Summary:
A rate-limiting step in breast cancer progression is acquisition of the invasive phenotype, which can precede metastasis. Expression of cell-surface proteases at the leading edge of a migrating cell provides cells with a mechanism to cross tissue barriers. A newly appreciated mechanism that may be relevant for breast cancer cell invasion is the formation of invadopodia, well-defined structures that project from the ventral membrane and promote degradation of the extracellular matrix, allowing the cell to cross a tissue barrier. Recently, there has been some controversy and discussion as to whether invadopodia, which are associated with carcinoma cells, are related to a similar structure called podosomes, which are associated with normal cells. Invadopodia and podosomes share many common characteristics, including a similar size, shape, subcellular localization and an ability to promote invasion. These two structures also share many common protein components, which we outline herein. It has been speculated that podosomes may be precursors to invadopodia and by extension both structures may be relevant to cancer cell invasion. Here, we compare and contrast the protein components of invadopodia and podosomes and discuss a potential role for these proteins and the evidence that supports a role for invadopodia and podosomes in breast cancer invasion.
Keywords: invadopodia, podosomes, invasion, breast cancer
Introduction
Breast cancer is a complex disease that is estimated to affect 182,460 women in 2008 with 40,480 predicted mortalities in the United States, alone. The most commonly diagnosed form of breast cancer is invasive ductal carcinoma, which is usually detected as a stage I disease. When treated with standard therapy (lumpectomy, radiation and tamoxifen) invasive ductal carcinoma has a five-year survival rate of approximately 80%. Initially, invasive ductal carcinoma begins as an atypical hyperplasia, typified by a loss of balance between growth and apoptosis of the epithelial cells that line the breast ducts. Here, the cells appear to fill the duct and show a characteristic pattern of increased mitotic activity throughout the hyperplasia. The disease can then progress to ductal carcinoma in situ where it remains contained within the ducts; however, mitotic activity is elevated throughout the tumor. Subsequently, these cells can become invasive. They can move as either a collective “sheet” of cells or they can separate away from the ductal carcinoma in situ and move independently. These newly invasive cells can breach the barrier of the ducts and move into the collagen matrix of the breast where they can establish a tumor. Invasion requires increased migratory capacity and protease expression. Ultimately, these cells may gain entry into the lymph nodes where they can metastasize, or they may intravasate directly into blood vessels, where they can be transported and trapped within the capillaries. Here, the cells can extravasate into surrounding tissue and potentially establish a distant site metastasis. Thus, a key feature in the progression of breast cancer is acquisition of the invasive phenotype. Clearly, if breast cancer invasion could be blocked, tumor growth would be confined and the disease rendered manageable.
Invasion occurs by different mechanisms. Migrating cells may express and secrete proteases at the leading edge of the carcinoma cell. These proteases degrade the extracellular matrix (ECM) and create a path of least resistance through which cells migrate and cross tissue barriers (Gimona et al. 2008). Alternatively, carcinoma cells can ‘push’ their way through a loose matrix, moving in a fashion that might be analogous to amoeboid motility, which can occur independent of protease activity (Sahai and Marshall, 2003). Invasive cells can also move ventrally, using podosomes or invadopodia, both of which promote the local release of protease activity and allow the cell to degrade the extracellular matrix and cross a tissue barrier.
Invadopodia and Podosomes
Invadopodia share many characteristics with podosomes, thus, there has been some controversy as to whether podosomes and invadopodia are related or distinct structures. Several very fine reviews have been written recently on this subject (Ayala et al. 2006; Yamaguchi and Condeelis, 2007; Linder, 2007; Gimona et al. 2008), that outline podosome and invadopodium structure and function and discuss some of the aspects of them that are common and distinct. At the core of this controversy is whether podosomes are precursors to invadopodia, and by extension, whether podosomes (like invadopodia) are relevant for cancer cell invasion. Alternatively, it has been speculated that podosomes and invadopodia could have both evolved from some common primordial structure. Here, we will review the protein components of podosomes and invadopodia and the data that indicate these structures may be related and relevant for breast cancer invasion.
Structural Features
Both podosomes and invadopodia are functional structures that form on the ventral membrane of cells and modulate the release and activation of proteases that degrade the extracellular matrix and promote the ability of cells to cross tissue barriers. The main differences are the types of cells in which they have been identified and their relative size. Podosomes are associated with normal cells, such as macrophages, osteoclasts, dendritic cells, epithelial cells, smooth muscle cells and fibroblasts. They are relatively small, about 1.0 μm in diameter and extend into the matrix 0.5 μm in length (Linder and Aepfelbacher, 2003). Podosomes can coalesce and form larger, ‘donut’ shaped structures that appear to be clusters of podosomes and are about 5 μm in diameter (Gringel et al. 2006; Gu et al. 2007). This difference in size could be related to changes in higher order structure or could correlate in part with a difference in the organization of actin filaments within them (Gimona et al. 2008). Interestingly, the size of the structure appears to correlate with half-life. Podosomes have a relatively short half-life, 2–10 minutes, however, larger podosomes appear to have a longer half-life (Gringel et al. 2006; Gu et al. 2007). In another level of higher organization, podosomes can cluster and form a larger ring structure called a rosette, which is characteristic of oncogene-transformed fibroblasts (Linder and Aepfelbacher, 2003). In yet a third higher order structure, podosomes can cluster together like a tightly connected rosette and form a ‘sealing zone’, which is a characteristic structure associated with osteoclasts and their bone resorption function.
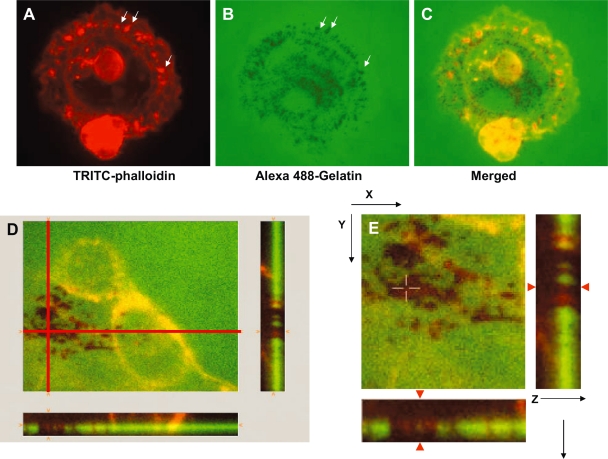
Invadopodia on the other hand are associated with carcinoma cells and have been described as larger structures, up to 8 μm in diameter and 2–5 μm in length based on immunofluorescence confocal microscopy analysis (Linder, 2007). Invadopodia can be detected, in part, by identifying F-actin in a structure of the appropriate size and shape, on the ventral membrane, using scanning confocal immunofluorescence microscopy (0.7 μm scanning thickness) (Fig. 1A-C). Herein, one can turn the cells on their side and detect the F-actin protruding into the extracellular matrix, which becomes degraded (no ‘green’) (Fig. 1D-E).
