Abstract
Receptor-mediated endocytosis is a dynamic process that is crucial for maintaining plasma membrane composition and controlling cell-signaling pathways. A variety of entry routes have evolved to ensure that the vast array of molecules on the cell surface can be differentially internalized by endocytosis. This diversity has extended to include a growing list of endocytic adaptor proteins, which are thought to initiate the internalization process. The key function of adaptors is to select the proteins that should be removed from the cell surface. Thus, they have a central role in defining the physiology of a cell. This has made the study of adaptor proteins a very active area of research that is ripe for exciting future discoveries. Here, we review recent work on how adaptors mediate endocytosis and address the following questions: what characteristics define an endocytic adaptor protein? What roles do these proteins fulfill in addition to selecting cargo and how might adaptors function in clathrin-independent endocytic pathways? Through the findings discussed in this Commentary, we hope to stimulate further characterization of known adaptors and expansion of the known repertoire by identification of new adaptors.
Key words: Adaptor, Clasp, Clathrin
Introduction
Endocytosis, the process of internalization of plasma membrane and extracellular materials, is required for numerous biological events ranging from maintaining membrane composition to controlling cell-signaling pathways (Le Roy and Wrana, 2005; Polo and Di Fiore, 2006). Because this process is important to many cellular functions, a variety of routes have evolved to accomplish these tasks (Howes et al., 2010).
Among the different forms of endocytosis, the clathrin-mediated pathway is the one that has been most extensively studied and is well-understood (Conner and Schmid, 2003). This highly coordinated process begins when membrane-associated complexes of adaptor proteins and clathrin select transmembrane cargos at the plasma membrane and, thereby, initiate the formation of clathrin-coated pits (CCPs) (Ehrlich et al., 2004). The forming pit is then joined by accessory and regulatory components that help to stabilize the early endocytic pit, and allow for recruitment of scission factors. Recruitment of these proteins in a temporally regulated manner leads to deformation of the plasma membrane into a cargo-filled CCP that is subsequently pinched-off and propelled into the cytoplasm (Fig. 1). The variety of transmembrane cargos concentrated into clathrin-coated vesicles at the plasma membrane requires the use of diverse sorting signals (Traub, 2009), and diverse adaptors to recognize them. This variety prevents competition for entry and allows plasticity in the selection of cargo for internalization.
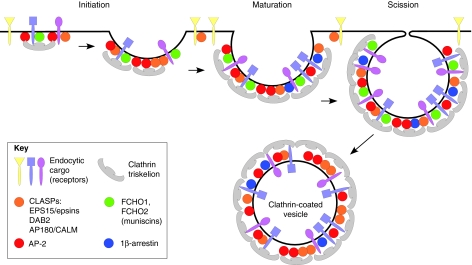
Fig. 1.
Diagram of clathrin-dependent endocytosis. Clathrin-dependent endocytosis begins when adaptor and clathrin complexes associate with cognate cargo, thus initiating the formation of a coated pit. As the pit matures, additional adaptor and scaffold proteins join the pit, providing a structural platform that helps regulate and synchronize interactions between the adaptors and the other endocytic proteins. Increasing membrane deformation attributed to BAR-domain-containing proteins (such as the muniscins) as well as from forces generated by polymerization of cytoskeletal elements, eventually leads to vesicle scission, which liberates a clathrin-coated vesicle into the cytoplasm (Maldonado-Baez and Wendland, 2006). Examples of different cargos are given in different colors. Blue and purple cargos contain sorting motifs that bind to clathrin-associated adaptors. This type of cargo can therefore be incorporated into the forming CCP. Yellow cargo, by contrast, is internalized through an alternative clathrin-independent pathway that might involve a select subset of CLASP adaptors.
The term endocytic adaptor was coined in 1981 (Pearse and Bretscher, 1981) to describe a yet-to-be-identified group of proteins that mediate the interactions between ‘address tickets’ on cargo proteins and clathrin, as clathrin cannot bind directly to cargo or membranes. At the time, it was hypothesized that these adaptors have three characteristics: interact with clathrin, recognize cargo ‘address tickets’, and perhaps have some signal directing them towards the desired organelle. The characterization of adaptor proteins began with the purification and identification of HA-2 (now known as AP-2) from clathrin-coated vesicles in 1984 (Pearse and Robinson, 1984). Amazingly, more than 25 years later, we are still discovering new features of this protein complex, and research on adaptors has led to the discoveries of over a dozen different types, each seemingly having a unique role in endocytosis.
Endocytic adaptors are divided into two main groups: multimeric adaptor proteins (for example, AP-2) and monomeric or non-classic adaptor proteins, such as the clathrin-associated sorting proteins (CLASPs). In this Commentary, we will focus on summarizing recent insights into well-known adaptor proteins, describing newly identified adaptor types and reviewing the molecular interactions in which adaptor proteins are involved. We will also examine the role of these adaptors in clathrin-dependent endocytosis, as well as their potential involvement in clathrin-independent pathways.
Binding motifs in endocytic adaptors
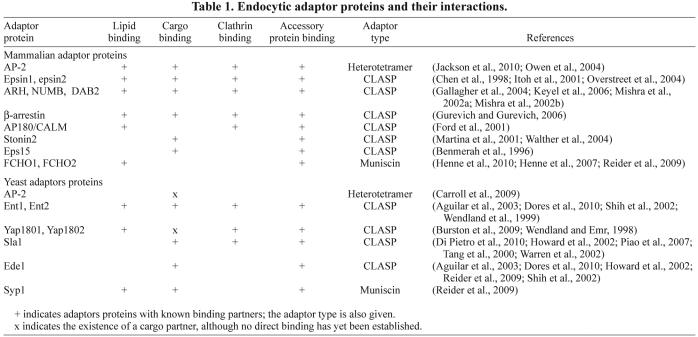
Endocytic adaptor proteins vary greatly in size (~300–3000 amino acids) and structure, but possess similar properties. Most of the clathrin adaptor proteins contain regions that interact with some or all of four types of binding partner: lipids, cargo, clathrin and accessory proteins (Table 1 and Fig. 2) (Owen et al., 2004). Cooperation between these interactions is required for efficient recruitment of adaptors to the plasma membrane and is crucial for progression of the internalization process. In recent years, many studies have focused on characterizing how endocytic adaptors bind to the plasma membrane (i.e. lipids), select cargo and recruit clathrin, and how these functions are coupled to promote deformation of the plasma membrane. The findings will be discussed here, beginning with the clathrin-binding motifs, followed by interactions with lipid, cargo and accessory proteins.
Table 1.
Endocytic adaptor proteins and their interactions.
