholestyramine. Atherosclerosis. 1990;83(1):69–80. doi: 10.1016/0021-9150(90)90132-3. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe Y, Ito T, Shiomi M, et al. Preventive effect of pravastatin sodium, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochimica et Biophysica Acta. 1988;960(3):294–302. [PubMed] [Google Scholar]
- 42.Shiomi M, Ito T, Tsukada T, et al. Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques: effect of pravastatin sodium on atherosclerosis in mature WHHL rabbits. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(11):1938–1944. doi: 10.1161/01.atv.15.11.1938. [DOI] [PubMed] [Google Scholar]
- 43.Shiomi M, Ito T. Effect of cerivastatin sodium, a new inhibitor of HMG-CoA reductase, on plasma lipid levels, progression of atherosclerosis, and the lesional composition in the plaques of WHHL rabbits. British Journal of Pharmacology. 1999;126(4):961–968. doi: 10.1038/sj.bjp.0702382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiomi M, Ito T, Hirouchi Y, Enomoto M. Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins. Atherosclerosis. 2001;157(1):75–84. doi: 10.1016/s0021-9150(00)00708-5. [DOI] [PubMed] [Google Scholar]
- 45.Shiomi M, Yamada S, Ito T. Atheroma stabilizing effects of simvastatin due to depression of macrophages or lipid accumulation in the atheromatous plaques of coronary plaque-prone WHHL rabbits. Atherosclerosis. 2005;178(2):287–294. doi: 10.1016/j.atherosclerosis.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Otake H, Shite J, Shinke T, et al. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. American Journal of Cardiology. 2008;101(1):1–7. doi: 10.1016/j.amjcard.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 47.Pepys MB, Baltz M, Gomer K. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- 48.Fan J, Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacology and Therapeutics. 2003;99(3):261–282. doi: 10.1016/s0163-7258(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Iden JB, Kovithavongs K, Gulamhusein R, Duff HJ, Kavanagh KM. In vivo temporal and spatial distribution of depolarization and repolarization and the illusive murine T wave. Journal of Physiology. 2004;555(1):267–279. doi: 10.1113/jphysiol.2003.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.London B. Cardiac arrhythmias: from (transgenic) mice to men. Journal of Cardiovascular Electrophysiology. 2001;12(9):1089–1091. doi: 10.1046/j.1540-8167.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- 51.Ness GC, Zhao Z, Keller RK. Effect of squalene synthase inhibition on the expression of hepatic cholesterol biosynthetic enzymes, LDL receptor, and cholesterol 7α hydroxylase. Archives of Biochemistry and Biophysics. 1994;311(2):277–285. doi: 10.1006/abbi.1994.1238. [DOI] [PubMed] [Google Scholar]
- 52.Schneider WJ, Brown MS, Goldstein JL. Kinetic defects in the processing of the low density lipoprotein receptor in fibroblasts from WHHL rabbits and a family with familial hypercholesterolemia. Molecular Biology & Medicine. 1983;1(3):353–367. [PubMed] [Google Scholar]
- 53.Subbiah MTR, Yunker RL, Rymaszewski Z, Kottke BA, Bale LK. Cholestyramine treatment in early life of low-density lipoprotein receptor deficient Watanabe rabbits: decreased aortic cholesteryl ester accumulation and atherosclerosis in adult life. Biochimica et Biophysica Acta. 1987;920(3):251–258. doi: 10.1016/0005-2760(87)90102-0. [DOI] [PubMed] [Google Scholar]