Figure 1.
Invadopodia formation in Src527F-expressing MDA-MB-231 breast carcinoma cells. (A) TRITC-phalloidin labeling of F-actin demonstrates actin-rich punctate structures around the cell peripheray (arrows) as detected by confocal immunofluorescence microscopy on the ventral membrane (0.7 μm scanning thickness). (B) The cells were plated on Alexa488-gelatin/fibronectin and allowed to degrade the extracellular matrix, as seen by zones of clearing in the ‘green’ extracellular matrix (arrows). (C) Merged image shows the overlap of F-actin with proteolytic activity. (D) Larger panel shows zones of clearing or active proteolysis. The rectangular images below and beneath illustrate a cross section of degraded extracellular matrix showing ‘red’ F-actin protruding into the ‘green’ extracellular matrix by both x-z and y-z images (note where the red lines intersect, cells are turned on the side and ‘red’ actin is detected in the zones of clearing which now lack ‘green’ extracellular matrix. (E) Close-up view of (D) where red arrow in x-z and y-z show ‘red’ F-actin protruding into the ‘green’ extracellular matrix as an invadopodia. Similarly, the rectangular images show ‘red’ actin present in zones of clearing where the ‘green’ extracellular matrix has been degraded. Cells were the kind gift of Susette Mueller (Bowden et al. 2006).
Interestingly, an electron microscopy ultrastructure study demonstrated that invadopodia had a slender structure, 0.8 μm–1.0 μm in diameter and 2 μm or more in length (Artym et al. 2006). However, another very thorough study by Buccione and colleagues using a combined electron microscopy and confocal light and immunofluorescence microscopy approach that appeared to show invadopodia can cluster together, which would make them appear as larger diameter structures by light microscopy (Baldassarre et al. 2003). This observation could be analogous to the difference between small and large podosomes (Gu et al. 2007). Invadopodia have a longer half-life than podosomes, estimated anywhere from 1–3 hours (Artym et al. 2006; Linder, 2007). However, invadopodia life span appears to correlate well with whether the carcinoma cell is migrating. Migrating cells showed shorter-lived invadopodia while static cells showed longer-lived invadopodia (Yamaguchi et al. 2005; Artym et al. 2006). It is not clear whether invadopodia formed in migrating cells have a different diameter relative to those that would form in a static cell. Nevertheless, it may be possible that carcinoma cells need to become static or less migratory (i.e. confront a tissue barrier) in order to generate a long-lived invadopodia.
Protein Biomarkers for Podosomes and Invadopodia
Recently, a meeting on podosomes and invadopodia was held at Cold Spring Harbor where the relationship of podosomes and invadopodia were discussed (“Podosomes and Invadopodia: Signatures of the wandering cell?”, November 26–29, 2007, John Condeelis, Ph.D., Chair). Although it was not resolved whether these are related or distinct structures, it was generally agreed that there should be a set of criteria used to define an ‘actin-rich dot’ on a ventral cell membrane as a podosome or an invadopodia. The consensus suggestion was that these structures should be imaged on the ventral membrane by confocal immunofluorescence microscopy at a scanning thickness of 0.7–1.0 μm. These structures should express actin in the core, as well as a reliable marker protein that differentiates invadopodia and podosomes from focal adhesions, such as cortactin, Tks5 or dynamin (Linder, 2007). Lastly, the ‘biomarkers’ should be detected in association with functional proteolytic activity by seeding the cells on a FITC-gelatin/fibronectin matrix and demonstrating that the biomarker for podosomes or invadopodia appear over zones of clearing where proteases have digested the matrix (Bowden et al. 2001). Using these criteria, a number of proteins have been described as associated with podosomes and invadopodia (Table 1). As podosomes are better studied than invadopodia, more protein components have been identified in association with podosomes. Nevertheless, it is clear that they share at least 32 common protein components, and likely more. In only one case did we find a controversy where one protein, tubulin, may be uniquely relevant for podosomes. Microtubular structures appear to be required for podosome dynamics but may be less important for invadopodia (Linder et al. 2000; Destaing et al. 2003; Destaing et al. 2005). In agreement with a role for microtubular structures supporting podosome dynamics, treatment of osteoclast cells with nocodazole did disrupt podosome location (Babb et al. 1997) and the microtubular-associated protein kinesin appears to be important for podosome dynamics (Kopp et al. 2006). Although there are data to indicate tubulin could be associated with invadopodia (Strohmaier et al. 2000), treatment of the Met-1 breast cancer cell line with colchicine did not inhibit invadopodia formation (Bourguignon et al. 1998). In this regard, it has been speculated that because podosomes are more dynamic structures than invadopodia, microtubules may not be required for invadopodia formation and function (Linder, 2007). If true, then it would be interesting to determine if there is a differential requirement of microtubular structures for larger, long-lived podosomes relative to smaller, short-lived podosomes. This is an understudied area that warrants a closer look. Otherwise, the protein components of podosomes and invadopodia listed in Table 1 tell a similar story. Actin filaments form the core of theses structures and an array of actin filament contractility, cross linking, branching and severing/capping proteins are represented in each structure and regulate the dynamic changes in actin filament organization, and by extension, the shape and the half-life of these structures in response to cellular signals. There are also proteins in place that can link the cytoskeleton to integrins and/or the membrane, which would promote interactions with the extra-cellular matrix. Adaptor proteins are present, which could serve to bridge interactions between signaling proteins such as tyrosine and serine kinases or phosphatases with the cytoskeleton are prevalent. These signaling proteins are predicted to regulate the architecture of these dynamic structures.