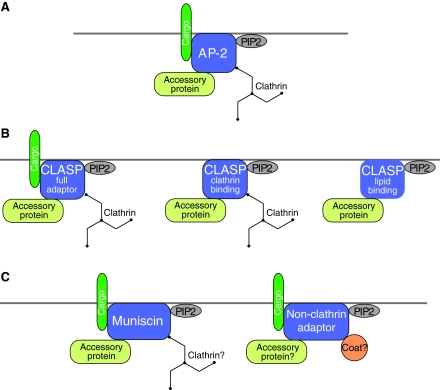
Fig. 2.
Schematic representations of different classes of adaptor proteins and their association with respective binding partners. (A) AP-2 and CLASP (full adaptor) proteins bind to lipids, cargo, accessory proteins and clathrin. (B) By contrast, other CLASP proteins are known to bind only some of these four partners. For example, some may not bind cargo, whereas others may not bind directly to clathrin. (C) The recently described muniscin adaptors bind cargo (for example, the yeast homolog Syp1), as well as lipids and accessory proteins. Another, new class of adaptors that selects cargo for internalization through non-clathrin pathways has been discovered recently. These non-clathrin adaptors proteins bind to cargo and lipids, and some may also, ultimately, associate with currently unknown coat and accessory proteins. PIP2, PtdIns (4,5)P2.
Clathrin-binding motifs
Early research identified a short consensus sequence, present in adaptor proteins, that was sufficient for binding to the N-terminal domain of the clathrin heavy chain (Dell'Angelica et al., 1998; Krupnick et al., 1997; Ramjaun and McPherson, 1998; Shih et al., 1995). This sequence, L[L/I][D/E/N][L/F][D/E], was termed the ‘clathrin box’ motif. Structural information and alignment of sequences present in other known clathrin-binding proteins led to the refinement of this sequence as LΦpΦ−, where ‘Φ’ denotes a bulky hydrophobic residue, ‘p’ a polar residue and ‘−’ a negatively charged residue (ter Haar et al., 2000). However, since the original definition of the clathrin box motif, several variant clathrin box motifs and non-canonical-binding motifs have been identified (Lafer, 2002).
A recent report by Lafer and colleagues (Zhuo et al., 2010) shed more light on clathrin-binding motifs. Using NMR and ultracentrifugation analysis, they identified two clathrin-binding motifs within the largely unstructured C-terminal domain of the adaptor protein clathrin coat assembly protein AP180 (also known as SNAP91) that both can bind weakly to the clathrin terminal domain. These two noncanonical clathrin-binding motifs DLF and DLL (Morgan et al., 2000), and ten additional, similar sequences are evenly dispersed throughout the C-terminal region of AP180. Interestingly, these binding motifs form a β-turn-like structure both in solution and when bound to substrate. These observations suggest that weak binding by many prestructured β-turn-like clathrin-binding regions allows for efficient recruitment of clathrin to endocytic sites.
In an assembled clathrin coat, three terminal domains are positioned under each vertex of the clathrin coat (Kirchhausen, 2000). In turn, many clathrin-binding proteins are known to contain two or more clathrin-binding motifs within disordered regions. Thus, it has been postulated that many clathrin-binding proteins recruit clathrin through a series of multivalent interactions, with terminal domains present at coat vertices. All structural information to date indicates that clathrin-binding motifs adopt a β-turn-like, or kinked, structure when bound to clathrin (Miele et al., 2004; ter Haar et al., 2000; Zhuo et al., 2010). The new data from the Lafer group indicates that these regions are prestructured in solution, allowing for faster association rates. This leads to the interesting idea that, rather than the precise sequence, it is instead the structure that better predicts clathrin-binding motifs. Clathrin-binding regions may only require a stretch of surface exposed acidic and bulky hydrophobic residues in a β-turn-like structure, making clathrin-binding motifs harder to predict than originally envisioned. Consequently, there might be additional proteins that possess unrecognized clathrin-binding regions.
Lipid, cargo and accessory protein binding
In addition to clathrin-binding motifs, endocytic adaptors are comprised of an array of discretely folded domains and unstructured regions that contain a multitude of ligands and/or ligand-binding regions (Fig. 3). This architecture provides the adaptors with the ability to simultaneously bind numerous partners, allowing for efficient internalization of cargo. The remarkable variation among plasma membrane cargo demands adaptors with diversity in their motifs and domains, and has led to the evolution of many families of adaptor proteins with various regions that bind to lipid, cargo and accessory proteins (Maldonado-Baez and Wendland, 2006).
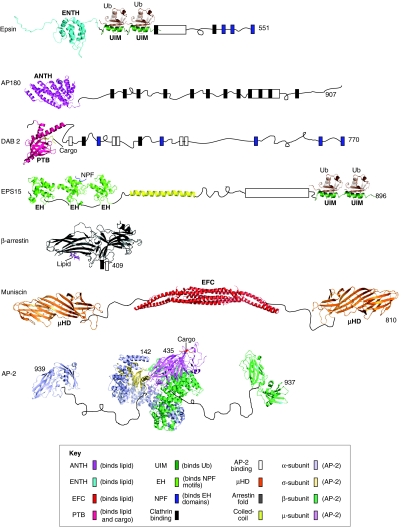
Fig. 3.
Representation of the overall domain and motif organization of human adaptor proteins. Multimeric and monomeric adaptor proteins consist of folded domains (represented by atomic structures), binding motifs (colored boxes), and relatively unstructured regions (represented by thin black curved lines). Selected ligands are shown and labeled in italics, when available in PDB. Most of the binding motifs on AP-2 were omitted for clarity. All structural domain images were generated de novo using Pymol software and coordinates through the protein data bank identification code (PDB). Epsin: ENTH domain of human epsin1 (PDB: 1INZ); S5a UIM (PDB: 1YX6) was used because the epsin UIM structure is not determined. AP180: ANTH domain of fly AP180 (PDB: 1HX8). Dab2: PTB domain of mouse Dab2 (PDB: 1M7E). EPS15: EH domain of mouse Eps15 (PDB: 1QJT); human ENTH2 Eps15 with NPF peptide (PDB: 1F8H); human ENTH3 Eps15 (PDB: 1C07). β-arrestin: cow arrestin 2 with lipid (PDB: 1ZSH). Muniscin: hybrid of human FCHO2 EFC (PDB: 2V0O) and yeast Syp1 μHD domain (PDB: 3G9H). AP-2: human AP-2 core (PDB: 2XA7), human AP-2 β-appendage (PDB: 2IV9), mouse AP-2 α-appendage (PDB:1QTS).