- 54.Shiomi M, Ito T. MTP inhibitor decreases plasma cholesterol levels in LDL receptor-deficient WHHL rabbits by lowering the VLDL secretion. European Journal of Pharmacology. 2001;431(1):127–131. doi: 10.1016/s0014-2999(01)01419-4. [DOI] [PubMed] [Google Scholar]
- 55.van Boven AJ, Jukema JW, Zwinderman AH, Crijns HJGM, Lie KI, Bruschke AVG. Reduction of transient myocardial ischemia with pravastatin in addition to the conventional treatment in patients with angina pectoris. Circulation. 1996;94(7):1503–1505. doi: 10.1161/01.cir.94.7.1503. [DOI] [PubMed] [Google Scholar]
- 56.Shiomi M, Yamada S, Amano Y, Nishimoto T, Ito T. Lapaquistat acetate, a squalene synthase inhibitor, changes macrophage/lipid-rich coronary plaques of hypercholesterolaemic rabbits into fibrous lesions. British Journal of Pharmacology. 2008;154(5):949–957. doi: 10.1038/bjp.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mortensen A, Hansen BF, Hansen JF, et al. Comparison of the effects of fish oil and olive oil on blood lipids and aortic atherosclerosis in Watanabe heritable hyperlipidaemic rabbits. British Journal of Nutrition. 1998;80(6):565–573. doi: 10.1017/s0007114598001664. [DOI] [PubMed] [Google Scholar]
- 58.Lichtenstein AH, Chobanian AV. Effect of fish oil on atherogenesis in Watanabe heritable hyperlipidemic rats. Arteriosclerosis. 1990;10(4):597–606. doi: 10.1161/01.atv.10.4.597. [DOI] [PubMed] [Google Scholar]
- 59.Pfister SL, Rosolowsky M, Schmitz JM, Clubb FJ, Campbell WB. Eicosapentaenoic acid alters vascular reactivity and platelet adhesion in Watanabe heritable hyperlipidemic rabbits. European Journal of Pharmacology. 1989;161(1):85–89. doi: 10.1016/0014-2999(89)90183-0. [DOI] [PubMed] [Google Scholar]
- 60.Rich S, Miller JF, Charous S, et al. Development of atherosclerosis in genetically hyperlipidemic rabbits during chronic fish-oil ingestion. Arteriosclerosis. 1989;9(2):189–194. doi: 10.1161/01.atv.9.2.189. [DOI] [PubMed] [Google Scholar]
- 61.Clubb FJ, Schmitz JM, Butler MM, Buja LM, Willerson JT, Campbell WB. Effect of dietary omega-3 fatty acid on serum lipids, platelet function, and atherosclerosis in Watanabe Heritable Hyperlipidemic rabbits. Arteriosclerosis. 1989;9(4):529–537. doi: 10.1161/01.atv.9.4.529. [DOI] [PubMed] [Google Scholar]
- 62.Kita T, Nagano Y, Yokode M, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carew TE, Schwenke DC, Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Nigris F, Youssef T, Ciafré S, et al. Evidence for oxidative activation of c-Myc-dependent nuclear signaling in human coronary smooth muscle cells and in early lesions of Watanabe heritable hyperlipidemic rabbits: protective effects of vitamin E. Circulation. 2000;102(17):2111–2117. doi: 10.1161/01.cir.102.17.2111. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida N, Murase H, Kunieda T, et al. Inhibitory effect of a novel water-soluble vitamin E derivative on atherosclerosis in rabbits. Atherosclerosis. 2002;162(1):111–117. doi: 10.1016/s0021-9150(01)00702-x. [DOI] [PubMed] [Google Scholar]
- 66.Yamada N, Ishibashi S, Shimano H, et al. Role of monocyte colony-stimulating factor in foam cell generation. Proceedings of the Society for Experimental Biology and Medicine. 1992;200(2):240–244. doi: 10.3181/00379727-200-43427. [DOI] [PubMed] [Google Scholar]
- 67.Shindo J, Ishibashi T, Yokoyama K, et al. Granulocyte-macrophage colony-stimulating factor prevents the progression of atherosclerosis via changes in the cellular and extracellular composition of atherosclerotic lesions in Watanabe heritable hyperlipidemic rabbits. Circulation. 1999;99(16):2150–2156. doi: 10.1161/01.cir.99.16.2150. [DOI] [PubMed] [Google Scholar]