Table 1.
Comparison of podosome and invadopodia associated proteins.
| Podosomes | Invadopodia | Function |
|---|---|---|
| Cytoskeletal components | ||
| Actin (Tarone et al. 1985) | Actin (Mueller et al. 1992) | Regulates cell contractility, motility and shape |
| Microtubules (Babb et al. 1997) | Unclear | Promote movement of motor proteins and vesicle transport |
| Intermediate Filaments (Correia et al. 1999) | Unclear | Cell shape and support |
| Actin filament contractility | ||
| Tropomyosin 4 (Burgstaller and Gimona, 2004) | Unknown | Regulates actin filament contraction |
| Caldesmon (Eves et al. 2006) | Caldesmon (Yoshio et al. 2007) | Regulates actin filament contraction |
| Calmodulin (Eves et al. 2006) | Calmodulin (Bourguignon et al. 1998) | Ca+2 and actin filament binding protein that can affect contraction |
| Myosin IIA ((Burgstaller and Gimona, 2004; Kopp et al. 2006) | Myosin II (implied in (Bourguignon et al. 1998) | Binds actin filaments, provides contractile force |
| Calponin (Gimona et al. 2003) | Unknown | Ca+2 binding protein and regulator of myosin II function |
| Actin filament cross linking | ||
| Sm22α (Transgelin) (Gimona et al. 2003) | Unknown | Regulates dynamic changes in actin filament cross linking and mesh-working |
| AFAP-110 (Gatesman et al. 2004) | AFAP-110 (Gatesman et al. 2004) | Regulates dynamic changes in actin filament cross linking and meshworking, src activating protein |
| Fimbrin (Messier et al. 1993; Babb et al. 1997) | Unknown | Actin filament cross linking protein |
| α-actinin (Chen, 1989) | α-actinin (Mueller et al. 1992) | Actin filament cross linking protein |
| Tensin (Hiura et al. 1995) | Tensin (Mueller et al.1992) | Actin filament cross linking protein |
| Palladin (Mykkanen et al. 2001) | Unknown | Actin filament cross linking, may link to VASP/mENA |
| Actin filament branching | ||
| VASP (Mykkanen et al. 2001, 2001; Spinardi and Marchisio, 2006) | Unknown | Actin filament barbed end binding protein, promote motility, reduce Arp2/3 formation |
| Arp2/3 (Mizutani et al. 2002) | Arp2/3 (Yamaguchi et al. 2005) | Actin filament polymerization and branching |
| WASp (Calle et al. 2006; Chellaiah, 2006) | WASp (Desai et al. 2008) | Modulates actin filament polymerization |
| N-Wasp (Mizutani et al. 2002) | N-Wasp (Yamaguchi et al. 2005) | Modulates actin filament polymerization |
| WIP (Anton et al. 2007; Chabadel et al. 2007) | Unknown | Modulator of WASp and N-WASp function |
| HSP90 (Park et al. 2005) | Unknown | Chaperones N-WASP and regulates its ability to affect actin filament branching |
| CDC42 (Tatin et al. 2006; Moreau et al. 2006) | CDC42 (Furmaniak-Kazmierczak et al. 2007) | Affector of actin filament branching and polymerization via Arp2/3 and N-WASp, Small GTP binding protein, regulates filopodia formation |
| Cortactin (Webb et al. 2006) | Cortactin (Bowden et al. 1999) | Promotes actin filament polymerization and branching as an Arp2/3 modulator |
| Actin filament severing/capping | ||
| Gelsolin (Biswas et al. 2004) | Unknown | Regulates actin filament severing and capping |
| Cofilin (Linder and Aepfelbacher, 2003) | Cofilin (Yamaguchi et al. 2005) | Regulates actin filament depolymerization and severing |
| Unknown | Nck (Yamaguchi et al. 2005) | Adaptor protein and regulator of actin filament polymerization |
| Actin filament bridging | ||
| Talin (Marchisio et al. 1988) | Talin (Mueller et al. 1992) | Links integrins to actin filaments |
| Vinculin (Chen, 1989) | Vinculin (Mueller et al. 1992) | Links integrins to actin filaments |
| Zyxin (Spinardi and Marchisio, 2006) | Unknown | Actin scaffolding protein, biosensor that can modulate transcriptional changes in response to adhesion |
| Unknown | Ankyrin (Bourguignon et al. 1998) | Links actin filaments with integral membrane proteins |
| Kindlins (Ussar et al. 2006) | Unknown | Links actin filaments to membrane |
| Intermediate Filaments | ||
| Vimentin (Correia et al. 1999) | Unknown | Intermediate filament protein, regulates positioning of organelles |
| Microtubules | ||
| Kinesin-3 (Kopp et al. 2006) | Unknown | Motor protein, vesicle transport |
| Cell Adhesion | ||
| β1, α3β1, α5β1, α6β1, αVβ3 Integrins (Marchisio et al. 1988; Spinardi and Marchisio, 2006; Calle et al. 2006) | β1, β3, αvβ3 Integrins (Deryugina et al. 2001) (Mueller et al. 1992; Nakahara et al. 1998) | Link cellular ventral membrane to the extracellular matrix |
| Unknown | Endoglin (Oxmann et al. 2008) | Transmembrane receptor part of TGFβ receptor complex and participates in cell adhesion |
| CD44 (Chabadel et al. 2007) | CD44 (Bourguignon et al. 1998) | Cell adhesion molecule that binds hyaluronic acid, MMP’s, collagen, osteopontin |
| Adaptor Proteins | ||
| Paxillin (Spinardi and Marchisio, 2006;Calle et al. 2006) | Paxillin (Mueller et al. 1992) | Fak binding partner. Transcriptional activator. |
| p130cas (Honda et al. 1998; Yogo et al. 2006) | Unknown | Src binding partner. Required for transformation and podosome formation |
| Tks5/FISH (Abram et al. 2003) | Tks5/FISH (Seals et al. 2005) | 5 SH3 domains, podosome ring protein |
| Eps8 (Goicoechea et al. 2006) | Unknown | Adaptor protein, binds receptors |
| Grb2 (Spinardi and Marchisio, 2006) | Unknown | Links to cell growth and RTK binding |
| Cbl (Bruzzaniti et al. 2005) | Cbl (Nam et al. 2007) | Adaptor, linked to ubiquitin machinery |
| STAT5 (Poincloux et al. 2007) | Unknown | Modulate transcription in response to cytosolic signaling |