Classic heterotetrameric AP adaptors
AP-2 is the most abundant non-clathrin component of endocytic vesicles purified from brain and is, consequently, the longest-studied and best-understood adaptor (Boehm and Bonifacino, 2001; Keen et al., 1979). The AP-2 complex consists of two large subunits (α, β2), one medium subunit (μ2) and one small subunit (σ2). The large subunits can be subdivided into a trunk domain (70–75 kDa) and an appendage domain (~30 kDa), which are connected by an extended, proteolytically sensitive, flexible linker (Kirchhausen et al., 1989; Zaremba and Keen, 1985). The large subunit trunk domains plus the entirety of the medium and small subunits comprise the biochemically stable AP-2 ‘core’ domain. The large subunits are involved in membrane targeting, clathrin binding (clathrin-binding motif: LΦ[D/E]Φ[D/E]) and the recruitment of accessory proteins (through binding to Dx[F/W] motifs) (Owen et al., 1999; Traub et al., 1999). The σ2 subunit stabilizes the complex and binds to the cargo motif [D/E]xxxL[L/I/M] (Doray et al., 2007; Kelly et al., 2008) and the μ2 subunit contains well-characterized lipid-binding and cargo-binding motifs (sorting YxxΦ containing cargo) (Owen et al., 2004).
AP-2 is recruited to the plasma membrane when basic residues within a structured lipid-binding pocket on the α-subunit interact with the head group of the lipid phosphatidylinositol-(4,5)-bisphosphate (PtdIns(4,5)P2), which is enriched in the plasma membrane (Collins et al., 2002; Gaidarov et al., 1996; Rohde et al., 2002). Subsequently, cargo-binding motifs within the adaptors select subsets of plasma membrane proteins to be internalized by binding to sorting signals in the cytoplasmic tails of membrane cargo proteins (Ohno et al., 1995; Owen and Evans, 1998).
The phosphorylation of T156 in the μ2 domain regulates the affinity of AP-2 for cargo at the plasma membrane (Olusanya et al., 2001). This phosphorylation is mediated by AP-2-associated kinase 1 (AAK1) (Conner and Schmid, 2002; Ricotta et al., 2002) that is homologous to the yeast endocytic kinases Ark1 and Prk1 (Smythe and Ayscough, 2003). In the unphosphorylated state, AP-2 binds plasma membrane lipids and cargo with low affinity. However, following phosphorylation, AP-2 shows an ~100-fold improvement in membrane and cargo binding (Fingerhut et al., 2001). When the crystal structure of the core AP-2 complex was first solved, it showed a closed or ‘locked’ conformation, in which the μ2-binding sites for cargos are blocked by residues from the β2 trunk, and also an incorrect orientation of the μ2 lipid-binding site to a face of the core that cannot interact with the membrane. This observation led to the proposal that the improved affinity for membranes and cargo occurs through a phosphorylation-induced conformational change in the μ2 domain that makes both the cargo-binding and lipid-binding sites more accessible and ‘open’ to their ligands (Collins et al., 2002).
A recent study has now elucidated the mechanism for the AP-2 conversion from its locked low-affinity state to its more-open, high-affinity conformation (Jackson et al., 2010). Using crystallography, it was shown that preincubation of the AP-2 core with a large excess of YxxΦ peptide causes the μ2 subunit to undergo a large conformational change, resulting in relocation of this subunit to a perpendicular face of the complex. Additionally, movement of the β2 subunit ‘unlatches’ the complex, exposing the cargo-binding sites of both the μ2 and σ2 subunits. These rearrangements result in the four PtdIns(4,5)P2-binding sites of AP-2 (two of which were newly identified in the study by Jackson and colleagues) and two cargo-binding sites becoming co-planar, which allows simultaneous multivalent interactions with membranes and cargo. Interestingly, this rearrangement did not require phosphorylation of T156, but was consistent with the phosphorylation that stabilizes the open conformation, resulting in apparent tighter binding to cargo-filled membranes.
Like its mammalian counterpart, the Saccharomyces cerevisiae AP-2 complex localizes to endocytic pits and possesses similar dynamics to other proteins that arrive early at nascent endocytic sites (Carroll et al., 2009; Kaksonen et al., 2005). However, contrary to mammalian AP-2, the S. cerevisiae β2 subunit does not bind clathrin, and until recently had no assigned function in the endocytic process (Yeung et al., 1999).
A new study indicates that the S. cerevisiae AP-2 complex has a crucial role in endocytosis and toxicity of the yeast K28 killer toxin (Carroll et al., 2009). Mutants in any of the four AP-2 subunits are unable to internalize the K28 toxin, but still internalize other cargos such as α-factor, suggesting a cargo-specific effect on the endocytosis of K28 toxin. The receptor for K28 toxin internalization and its potential AP-2-binding motif remain elusive. Identifying the receptor will be crucial to uncovering a sorting signal recognized by the yeast AP-2 complex and may lead to identification of other cargo that rely on AP-2 for endocytosis in yeast.
Non-classic and CLASP adaptor proteins
In addition to the tetrameric AP-2 complex, all eukaryotes also contain a plethora of other adaptors that participate in clathrin-mediated endocytosis. These CLASP adaptors are mono- or dimeric and vary in structure and binding properties. Most of them contain regions that interact with each of the four types of binding partner; however, there are some exceptions that are presently known to bind cargo, clathrin or lipids.
Ubiquitin-binding adaptors
Monoubiquitin or short chains of ubiquitin covalently bound to lysine residues (K63-linked ubiquitin) in transmembrane receptors, can signal the internalization of cargo through the endocytic pathway (Galan and Haguenauer-Tsapis, 1997; Haglund et al., 2003; Shih et al., 2000; Terrell et al., 1998). Mounting evidence suggests that short chains of ubiquitin are needed for the internalization of receptors in higher eukaryotes, because a single-ubiquitin moiety is insufficient to elicit efficient internalization of many receptors (Duncan et al., 2006; Hawryluk et al., 2006).
Ubiquitin-binding adaptor proteins use one of two small regions – the ubiquitin-interacting motif (UIM) or the ubiquitin-associated domain (UBA) – to bind ubiquitin (Hofmann and Falquet, 2001; Polo et al., 2002; Traub and Lukacs, 2007). In mammals and flies, epsin 1, epsin 2 (EPN1, EPN2) and the epidermal growth factor receptor pathway substrate 15 (EPS15), bind and traffic ubiquitylated cargo (Itoh et al., 2001; Overstreet et al., 2004). The molecular architecture of epsins 1 and 2 is similar: both contain an N-terminal epsin N-terminal homology (ENTH) domain followed by two UIMs and an unstructured region containing motifs for clathrin-binding, accessory-protein-binding and AP-2-binding. The ENTH domain binds to PtdIns(4,5)P2 (Chen et al., 1998; Itoh et al., 2001) and promotes membrane curvature (Ford et al., 2002). Additionally, epsin 1 and epsin 2 each contain numerous Dx[F/W] motifs for AP-2-binding, and EPS15 homology (EH) domain-binding Asp-Pro-Phe (NPF) motifs that are crucial for correct localization and endocytic progression (Benmerah et al., 1996; Chen et al., 1998; Maldonado-Báez et al., 2008).