- 68.Chobanian AV, Haudenschild CC, Nickerson C, Hope S. Trandolapril inhibits atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1992;20(4):473–477. doi: 10.1161/01.hyp.20.4.473. [DOI] [PubMed] [Google Scholar]
- 69.Hope S, Brecher P, Chobanian AV. Comparison of the effects of AT receptor blockade and angiotensin converting enzyme inhibition on atherosclerosis. American Journal of Hypertension. 1999;12(1 I):28–34. doi: 10.1016/s0895-7061(98)00203-9. [DOI] [PubMed] [Google Scholar]
- 70.Koike H. New pharmacologic aspects of CS-866, the newest angiotensin II receptor antagonist. American Journal of Cardiology. 2001;87(8, supplement 1):33c–36c. doi: 10.1016/s0002-9149(01)01540-5. [DOI] [PubMed] [Google Scholar]
- 71.Imanishi T, Kuroi A, Ikejima H, et al. Effects of angiotensin converting enzyme inhibitor and angiotensin II receptor antagonist combination on nitric oxide bioavailability and atherosclerotic change in Watanabe heritable hyperlipidemic rabbits. Hypertension Research. 2008;31(3):575–584. doi: 10.1291/hypres.31.575. [DOI] [PubMed] [Google Scholar]
- 72.Imanishi T, Tsujioka H, Ikejima H, et al. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52(3):563–572. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 73.van Niekerk JLM, Hendriks Th, de Boer HHM, Van ’t Laar A. Does nifedipine suppress atherogenesis in WHHL rabbits? Atherosclerosis. 1984;53(1):91–98. doi: 10.1016/0021-9150(84)90109-6. [DOI] [PubMed] [Google Scholar]
- 74.Chobanian AV. The effects of ACE inhibitors and other antihypertensive drugs on cardiovascular risk factors and atherogenesis. Clinical Cardiology. 1990;13(6):VII43–VII48. doi: 10.1002/clc.4960131409. [DOI] [PubMed] [Google Scholar]
- 75.Hosomi H, Katsuda S, Watanabe Y. Effect of atherosclerosis on the responsiveness of the rapidly acting arterial pressure control system in WHHL rabbits. Cardiovascular Research. 1986;20(3):195–200. doi: 10.1093/cvr/20.3.195. [DOI] [PubMed] [Google Scholar]
- 76.Sata M, Fukuda D. Crucial role of renin-angiotensin system in the pathogenesis of atherosclerosis. Journal of Medical Investigation. 2010;57(1-2):12–25. doi: 10.2152/jmi.57.12. [DOI] [PubMed] [Google Scholar]
- 77.Ogawa M, Ishino S, Mukai T, et al. F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. Journal of Nuclear Medicine. 2004;45(7):1245–1250. [PubMed] [Google Scholar]
- 78.Meding J, Urich M, Licha K, et al. Magnetic resonance imaging of atherosclerosis by targeting extracellular matrix deposition with Gadofluorine M. Contrast Media & Molecular Imaging. 2007;2(3):120–129. doi: 10.1002/cmmi.137. [DOI] [PubMed] [Google Scholar]
- 79.Steen H, Lima JAC, Chatterjee S, et al. High-resolution three-dimensional aortic magnetic resonance angiography and quantitative vessel wall characterization of different atherosclerotic stages in a rabbit model. Investigative Radiology. 2007;42(9):614–621. doi: 10.1097/RLI.0b013e3180592a93. [DOI] [PubMed] [Google Scholar]
- 80.Iwata A, Miura SI, Imaizumi S, Zhang B, Saku K. Measurement of atherosclerotic plaque volume in hyperlipidemic rabbit aorta by intravascular ultrasound. Journal of Cardiology. 2007;50(4):229–234. [PubMed] [Google Scholar]
- 81.Ogawa M, Magata Y, Kato T, et al. Application of F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. Journal of Nuclear Medicine. 2006;47(11):1845–1850. [PubMed] [Google Scholar]