| Calcitonin (Shyu et al. 2007) | Unknown | 32 amino acid polypeptide that binds Ca+2 and reduces local Ca+2 levels |
| Caveolin 1 (Colonna and Podesta, 2005) | Unknown | Scaffolding protein, links integrins to tyrosine kinases, component of lipid rafts |
| Tyrosine kinases | ||
| Src (Tarone et al. 1985) | Src (Chen, 1989) | PTK |
| Pyk2 (Chiusaroli et al. 2004) | Unknown | PTK, Fak-like |
| Csk (Howell and Cooper, 1994) | Unknown | Regulator of Src |
| Fak (Seals et al. 2005) | Fak (Hauck et al. 2002) | Integrin associated. Controversial association with invadopodia or podosomes |
| Tyrosine Phosphatases | ||
| Unknown | PTP1B (Cortesio et al. 2008) | Regulator of cSrc |
| PTP epsilon (Chiusaroli et al. 2004) | Unknown | Regulator of cSrc |
| Ser/thr kinases | ||
| Pak4 (Gringel et al. 2006) | Unknown | Effector of actin filament cross linking |
| Unknown | PKCmu (Bowden et al. 1999) | Effector of actin filament cross linking |
| Erk/Mek (Redondo-Munoz et al. 2006) | Erk/Mek (Furmaniak-Kazmierczak et al. 2007) | Effector of actin filament integrity |
| Effectors of small GTP binding proteins and related signaling | ||
| αPIX (Gringel et al. 2006) | PIX (Furmaniak-Kazmierczak et al. 2007) | Pak binding partner and guanine nucleotide exchange factor (GEF) |
| ASAP1 (Bharti et al. 2007) | ASAP1 (Nam et al. 2007) | Arf GAP that uses lipids to become active (bind PH domain) |
| p190RhoGap (Burgstaller and Gimona, 2004) | p190RhoGap (Nakahara et al. 1998) | Negatively Regulates Rho function as a GAP |
| Unknown | Rock II (Vishnubhotla et al. 2007) | Positively Regulates Rho function |
| Dynamin2 (Ochoa et al. 2000) | Dynamin 2 (McNiven et al. 2004) | Affect vesicles and membrane invaginations that secrete MMPs, GTPase |
| Endophilin2 (Ochoa et al. 2000) | Unknown | Dynamin 2 and synaptojanin binding partner |
| Lipid signaling | ||
| SHIP-2 (Yogo et al. 2006) | Unknown | Phosphoinositide 5′ phosphatase with SH2 domain |
| Unknown | Synaptojanin 2 (Chuang et al. 2004) | Phosphoinositide 5′-phosphatase, vesicle uncoating, effector of Rac1 |
| CIN85 (Gaidos et al. 2007) | CIN85 (Nam et al. 2007) | Component of endocytic vesicles and binds Arf6 and ASAP1 (Arf6 GAP), associates with Cbl E3- ligase |
| Proteases | ||
| MT1-MMP (Sato et al. 1997) | MT1-MMP (Chen and Wang, 1999) | Membrane bound metalloproteinase |
| ADAM12 (Abram et al. 2003) | Unknown | A type of MMP |
| MMP2 (Tatin et al. 2006) | MMP2 (Deryugina et al. 2002) | Soluble metalloproteinase, colla-genase and gelatinase |
| MMP9 (Linder, 2007) | MMP9 (Linder, 2007) | Soluble metalloproteinase, colla-genase and gelatinase |
| Calpain 2 (Calle et al. 2006) | Calpain 2 (Cortesio et al. 2008) | Ca+2 dependent cysteine protease |
| Unknown | Seprase (Ghersi et al. 2006) | Gelatinase and serine protease |
| Unknown | DPP4/CD26 (O’Brien and O’Connor, 2008) | Broad spectrum protease, degrades incretins |
| Unknown | uPAR (Kindzelskii et al. 2004) | Can link to integrins via UPARAP, binds uPA |
| Unknown | uPA (urokinase) (Kindzelskii et al. 2004) | Serine protease |
| Unknown | Type II serine protease (O’Brien and O’Connor, 2008) | Serine protease |
| Unknown | Invadolysin (likely) (McHugh et al. 2004) | Metalloprotease, cleaves lamin |
| Unknown | Legumain (Liu et al. 2003) | Cysteine protease |
Unknown means unknown.
Vesicle Transport and Podosome/Invadopodia Formation
Both podosomes and invadopodia contain small GTP binding proteins and regulatory proteins that that control their function. Within this class of proteins, dynamin and endophilin stand out as proteins that could bridge interactions of GTP binding proteins with membranes and promote the formation of a secretory canaliculi or the docking of vesicle membranes. Lastly, a variety of proteases are apparent, and most of them have been detected in invadopodia. To this end it is noteworthy that in invadopodia, TIMP-2 is able to block protease activity whereas TIMP-1 was not, indicating that invadopodia are more dependent upon membrane bound proteases than secreted proteases (Chen and Wang, 1999). Interestingly, both podosomes and invadopodia formation may require exocytosis, as brefeldin A and Exo 1 will block the formation of invadopodia and podosomes (Ayala et al. 2006; Walker et al. 2007). In this regard, it is also noteworthy that several proteins found associated with podosomes or invadopodia are normally associated with perinuclear vesicles in quiescent, normal cells, including cSrc, cortactin, Pyk2, dynamin 2, ADAM12, MT1-MMP and Tks5 (Kaplan et al. 1992; Redmond et al. 1992; Howell and Cooper, 1994; Fincham et al. 1996; Nicoziani et al. 2000; Hougaard et al. 2000; Kang et al. 2001). Thus, we would speculate that when cells make a decision to form a podosome or an invadopodia, outside-in signals could stimulate the movement of vesicles to the ventral membrane which in turn would deliver ‘cargo’ or protein components necessary for the formation of these structures. As podosomes and invadopodia will form rapidly, in less than 15 minutes after treatment with phorbol esters, and further, the formation of these structures do not require de novo protein synthesis (Linder and Aepfelbacher, 2003), and vesicle transport can be achieved rapidly and in less than 15 minutes, it may be possible that vesicle transport could facilitate the trafficking of podosome or invadopodia-associated proteins to the ventral membrane, which would allow construction of these structures and could offer a novel mechanism for the formation of an invasive structure.