Ubiquitylation is one of the principal internalization signals in S. cerevisiae. The yeast epsins Ent1 and Ent2, as well as the yeast EPS15-like protein Ede1 use ubiquitin-binding regions to promote rapid endocytosis (Dores et al., 2010; Shih et al., 2002). Surprisingly, cells that lack all three ubiquitin-binding adaptors can still internalize the ubiquitylated receptor Ste2, indicating the existence of additional, unknown ubiquitin-binding adaptors (Dores et al., 2010). The yeast adaptor Sla1 might be one protein having this role, because its SH3 domain binds ubiquitin (Stamenova et al., 2007) and it arrives at endocytic sites together with the ubiquitin-binding adaptors Ent1 and Ent2 (Toret et al., 2008).
Cargo-specific adaptors
Some CLASPs act as cargo-specific adaptors that recognize a single transmembrane receptor or a small family of receptors. Numb, LDLRAP1 (also known as and, hereafter, referred to as ARH) and DAB2 all contain an N-terminal phosphotyrosine binding (PTB) domain that preferentially binds to non-phosphorylated tyrosines within [F/Y]xNPxY cargo-sorting motifs (Uhlik et al., 2005), followed by several hundred amino acids predicted to lack a defined secondary structure. ARH and DAB2 cargo recognition sites are essential for clathrin-dependent internalization of megalin and members of the low-density lipoprotein receptor (LDLR) family (Gallagher et al., 2004; Keyel et al., 2006; Mishra et al., 2002a). In addition, the PTB domain also contains a basic PtdIns(4,5)P2-binding site opposite to the peptide groove of the cargo-sorting motif; therefore, two ligands can simultaneously bind the domain in a manner that is mechanistically analogous to the arrangement of the AP-2 μ-subunit. The large unstructured region, which is essential for LDLR uptake, contains motifs for binding to clathrin and AP-2 (Mishra et al., 2002a; Mishra et al., 2002b; Zhou et al., 2003). It will be interesting to learn whether other physiologically important receptor families have dedicated adaptors, and to explore the potential co-evolution of these cognate receptor–adaptor pairs.
Lipid-binding adaptors that lack cargo selectivity
Whereas some adaptors only recognize one receptor family, it appears that other adaptors do not recognize cargo at all. Although neuronal AP180 and its ubiquitous counterpart CALM (officially known as PICALM) are referred to as adaptor proteins, so far there is no evidence to suggest that they directly bind to cargo. Therefore, it seems that the sole purpose of these proteins is to link clathrin to membrane phospholipids. Varying forms of AP180 and/or CALM are expressed from yeast to mammals, and all possess an AP180 N-terminal homology (ANTH) domain (which is structurally similar to the ENTH domain) that binds PtdIns(4,5)P2 (Norris et al., 1995; Ye and Lafer, 1995). This structured domain is followed by a long unstructured region that contains NPF motifs, a series of DLL and DLF variant clathrin-binding motifs and, in non-yeast species, Dx[F/W] AP-2-binding motifs (Morgan et al., 2000; Zhuo et al., 2010).
Interestingly, deletion of the AP180 homologs in yeast (Yap1801 and Yap1802), results in defects in the uptake of the v-SNARE Snc1 (Burston et al., 2009). Studies in other eukaryotes also support a requirement for AP180 and its homologs in the internalization of the v-SNARE VAMP (also known as synaptobrevin) (Bao et al., 2005; Dittman and Kaplan, 2006; Harel et al., 2008), suggesting a cargo-specific role for these proteins. However, to date, no direct physical interaction has been demonstrated between v-SNAREs and AP180s. Therefore, although in vivo experiments with AP180 proteins indicate a cargo-specific role in v-SNARE internalization – they might not themselves bind the transmembrane cargo. AP180 proteins might instead associate with an undetermined accessory protein or with other adaptor proteins that bind cargo directly. In the internalization of the yeast pheromone receptor Ste3, the yeast AP180 homologs also share a redundant role with epsins, implicating a role of AP180 in trafficking other non-SNARE cargos (Maldonado-Báez et al., 2008).
Cargo-binding adaptors that lack lipid- and clathrin-binding selectivity
There are a few proteins that have been classified as adaptor proteins on the basis of their ability to bind cargo but that do not contain any known clathrin or lipid-binding motifs. These proteins include EPS15 (Ede1 in yeast) that selects ubiquitylated cargo, and stonins that bind to and sort synaptotagmin family cargos.
Stonins are conserved from Caenorhabditis elegans to humans but are not found in prokaryotes or yeast (Maritzen et al., 2010). The N-terminal domain of stonins, which binds AP-2 (Walther et al., 2004), is followed by a conserved region of unknown function (the stonin homology domain) that is exclusively found in stonins. The C-terminus contains a μ-homology domain (μHD) that is homologous to the cargo-binding C-terminus of the μ2 subunit of AP-2 and essential for selecting synaptotagmin cargo. Stonin 2 (STON2), the best-studied stonin, also contains two NPF motifs that serve as potential binding partners for EH-domain proteins, such as EPS15 and intersectin (Martina et al., 2001).
The arrestin adaptor family
Arrestins are adaptor proteins that are crucial in trafficking nutrient and signaling receptors (Lefkowitz and Shenoy, 2005; Ma and Pei, 2007). They regulate the inactivation, internalization, and signaling of transmembrane G-protein-coupled receptors (GPCRs) (Gurevich and Gurevich, 2006). On the basis of phylogenetic and structural analyses, the arrestin family of proteins can be divided into two sub-classes: ancient α-arrestins and, more recently evolved, β-arrestins (Alvarez, 2008). α-arrestins were predicted to possess similar structures to those solved for the β sub-class of arrestins, with one important difference: β-arrestins contain tail domains with conserved clathrin-interacting motifs, whereas α-arrestins contain tail domains with PxY motifs, which bind WW motifs that are present in ubiquitin ligases (Mittal and McMahon, 2009).
The β-arrestins help control the strength and duration of GPCR signaling by binding to phosphorylated GPCRs and attenuating signaling by blocking receptor interactions with cognate G-proteins (Lohse et al., 1990). Moreover, β-arrestins promote receptor endocytosis through association with other endocytic proteins (Goodman et al., 1996; Laporte et al., 2000). Upon binding a GPCR, β-arrestin undergoes a conformational change, thereby exposing a C-terminal region that contains motifs for AP-2 and clathrin binding; this, in turn, allows for efficient clathrin-mediated endocytosis of the receptor to take place (Edeling et al., 2006; Gurevich and Gurevich, 2003).
Although β-arrestins are well-studied, until recently, very little was known about the functions of α-arrestins. There are six known α-arrestins in mammals: thioredoxin interacting protein (TXNIP) and the five arrestin-domain-containing proteins Arrdc1 to Arrdc5 (Alvarez, 2008), and twelve in S. cerevisiae – the arrestin-related trafficking adaptor proteins Art1 to Art10, and the arrestin proteins Bul1 and Bul2 (Lin et al., 2008; Nikko and Pelham, 2009; O'Donnell et al., 2010). Yeast and mammalian α-arrestins share a conserved structural arrangement: an N-terminal arrestin domain followed by a C-terminal tail that contains multiple PxY motifs. In yeast, which do not possess the more recently evolved β-arrestins, the ART proteins have been shown to function as ubiquitin-ligase adaptors, linking the E3 ubiquitin ligase Rsp5 to the tails of transmembrane receptors (Herrador et al., 2010; Lin et al., 2008; Liu et al., 2007). The PxY motifs in ART proteins interact with WW domains in Rsp5, allowing for subsequent ubiquitylation of the target receptor and initiation of receptor endocytosis. ART proteins directly downregulate specific nutrient transporters, thereby providing cargo-specific regulation following appropriate stimulation. Addition of PxY motifs to the tail of the receptor arginine permease Can1 is sufficient for its internalization, bypassing the need for Art1. Thus, ART proteins are not needed for interactions with other endocytic proteins, which indicates that they function only as ubiquitin adaptors and not as true endocytic adaptors that link cargo to clathrin. Recent data suggest that Bul1 and Bul2 are functionally redundant with some of the ART proteins, acting as ubiquitin-ligase adaptors at the plasma membrane (Liu et al., 2007; Nikko and Pelham, 2009; Risinger and Kaiser, 2008).
Although some of the mammalian α-arrestins have also been implicated in regulation of nutrient uptake (e.g. the uptake of glucose), other studies indicate that their PxY motifs are dispensable in the regulation of glucose uptake. This suggests that mammalian α-arrestins do not all share a conserved function as ubiquitin-ligase adaptors (Patwari et al., 2009; Patwari et al., 2006). However, recent reports have implicated mammalian α-arrestins in integrin trafficking (Draheim et al., 2010).
Not all α-arrestins localize to the plasma membrane – some members of this family carry out functions on intracellular membranes. For example, Art6 and Art3 (also known as Aly1 and Aly2, respectively) control intracellular sorting of the amino acid permease Gap1 from endosomes to the Golgi complex (O'Donnell et al., 2010). Interestingly, Aly1 and Aly2 associate with the clathrin heavy chain and directly bind a subunit of the yeast adaptor complex AP-1. However, so far there is no evidence to suggest that plasma membrane α-arrestins harbor similar interactions with clathrin and other adaptor proteins.
Sla1 – an adaptor protein in yeast
The yeast protein Sla1 has no obvious homolog in mammalian cells, but has been compared with intersectin and CIN85 (also known as SH3KBP1) (Stamenova et al., 2004; Yu and Cai, 2004). Sla1 possesses many features of an endocytic clathrin adaptor: it has cortical patch dynamics similar to those of the yeast adaptor proteins Yap1801, Yap1802, Ent1 and Ent2, and binds to many endocytic accessory proteins (Gourlay et al., 2003; Kaksonen et al., 2003; Warren et al., 2002). Sla1 is required for clathrin-dependent internalization of certain transmembrane cargos by directly binding to NPFxD sorting-motifs that are present in their cytosolic tails (Howard et al., 2002; Piao et al., 2007). More recently, Sla1 was found to directly bind to clathrin through a LLDLQ variant clathrin box (Di Pietro et al., 2010), thereby confirming its role as a bona fide clathrin adaptor. Interestingly, the Sla1 clathrin-binding motif is negatively regulated by another domain in Sla1 termed the Sla1-homology domain 2 (SHD2) – suggesting an intriguing mode to control Sla1 adaptor activities. The structure of SHD2 revealed a sterile α-motif (SAM) domain fold that was previously unknown to have a role in endocytosis (Di Pietro et al., 2010). This leads to the possibility that other proteins with folds similar to that of the SAM domain will be found to exert autoregulatory effects.
The muniscin family of endocytic adaptors
Muniscins are the latest addition to the growing list of endocytic adaptor proteins. This newly discovered family of proteins is conserved from yeast (Syp1) to humans (FCHO1, FCHO2, SGIP) and was identified through homology comparison of Syp1 domains with domains within the μ-subunits of the AP complexes (Reider et al., 2009). Interestingly, with the exception of SGIP, these proteins also contain an N-terminally positioned F-BAR domain that binds to and tubulates membranes, a process that is important for the deformation of the plasma membrane into a clathrin-coated vesicle (Henne et al., 2007; Reider et al., 2009).
Studies in yeast have revealed that Syp1 is one of the earliest proteins recruited to the endocytic pit and that it directly binds the scaffold protein Ede1, which may stabilize coat formation (Reider et al., 2009; Stimpson et al., 2009). The Syp1 μHD also binds directly to and traffics the transmembrane cargo protein Mid2, which makes Syp1 one the first BAR-domain-containing proteins known to bind cargo. The unstructured middle linker region of Syp1 was also shown to be important in the regulation of actin polymerization through inhibition of Las17 (the yeast ortholog of mammalian WASP) (Boettner et al., 2009).
On the basis of these data, a model has been proposed, in which early-arriving Syp1 induces membrane curvature and clusters cargo in endocytic pits while, at same time, also interacting with the scaffold protein Ede1 in order to allow maturation of the pit and inhibit premature actin polymerization. Syp1 dissociates from the pit before invagination of the vesicle, thereby alleviating the inhibition of Las17 and allowing for a burst in actin polymerization to take place, which in turn promotes vesicle invagination and scission.