Invadopodia and Breast Cancer
Breast cancer cells will generate invadopodia in response to signals stimulated by growth factors, phorbol esters or interactions with the extracellular matrix (Yamaguchi et al. 2005; Yamaguchi and Condeelis, 2007). MDA-MB-231 breast carcinoma cells are an excellent system for studying invasion and metastasis and they will form invadopodia in response to stimuli. It is noteworthy that many proteins required for or associated with invadopodia formation are also associated with breast cancer progression, either through activation of signaling potential or changes in expression levels. In MDA-MB-231 cells, the expression levels and the signaling potential of the small GTP binding protein Arf6 was required for breast cancer invasion (Hashimoto et al. 2004; Onodera et al. 2005; Nam et al. 2007). Interestingly, Arf6 will relay signals from phorbol esters that promote phospholipase D activation and phosphatidic acid production, the latter of which is a component of vesicle membranes (Xu et al. 2003). Arf6 will couple with RalA, which can regulate the transport of vesicles to the ventral membrane (Caumont et al. 1998). Thus, it may be possible that Arf6 signaling is required for promoting phorbol ester or growth factor directed transport of vesicles to the ventral membrane that promote invadopodia formation. Another important signaling protein in breast cancer and invadopodia formation is cSrc, which exists on perinuclear vesicles and becomes activated upon trafficking to the membrane (Sandilands et al. 2004). cSrc is activated in breast cancer and will promote breast cancer formation in animal models. cSrc activation is a requirement for podosome and invadopodia formation (Linder, 2007). Indeed, the initial description of podosomes was associated with expression of the constitutively activated v-Src in fibroblasts (Tarone et al. 1985; Marchisio et al. 1988; Gavazzi et al. 1989). cSrc will phosphorylate a number of proteins on tyrosine and many of those substrates are relevant to breast cancer progression and are also found associated with both podosomes and invadopodia. Interestingly, phosphotyrosine signals will coalesce in podosomes and invadopodia (Kanner et al. 1991; Bowden et al. 2006). To this end, it is noteworthy that expression of the cSrc substrates cortactin and Tks5 are required for podosome formation (Seals et al. 2005; Webb et al. 2006). cSrc appears to play a role in podosome turnover and both the cSrc regulating protein CSK, as well as the tyrosine phosphatase PTP1B are required for podosome formation, likely by regulating dynamic changes in cSrc activity (Howell and Cooper, 1994; Cortesio et al. 2008). Each of these proteins are upregulated in breast cancer tissues and thus, could be well positioned to promote the formation of invasive structures and progression to an invasive phenotype. Thus, the protein components of podosomes and invadopodia may be very relevant to breast cancer.
Podosome and Invadopodia Proteins May React to the Tumor Microenvironment
Other podosome and invadopodia associated proteins may also play important roles in the interpretation of outside-in signals that promote invasive potential. In the Met-1 breast cancer model system, the adhesion protein CD44 plays a role in invadopodia formation by linking ankyrin to the contractile actomyosin system (Bourguignon et al. 1998). In this regard, it may be possible that the adhesion aspects of podosomes, which do appear to differentiate them from invadopodia, could be regulated by contractile forces, much like focal adhesion plaques require negative contractile forces to promote adhesion (Dorfleutner et al. 2007). Studies in the MDA-MB-231 breast cancer cell line demonstrated that invadopodia will form in a stepwise fashion and promote invasive activity of breast carcinoma cells via expression of MT1-MMP (Kelly et al. 1998; Artym et al. 2006).
The ability of breast cancer cell lines to promote invasion and degradation of the extracellular matrix also correlated with an ability to phagocytose digested extracellular matrix proteins (Coopman et al. 1998). This function may be regulated by endophilin-2, SHIP-2, CIN85 and/or synaptojanin-2. SHIP-2 is an inositol 5-phosphatase found in podosomes, which removes the 5′ phosphate from phosphatidlyinositol-3,4,5-phosphate (Pesesse et al. 1998; Erneux et al. 1998). SHIP-2 is able to down regulate Fcγ-receptor mediated phagocytosis (independent of SHIP-1) and does this via an ability to down regulate Rac activity (Ai et al. 2006). Similarly, synaptojanin-2 is an inositol 5′-phosphatase found in invadopodia that regulates endocytic vesicle trafficking (Singer-Kruger et al. 1998). Synaptojanin-2 will bind to activated Rac and negatively regulate endocytosis (Malecz et al. 2000). Synaptojanin-2 is recruited to the membrane and stabilized by interactions with endophilin, which promotes clathrin-mediated endocytosis (Song and Zinsmaier, 2003). Interestingly, endophilin will also bridge interactions with dynamin 2 in podosomes (Ochoa et al. 2000) as well as with CIN85 (Petrelli et al. 2002). Here, a CIN85/endophilin complex was shown to affect changes in membrane curvature, which is consistent with a role for dynamin 2. Thus, the SHIP2 and/or synaptojanin-2/endophilin/dynamin-2/CIN85 proteins may play an important role in regulating the phagocytic activity associated with invasion by invadopodia as well as changes in membrane curvature that may promote vesicle trafficking or protease release. By this rationale, their appearance and association with invadopodia may be consistent with the function of these invasive structures. Further, it could be speculated that both invadopodia and podosomes utilize these signaling proteins in a similar manner to promote invasive potential. If true, then each of these proteins might be interesting drug targets that could be exploited to control breast cancer invasion.