Consistent with findings in yeast, the mammalian counterparts of Syp1 – FCHO1 and FCHO2 – were found to bind to the plasma membrane and to directly recruit the scaffold proteins EPS15 (Ede1 in yeast) and intersectin (Pan1 in yeast) (Henne et al., 2010; Reider et al., 2009). These proteins are then joined by AP-2, clathrin and the rest of the endocytic machinery to complete the formation of a coated vesicle. A substantial reduction of FCHO1 and FCHO2 results in the complete loss of CCP formation, which can be rescued by reintroduction of FCHO2 (Henne et al., 2010). This information led the authors to suggest that FCHO1 and FCHO2 are nucleators of CCPs in mammalian cells. However, loss of Syp1 does not alter the initiation or progression of endocytic vesicle formation in yeast, suggesting that other proteins have an – albeit redundant – role in vivo or that the mammalian proteins have evolved an additional function. It remains to be tested whether mammalian muniscins share similarly conserved cargo-binding and actin regulatory functions with the yeast homolog Syp1.
It is also yet to be determined whether muniscins bind to clathrin. Syp1 and its mammalian homologs contain no canonical or variant clathrin-binding motifs. However, our recent analysis suggests that Syp1 contains several conserved surface-exposed acidic and bulky hydrophobic amino acid clusters in regions of β-turn-like structures within its μHD, and these may represent unrecognized clathrin-binding motifs (A.R. and B.W., unpublished data).
Adaptors in clathrin-independent pathways
In addition to clathrin-mediated endocytosis, several other types of endocytosis exist, including caveolin-mediated internalization and a variety of other non-clathrin based routes (Hansen and Nichols, 2009). Many of the endocytic adaptor proteins described either do not bind clathrin or might contain unrecognized clathrin-binding motifs (e.g. muniscins, stonin and EPS15 in mammals, and Ede1 and AP-2 in yeast). This leads to many interesting questions. Why, for example, have some adaptor proteins evolved the ability to bind clathrin, whereas others have not? Do adaptor proteins participate in non-clathrin mediated endocytosis? Can one adaptor protein function in both clathrin-dependent and clathrin-independent internalization?
Perhaps some of the answers lie in the need for flexibility of cargo internalization owing to the ever-changing cellular environment. Environmental conditions and cellular needs can change quickly; consequently, the cell must demonstrate plasticity in cargo internalization to allow for rapid changes in nutrient uptake and cell signaling. This not only avoids competition between distinct cargos, but also allows precise fine-tuning of the cellular physiology. We know that this flexibility is, at least in part, attained through the use of diverse sorting signals and adaptors to sense these signals. Additionally, this adaptability could be acquired by cargo and/or adaptor pairs that are internalized through distinct endocytic pathways, depending on the stimulation level and/or by having adaptor proteins that function in multiple types of internalization. There is evidence to suggest that both types of regulation help govern the process of endocytosis.
One receptor, many internalization routes
Previous studies have suggested that one receptor can be internalized through different routes depending on nutrient or ligand availability. When epidermal growth factor (EGF) receptor is stimulated with EGF at a low concentration, the receptor is endocytosed in a clathrin-dependent manner and recycled back to the cell surface. By contrast, at high concentrations of EGF, the clathrin-dependent pathway becomes saturated and a substantial amount of the receptor is ubiquitylated, internalized and degraded through a clathrin-independent route (Sigismund et al., 2008; Sigismund et al., 2005; Yamazaki et al., 2002). Surprisingly, the clathrin adaptors EPS15 and epsin are necessary for this clathrin-independent internalization of ubiquitylated EGF receptor, providing evidence that some adaptors function in both clathrin-dependent and clathrin-independent internalization (see below).
It has also been reported that, at extremely high concentrations of EGF, the receptor can be internalized in a clathrin-dependent manner (Kazazic et al., 2006). A similar pattern has been observed for other receptors, such as transforming growth factor-β (TGF-β) receptor (Di Guglielmo et al., 2003), supporting the idea that differential sorting of receptors between different endocytic pathways is a common phenomenon.
One adaptor, many internalization routes
Recent evidence indicates that – in addition to EPS15 and epsin – DAB1, a neuron-specific protein similar to DAB2, is an adaptor that functions in both clathrin-dependent and clathrin-independent sorting events. The PTB domain within DAB1 binds with high affinity to FxNPxY-sorting-signals that are present in both amyloid precursor proteins and in LDL receptors (Homayouni et al., 1999; Howell et al., 1999; Trommsdorff et al., 1998). These transmembrane proteins are known to be internalized through clathrin-mediated endocytosis, thereby linking DAB1 to clathrin-dependent cargo sorting (Chen et al., 1990; Cirrito et al., 2008).
However, a new report also implicates DAB1 in the caveolin-dependent internalization of albumin in astrocytes (Bento-Abreu et al., 2009). Albumin is internalized through the transmembrane receptor megalin, which contains two FxNPxY-sorting-signals in its cytoplasmic tail (Bento-Abreu et al., 2008). DAB1 has been observed in an immunocomplex with megalin and caveolin-1, and knockdown of Dab1 leads to a proportional reduction in albumin uptake. This internalization process is dependent on caveolin and DAB1 but not clathrin. These observations link DAB1 to clathrin-independent sorting events (Bento-Abreu et al., 2009).
If one adaptor can, indeed, function in both clathrin-dependent and clathrin-independent internalization, it will be exciting to investigate the mechanism(s) that determine when one endocytic pathway is preferred to another.
Concluding remarks
In this Commentary we have revisited the definition of a clathrin adaptor and examined the features of some of these proteins. One of the fundamental functions of adaptor proteins is the ability to select transmembrane cargo. We have described several proteins that are unable to bind clathrin but still have important roles in clathrin-dependent endocytosis. In addition to the bona fide clathrin adaptors we, therefore, propose to include these proteins in the group of clathrin adaptors.
In recent years, the knowledge of the roles for adaptor proteins in clathrin-dependent endocytic pathways has been substantially enhanced and several new adaptors have been identified. Future research will undoubtedly discover and confirm the existence of even more adaptor proteins – such as that of the putative adaptor RALT/MIG6 (Goh et al., 2010) – and may also uncover new binding partners for known adaptors. Despite the fact that, regarding the mechanisms of clathrin-dependent endocytosis, many questions are still unanswered, the emphasis on clathrin-independent pathways is likely to grow in the future. Some of the research will presumably involve proteins that select transmembrane cargo for internalization through these clathrin-independent routes. It seems reasonable to include these non-clathrin-associated cargo-selecting proteins in the ‘adaptor’ group, especially if they bind to a type of coat protein that mediates the formation of clathrin-independent carriers. We predict this will be an exciting frontier for future research on endocytic mechanisms.
Finally, it is intriguing to speculate that some clathrin adaptors have a second role in selecting cargo for clathrin-independent pathways, as suggested by the interesting observations about Dab1, EPS15 and epsin. Perhaps other proteins will turn out to have similar multifunctionality. It is, therefore, important to keep an open mind regarding the functions of adaptors, because they may be among the most adaptable of proteins.