Summary
We have contrasted the differences and similarities between podosomes and invadopodia by cataloging the proteins found in these invasive structures and comparing their known and predicted functions for normal cells (podosomes) and carcinoma cells (invadopodia) in an effort to address the hypothesis that these two invasive structures may be related. To date, their is no evidence to indicate that invadopodia are derived from podosomes, or that each of these structures are derived from a common precursor structure. The major differences between the two are size, dependence on microtubular structures and subcellular localization upon the ventral membrane, whereby invadopodia are found below the Golgi bodies, while podosomes can be found either centrally located or at the leading edge of a migrating cell (Gimona et al. 2008). However, we speculate that given the common cellular signals that regulate their construction, common protein components and architecture, common size, shape and ventral membrane location, that these two structures are related. Further, a number of studies have shown the requirement for specific protein components in podosome and invadopodium formation and these same proteins are required for breast carcinoma cell invasion and are also expressed at high levels in breast cancer tissues. Probably the most interesting of these results were those done by Courtneidge and colleagues who have shown quite nicely the correlation between Tks5 in expression in breast cancer cells and its role in podosome formation and invasion (Seals et al. 2005). Future studies should focus on determining if theses structures are related and their role in breast cancer invasion, which will foster studies designed to create inhibitors that block invadopodia and podosome formation that may prevent breast carcinoma cells from invading.
Acknowledgments
This work was supported by a grant from the NIH, CA06731 (DCF) and RR166640 (DCF and JMC) as well as a training grant from the WVEpscor (DV). We thank Scott Weed for many helpful discussions.
References
- Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 2003;278:16844–51. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- Ai J, Maturu A, Johnson W, Wang Y, Marsh CB, Tridandapani S. The inositol phosphatase SHIP-2 down-regulates FcgammaR-mediated phagocytosis in murine macrophages independently of SHIP-1. Blood. 2006;107:813–20. doi: 10.1182/blood-2005-05-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton IM, Jones GE, Wandosell F, Geha R, Ramesh N. WASP-interacting protein (WIP): working in polymerisation and much more. Trends Cell. Biol. 2007;17:555–62. doi: 10.1016/j.tcb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Ayala I, Baldassarre M, Caldieri G, Buccione R. Invadopodia: a guided tour. Eur. J. Cell. Biol. 2006;85:159–64. doi: 10.1016/j.ejcb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Babb SG, Matsudaira P, Sato M, Correia I, Lim SS. Fimbrin in podosomes of monocyte-derived osteoclasts. Cell. Motil. Cytoskeleton. 1997;37:308–25. doi: 10.1002/(SICI)1097-0169(1997)37:4<308::AID-CM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell. 2003;14:1074–84. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S, et al. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol. Cell. Biol. 2007;27:8271–83. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas RS, Baker D, Hruska KA, Chellaiah MA. Polyphosphoinositides-dependent regulation of the osteoclast actin cytoskeleton and bone resorption. BMC. Cell. Biol. 2004;5:19. doi: 10.1186/1471-2121-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu D, Zhu H. CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Front Biosci. 1998;3:d637–d649. doi: 10.2741/a308. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell. Biol. 2001;63:613–27. doi: 10.1016/s0091-679x(01)63033-4. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, Mueller SC. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp. Cell. Res. 2006;312:1240–53. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A, Neff L, Sanjay A, Horne WC, De CP, Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol. Biol. Cell. 2005;16:3301–13. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J. Cell. Sci. 2004;117:223–31. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J. Cell. Sci. 2006;119:2375–85. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- Caumont AS, Galas MC, Vitale N, Aunis D, Bader MF. Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem. 1998;273:1373–9. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- Chabadel A, Banon-Rodriguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, Jurdic P, Anton IM, Saltel F. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol. Biol. Cell. 2007;18:4899–910. doi: 10.1091/mbc.E07-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah MA. Regulation of podosomes by integrin alphavbeta3 and Rho GTPase-facilitated phosphoinositide signaling. Eur. J. Cell. Biol. 2006;85:311–7. doi: 10.1016/j.ejcb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 1989;251:167–85. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann. N. Y. Acad. Sci. 1999;878:361–71. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- Chiusaroli R, Knobler H, Luxenburg C, Sanjay A, Granot-Attas S, Tiran Z, Miyazaki T, Harmelin A, Baron R, Elson A. Tyrosine phosphatase epsilon is a positive regulator of osteoclast function in vitro and in vivo. Mol. Biol. Cell. 2004;15:234–44. doi: 10.1091/mbc.E03-04-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004;64:8271–5. doi: 10.1158/0008-5472.CAN-04-2097. [DOI] [PubMed] [Google Scholar]
- Colonna C, Podesta EJ. ACTH-induced caveolin-1 tyrosine phosphorylation is related to podosome assembly in Y1 adrenal cells. Exp. Cell. Res. 2005;304:432–42. doi: 10.1016/j.yexcr.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Coopman PJ, Do MT, Thompson EW, Mueller SC. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin. Cancer Res. 1998;4:507–15. [PubMed] [Google Scholar]
- Correia I, Chu D, Chou YH, Goldman RD, Matsudaira P. Integrating the actin and vimentin cytoskeletons. adhesion-dependent formation of fimbrinvimentin complexes in macrophages. J. Cell. Biol. 1999;146:831–42. doi: 10.1083/jcb.146.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J. Cell. Biol. 2008;180:957–71. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp. Cell. Res. 2001;263:209–23. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J. Biol. Chem. 2002;277:9749–56. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- Desai B, Ma T, Chellaiah MA. Invadopodia and matrix degradation: a new property of prostate cancer cells during migration and invasion. J. Biol. Chem. 2008 doi: 10.1074/jbc.M709401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell. 2003;14:407–16. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell. Sci. 2005;118:2901–11. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J. Cell. Physiol. 2007;213:740–9. doi: 10.1002/jcp.21143. [DOI] [PubMed] [Google Scholar]
- Erneux C, Govaerts C, Communi D, Pesesse X. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim. Biophys. Acta. 1998;1436:185–99. doi: 10.1016/s0005-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- Eves R, Webb BA, Zhou S, Mak AS. Caldesmon is an integral component of podosomes in smooth muscle cells. J. Cell. Sci. 2006;119:1691–702. doi: 10.1242/jcs.02881. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J. Cell. Biol. 1996;135:1551–64. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Cote GP. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ. Res. 2007;100:1328–36. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- Gaidos G, Soni S, Oswald DJ, Toselli PA, Kirsch KH. Structure and function analysis of the CMS/CIN.85 protein family identifies actin-bundling properties and heterotypic-complex formation. J. Cell. Sci. 2007;120:2366–77. doi: 10.1242/jcs.004333. [DOI] [PubMed] [Google Scholar]
- Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC. Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol. Cell. Biol. 2004;24:7578–97. doi: 10.1128/MCB.24.17.7578-7597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Nermut MV, Marchisio PC. Ultrastructure and gold-immunolabelling of cell-substratum adhesions (podosomes) in RSV-transformed BHK cells. J. Cell. Sci. 1989;94(1):85–99. doi: 10.1242/jcs.94.1.85. [DOI] [PubMed] [Google Scholar]
- Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT. The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res. 2006;66:4652–61. doi: 10.1158/0008-5472.CAN-05-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell. Biol. 2008 doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Gimona M, Kaverina I, Resch GP, Vignal E, Burgstaller G. Calponin repeats regulate actin filament stability and formation of podosomes in smooth muscle cells. Mol. Biol. Cell. 2003;14:2482–91. doi: 10.1091/mbc.E02-11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J. Cell. Sci. 2006;119:3316–24. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, Linder S. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J. Cell. Physiol. 2006;209:568–79. doi: 10.1002/jcp.20777. [DOI] [PubMed] [Google Scholar]
- Gu Z, Kordowska J, Williams GL, Wang CL, Hai CM. Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells. Exp. Cell. Res. 2007;313:849–66. doi: 10.1016/j.yexcr.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Onodera Y, Hashimoto A, Tanaka M, Hamaguchi M, Yamada A, Sabe H. Requirement for Arf6 in breast cancer invasive activities. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6647–52. doi: 10.1073/pnas.0401753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 2002;277:12487–90. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- Hiura K, Lim SS, Little SP, Lin S, Sato M. Differentiation dependent expression of tensin and cortactin in chicken osteoclasts. Cell. Motil. Cytoskeleton. 1995;30:272–84. doi: 10.1002/cm.970300405. [DOI] [PubMed] [Google Scholar]
- Honda H, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 1998;19:361–5. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Hougaard S, Loechel F, Xu X, Tajima R, Albrechtsen R, Wewer UM. Trafficking of human ADAM 12-L: retention in the trans-Golgi network. Biochem. Biophys. Res. Commun. 2000;275:261–7. doi: 10.1006/bbrc.2000.3295. [DOI] [PubMed] [Google Scholar]
- Howell BW, Cooper JA. Csk suppression of Src involves movement of Csk to sites of Src activity. Mol. Cell. Biol. 1994;14:5402–11. doi: 10.1128/mcb.14.8.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T, Yi J, Guo A, Wang X, Overall CM, Jiang W, Elde R, Borregaard N, Pei D. Subcellular distribution and cytokine- and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. J. Biol. Chem. 2001;276:21960–8. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- Kanner SB, Reynolds AB, Wang HC, Vines RR, Parsons JT. The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 1991;10:1689–98. doi: 10.1002/j.1460-2075.1991.tb07693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KB, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J. Cell. Biol. 1992;118:321–33. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T, Kechelava S, Rozypal TL, West KW, Korourian S. Seprase, a membrane-bound protease, is overexpressed by invasive ductal carcinoma cells of human breast cancers. Mod. Pathol. 1998;11:855–63. [PubMed] [Google Scholar]
- Kindzelskii AL, Amhad I, Keller D, Zhou MJ, Haugland RP, Garni-Wagner BA, Gyetko MR, Todd RF, Petty HR. Pericellular proteolysis by leukocytes and tumor cells on substrates: focal activation and the role of urokinase-type plasminogen activator. Histochem. Cell. Biol. 2004;121:299–310. doi: 10.1007/s00418-004-0639-3. [DOI] [PubMed] [Google Scholar]
- Kopp P, Lammers R, Aepfelbacher M, Woehlke G, Rudel T, Machuy N, Steffen W, Linder S. The kinesin KIF1C and micro-tubule plus ends regulate podosome dynamics in macrophages. Mol. Biol. Cell. 2006;17:2811–23. doi: 10.1091/mbc.E05-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell. Biol. 2007;17:107–17. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell. Biol. 2003;13:376–85. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Linder S, Hufner K, Wintergerst U, Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell. Sci. 2000;113(23):4165–76. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- Liu C, Sun C, Huang H, Janda K, Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003;63:2957–64. [PubMed] [Google Scholar]
- Malecz N, McCabe PC, Spaargaren C, Qiu R, Chuang Y, Symons M. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr. Biol. 2000;10:1383–6. doi: 10.1016/s0960-9822(00)00778-8. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Bergui L, Corbascio GC, Cremona O, D’Urso N, Schena M, Tesio L, Caligaris-Cappio F. Vinculin, talin, and integrins are localized at specific adhesion sites of malignant B. lymphocytes. Blood. 1988;72:830–3. [PubMed] [Google Scholar]
- McHugh B, Krause SA, Yu B, Deans AM, Heasman S, McLaughlin P, Heck MM. Invadolysin: a novel, conserved metalloprotease links mitotic structural rearrangements with cell migration. J. Cell. Biol. 2004;167:673–86. doi: 10.1083/jcb.200405155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA, Baldassarre M, Buccione R. The role of dynamin in the assembly and function of podosomes and invadopodia. Front Biosci. 2004;9:1944–53. doi: 10.2741/1348. [DOI] [PubMed] [Google Scholar]
- Messier JM, Shaw LM, Chafel M, Matsudaira P, Mercurio AM. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell. Motil Cytoskeleton. 1993;25:223–33. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–74. [PubMed] [Google Scholar]
- Moreau V, Tatin F, Varon C, Anies G, Savona-Baron C, Genot E. Cdc42-driven podosome formation in endothelial cells. Eur. J. Cell. Biol. 2006;85:319–25. doi: 10.1016/j.ejcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Yeh Y, Chen WT. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J. Cell. Biol. 1992;119:1309–25. doi: 10.1083/jcb.119.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, Suila H, Carpen O. Characterization of human palladin, a microfilament-associated protein. Mol. Biol. Cell. 2001;12:3060–73. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Biol. Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- Nam JM, Onodera Y, Mazaki Y, Miyoshi H, Hashimoto S, Sabe H. CIN85, a Cbl-interacting protein, is a component of AMAP1-mediated breast cancer invasion machinery. EMBO J. 2007;26:647–56. doi: 10.1038/sj.emboj.7601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoziani P, Vilhardt F, Llorente A, Hilout L, Courtoy PJ, Sandvig K, van DB. Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol. Biol. Cell. 2000;11:481–95. doi: 10.1091/mbc.11.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien P, O’Connor BF. Seprase: An overview of an important matrix serine protease. Biochim. Biophys. Acta. 2008 doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Ochoa GC, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell. Biol. 2000;150:377–89. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–73. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxmann D, Held-Feindt J, Stark AM, Hattermann K, Yoneda T, Mentlein R. Endoglin expression in metastatic breast cancer cells enhances their invasive phenotype. Oncogene. 2008 doi: 10.1038/sj.onc.1211025. [DOI] [PubMed] [Google Scholar]
- Park SJ, Suetsugu S, Takenawa T. Interaction of HSP90 to N.-WASP leads to activation and protection from proteasome-dependent degradation. EMBO J. 2005;24:1557–70. doi: 10.1038/sj.emboj.7600586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesesse X, Moreau C, Drayer AL, Woscholski R, Parker P, Erneux C. The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett. 1998;437:301–3. doi: 10.1016/s0014-5793(98)01255-1. [DOI] [PubMed] [Google Scholar]
- Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–90. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- Poincloux R, Cougoule C, Daubon T, Maridonneau-Parini I, Le C. Tyrosine-phosphorylated STAT5 accumulates on podosomes in Hck-transformed fibroblasts and chronic myeloid leukemia cells. J. Cell. Physiol. 2007;213:212–20. doi: 10.1002/jcp.21112. [DOI] [PubMed] [Google Scholar]
- Redmond T, Brott BK, Jove R, Welsh MJ. Localization of the viral and cellular Src kinases to perinuclear vesicles in fibroblasts. Cell. Growth Differ. 1992;3:567–76. [PubMed] [Google Scholar]
- Redondo-Munoz J, Escobar-Diaz E, Samaniego R, Terol MJ, Garcia-Marco JA, Garcia-Pardo A. MMP-9 in B-cell chronic lymphocytic leukemia is up-regulated by alpha4beta1 integrin or CXCR4 engagement via distinct signaling pathways, localizes to podosomes, and is involved in cell invasion and migration. Blood. 2006;108:3143–51. doi: 10.1182/blood-2006-03-007294. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell. Biol. 2003;5:711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endo-some-mediated delivery to the membrane. Dev. Cell. 2004;7:855–69. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Sato T, del Carmen OM, Hou P, Heegaard AM, Kumegawa M, Foged NT, Delaisse JM. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J. Cell. Sci. 1997;110(5):589–96. doi: 10.1242/jcs.110.5.589. [DOI] [PubMed] [Google Scholar]
- Seals DF, Azucena EF, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–65. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Shyu JF, Shih C, Tseng CY, Lin CH, Sun DT, Liu HT, Tsung HC, Chen TH, Lu RB. Calcitonin induces podosome disassembly and detachment of osteoclasts by modulating Pyk2 and Src activities. Bone. 2007;40:1329–42. doi: 10.1016/j.bone.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B, Nemoto Y, Daniell L, Ferro-Novick S, De CP. Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J. Cell. Sci. 1998;111(22):3347–56. doi: 10.1242/jcs.111.22.3347. [DOI] [PubMed] [Google Scholar]
- Song W, Zinsmaier KE. Endophilin and synaptojanin hook up to promote synaptic vesicle endocytosis. Neuron. 2003;40:665–7. doi: 10.1016/s0896-6273(03)00726-8. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Marchisio PC. Podosomes as smart regulators of cellular adhesion. Eur. J. Cell. Biol. 2006;85:191–4. doi: 10.1016/j.ejcb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Strohmaier AR, Porwol T, Acker H, Spiess E. Three-dimensional organization of microtubules in tumor cells studied by confocal laser scanning microscopy and computer-assisted deconvolution and image reconstruction. Cells Tissues. Organs. 2000;167:1–8. doi: 10.1159/000016760. [DOI] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell. Res. 1985;159:141–57. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J. Cell. Sci. 2006;119:769–81. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- Ussar S, Wang HV, Linder S, Fassler R, Moser M. The Kindlins: subcellular localization and expression during murine development. Exp. Cell. Res. 2006;312:3142–51. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Vishnubhotla R, Sun S, Huq J, Bulic M, Ramesh A, Guzman G, Cho M, Glover SC. ROCK-II mediates colon cancer invasion via regulation of MMP-2 and MMP-13 at the site of invadopodia as revealed by multiphoton imaging. Lab. Invest. 2007;87:1149–58. doi: 10.1038/labinvest.3700674. [DOI] [PubMed] [Google Scholar]
- Walker VG, Ammer A, Cao Z, Clump AC, Jiang BH, Kelley LC, Weed SA, Zot H, Flynn DC. PI3K activation is required for PMA-directed activation of cSrc by AFAP-110. Am. J. Physiol. Cell. Physiol. 2007;293:C119–C132. doi: 10.1152/ajpcell.00525.2006. [DOI] [PubMed] [Google Scholar]
- Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: roles of the protein interaction domains. Exp. Cell. Res. 2006;312:760–9. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Xu L, Frankel P, Jackson D, Rotunda T, Boshans RL, Souza-Schorey C, Foster DA. Elevated phospholipase D activity in H-Ras- but not K-Ras-transformed cells by the synergistic action of RalA and ARF6. Mol. Cell. Biol. 2003;23:645–54. doi: 10.1128/MCB.23.2.645-654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell. Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo K, Mizutamari M, Mishima K, Takenouchi H, Ishida-Kitagawa N, Sasaki T, Takeya T. Src homology 2 (SH2)-containing 5′-inositol phosphatase localizes to podosomes, and the SH2 domain is implicated in the attenuation of bone resorption in osteoclasts. Endocrinology. 2006;147:3307–17. doi: 10.1210/en.2005-1309. [DOI] [PubMed] [Google Scholar]
- Yoshio T, Morita T, Kimura Y, Tsujii M, Hayashi N, Sobue K. Caldesmon suppresses cancer cell invasion by regulating podo-some/invadopodium formation. FEBS Lett. 2007;581:3777–82. doi: 10.1016/j.febslet.2007.06.073. [DOI] [PubMed] [Google Scholar]