Acknowledgments
We thank Linton Traub for insightful comments and the members of the Wendland laboratory for helpful discussions. This work was supported by grants from the NIH (A.R.) and the CMDB Program Training T32GM007231, R01 GM060979 (B.W.). Deposited in PMC for release after 12 months.
References
- Aguilar R. C., Watson H. A., Wendland B. (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737-10743 [DOI] [PubMed] [Google Scholar]
- Alvarez C. E. (2008). On the origins of arrestin and rhodopsin. BMC Evol. Biol. 8, 222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Daniels R. W., MacLeod G. T., Charlton M. P., Atwood H. L., Zhang B. (2005). AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J. Neurophysiol. 94, 1888-1903 [DOI] [PubMed] [Google Scholar]
- Benmerah A., Begue B., DautryVarsat A., CerfBensussan N. (1996). The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J. Biol. Chem. 271, 12111-12116 [DOI] [PubMed] [Google Scholar]
- Bento-Abreu A., Velasco A., Polo-Hernández E., Pérez-Reyes P. L., Tabernero A., Medina J. M. (2008). Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J. Neurochem. 106, 1149-1159 [DOI] [PubMed] [Google Scholar]
- Bento-Abreu A., Velasco A., Polo-Hernández E., Lillo C., Kozyraki R., Tabernero A., Medina J. M. (2009). Albumin endocytosis via megalin in astrocytes is caveola- and Dab-1 dependent and is required for the synthesis of the neurotrophic factor oleic acid. J. Neurochem. 111, 49-60 [DOI] [PubMed] [Google Scholar]
- Boehm M., Bonifacino J. S. (2001). Adaptins: the final recount. Mol. Biol. Cell 12, 2907-2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner D. R., D'Agostino J. L., Torres O. T., Daugherty-Clarke K., Uygur A., Reider A., Wendland B., Lemmon S. K., Goode B. L. (2009). The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation. Curr. Biol. 19, 1979-1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston H. E., Maldonado-Baez L., Davey M., Montpetit B., Schluter C., Wendland B., Conibear E. (2009). Regulators of yeast endocytosis identified by systematic quantitative analysis. J. Cell Biol, 185, 1097-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. Y., Stirling P. C., Stimpson H. E. M., Giesselmann E., Schmitt M. J., Drubin D. G. (2009). A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev. Cell 17, 552-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V. I., Capua M. R., Takei K., Butler M. H., Di Fiore P. P., De Camilli P. (1998). Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793-797 [DOI] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. (1990). Npxy, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low-density-lipoprotein receptor. J. Biol. Chem. 265, 3116-3123 [PubMed] [Google Scholar]
- Cirrito J. R., Kang J.-E., Lee J., Stewart F. R., Verges D. K., Silverio L. M., Bu G., Mennerick S., Holtzman D. M. (2008). Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58, 42-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J. (2002). Molecular architecture and functional model of the endocytic AP2 complex. Cell 109, 523-535 [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. (2002). Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 156, 921-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. (2003). Regulated portals of entry into the cell. Nature 422, 37-44 [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Klumperman J., Stoorvogel W., Bonifacino J. S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, 431-434 [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410-421 [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Cascio D., Feliciano D., Bowie J. U., Payne G. S. (2010). Regulation of clathrin adaptor function in endocytosis: novel role for the SAM domain. EMBO J. 29, 1033-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman J. S., Kaplan J. M. (2006). Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc. Natl. Acad. Sci. USA 103, 11399-11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B., Lee I., Knisely J., Bu G. J., Kornfeld S. (2007). The gamma/sigma 1 and alpha/sigma 2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell 18, 1887-1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores M. R., Schnell J. D., Maldonado-Baez L., Wendland B., Hicke L. (2010). The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic 11, 151-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim K. M., Chen H. B., Tao Q., Moore N., Roche M., Lyle S. (2010). ARRDC3 suppresses breast cancer progression by negatively regulating integrin beta4. Oncogene 29, 5032-5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L. M., Piper S., Dodd R. B., Saville M. K., Sanderson C. M., Luzio J. P., Lehner P. J. (2006). Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 25, 1635-1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling M. A., Mishra S. K., Keyel P. A., Steinhauser A. L., Collins B. M., Roth R., Heuser J. E., Owen D. J., Traub L. M. (2006). Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell 10, 329-342 [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. (2004). Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591-605 [DOI] [PubMed] [Google Scholar]
- Fingerhut A., von Figura K., Honing S. (2001). Binding of AP2 to sorting signals is modulated by AP2 phosphorylation. J. Biol. Chem. 276, 5476-5482 [DOI] [PubMed] [Google Scholar]
- Ford M. G., Pearse B. M., Higgins M. K., Vallis Y., Owen D. J., Gibson A., Hopkins C. R., Evans P. R., McMahon H. T. (2001). Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051-1055 [DOI] [PubMed] [Google Scholar]
- Ford M. G. J., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J. K., Evans P. R., McMahon H. T. (2002). Curvature of clathrin-coated pits driven by epsin. Nature 419, 361-366 [DOI] [PubMed] [Google Scholar]
- Gaidarov I., Chen Q., Falck J. R., Reddy K. K., Keen J. H. (1996). A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit-implications for the endocytic pathway. J. Biol. Chem. 271, 20922-20929 [DOI] [PubMed] [Google Scholar]
- Galan J. M., Haguenauer-Tsapis R. (1997). Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16, 5847-5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H., Oleinikov A. V., Fenske C., Newman D. J. (2004). The adaptor disabled-2 binds to the third Psi xNPxY sequence on the cytoplasmic tail of megalin. Biochimie 86, 179-182 [DOI] [PubMed] [Google Scholar]
- Goh L. K., Huang F., Kim W., Gygi S., Sorkin A. (2010). Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J. Cell Biol. 189, 871-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman O. B., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. (1996). beta-arrestin acts as a clathrin adaptor in endocytosis of the beta(2)-adrenergic receptor. Nature 383, 447-450 [DOI] [PubMed] [Google Scholar]
- Gourlay C. W., Dewar H., Warren D. T., Costa R., Satish N., Ayscough K. R. (2003). An interaction between Sla1p and Sla2p plays a role in regulating actin dynamics and endocytosis in budding yeast. J. Cell Sci. 116, 2551-2564 [DOI] [PubMed] [Google Scholar]
- Gurevich E. V., Gurevich V. V. (2006). Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 7, 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V. V., Gurevich E. V. (2003). The new face of active receptor bound minireview arrestin attracts new partners. Structure 11, 1037-1042 [DOI] [PubMed] [Google Scholar]
- Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. (2003). Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461-466 [DOI] [PubMed] [Google Scholar]
- Hansen C. G., Nichols B. J. (2009). Molecular mechanisms of clathrin-independent endocytosis. J. Cell Sci. 122, 1713-1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel F., Denault A., Ngo Q., Dupuis J., Khairy P. (2008). Near-infrared spectroscopy to monitor peripheral blood flow perfusion. J. Clin. Monit. Comput. 1, 37-43 [DOI] [PubMed] [Google Scholar]
- Hawryluk M. J., Keyel P. A., Mishra S. K., Watkins S. C., Heuser J. E., Traub L. M. (2006). Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic 7, 262-281 [DOI] [PubMed] [Google Scholar]
- Henne W. M., Kent H. M., Ford M. G. J., Hegde B. G., Daumke O., Butler P. J. G., Mittal R., Langen R., Evans P. R., McMahon H. T. (2007). Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure 15, 839-852 [DOI] [PubMed] [Google Scholar]
- Henne W. M., Boucrot E., Meinecke M., Evergren E., Vallis Y., Mittal R., McMahon H. T. (2010). FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328, 1281-1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrador A., Herranz S., Lara D., Vincent O. (2010). Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol. Cell. Biol. 30, 897-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K., Falquet L. (2001). A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem. Sci. 26, 347-350 [DOI] [PubMed] [Google Scholar]
- Homayouni R., Rice D. S., Sheldon M., Curran T. (1999). Disabled-1 binds to the cytoplasmic domain of amyloid precursor-like protein 1. J. Neurosci. 19, 7507-7515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. P., Hutton J. L., Olson J. M., Payne G. S. (2002). Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J. Cell Biol. 157, 315-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. W., Lanier L. M., Frank R., Gertler F. B., Cooper J. A. (1999). The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol. 19, 5179-5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes M. T., Mayor S., Parton R. G. (2010). Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 22, 519-527 [DOI] [PubMed] [Google Scholar]
- Itoh T., Koshiba S., Kigawa T., Kikuchi A., Yokoyama S., Takenawa T. (2001). Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291, 1047-1051 [DOI] [PubMed] [Google Scholar]
- Jackson L. P., Kelly B. T., Mccoy A. J., Gaffry T., James L. C., Collins B. M., Höning S., Evans P. R., Owen D. J. (2010). A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141, 1220-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G. (2003). A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475-487 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. (2005). A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123, 305-320 [DOI] [PubMed] [Google Scholar]
- Kazazic M., Roepstorff K., Johannessen L. E., Pedersen N. M., van Deurs B., Stang E., Madshus I. H. (2006). EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. aTraffic 7, 1518-1527 [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. (1979). Clathrin-coated vesicles-isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell 16, 303-312 [DOI] [PubMed] [Google Scholar]
- Kelly B. T., McCoy A. J., Spate K., Miller S. E., Evans P. R., Honing S., Owen D. J. (2008). A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456, 976-979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel P. A., Mishra S. K., Roth R., Heuser J. E., Watkins S. C., Traub L. M. (2006). A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell 17, 4300-4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. (2000). Clathrin. Annu. Rev. Biochem. 69, 699-727 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Nathanson K. L., Matsui W., Vaisberg A., Chow E. P., Burne C., Keen J. H., Davis A. E. (1989). Structural and functional division into 2 domains of the large (100-Kda to 115-Kda) chains of the clathrin-associated protein complex Ap-2. Proc. Natl. Acad. Sci. USA 86, 2612-2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick J. G., Goodman O. B., Keen J. H., Benovic J. L. (1997). Arrestin/clathrin interaction-Localization of the clathrin binding domain of nonvisual arrestins to the carboxyl terminus. J. Biol. Chem. 272, 15011-15016 [DOI] [PubMed] [Google Scholar]
- Lafer E. M. (2002). Clathrin-protein interactions. Traffic 3, 513-520 [DOI] [PubMed] [Google Scholar]
- Laporte S. A., Oakley R. H., Holt J. A., Barak L. S., Caron M. G. (2000). The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta(2)-adrenergic receptor into clathrin-coated pits. J. Biol. Chem. 275, 23120-23126 [DOI] [PubMed] [Google Scholar]
- Le Roy C., Wrana J. L. (2005). Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112-126 [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Shenoy S. K. (2005). Transduction of receptor signals by beta-arrestins. Science 308, 512-517 [DOI] [PubMed] [Google Scholar]
- Lin C. H., MacGurn J. A., Chu T., Stefan C. J., Emr S. D. (2008). Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135, 714-725 [DOI] [PubMed] [Google Scholar]
- Liu J., Sitaram A., Burd C. G. (2007). Regulation of copper-dependent endocytosis and vacuolar degradation of the yeast copper transporter, Ctr1p, by the Rsp5 ubiquitin ligase. Traffic 8, 1375-1384 [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. (1990). Beta-Arrestin – a protein that regulates Beta-Adrenergic-Receptor function. Science 248, 1547-1550 [DOI] [PubMed] [Google Scholar]
- Ma L., Pei G. (2007). beta-arrestin signaling and regulation of transcription. J. Cell Sci. 120, 213-218 [DOI] [PubMed] [Google Scholar]
- Maldonado-Baez L., Wendland B. (2006). Endocytic adaptors: recruiters, coordinators and regulators. Trends Cell Biol. 16, 505-513 [DOI] [PubMed] [Google Scholar]
- Maldonado-Báez L., Dores M. R., Perkins E. M., Drivas T. G., Hicke L., Wendland B. (2008). Interaction between Epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis. Mol. Biol. Cell 19, 2936-2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritzen T., Podufall J., Haucke V. (2010). Stonins-specialized adaptors for synaptic vesicle recycling and beyond? Traffic 11, 8-15 [DOI] [PubMed] [Google Scholar]
- Martina J. A., Bonangelino C. J., Aguilar R. C., Bonifacino J. S. (2001). Stonin 2, an adaptor-like protein that interacts with components of the endocytic machinery. J. Cell Biol. 153, 1111-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele A. E., Watson P. J., Evans P. R., Traub L. M., Owen D. J. (2004). Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain beta-propeller. Nat. Struct. Mol. Biol. 11, 242-248 [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Keyel P. A., Hawryluk M. J., Agostinelli N. R., Watkins S. C., Traub L. M. (2002a). Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 21, 4915-4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. K., Watkins S. C., Traub L. M. (2002b). The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc. Natl. Acad. Sci. USA 99, 16099-16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., McMahon H. T. (2009). Arrestins as adaptors for ubiquitination in endocytosis and sorting. EMBO Rep. 1, 41-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. R., Prasad K., Hao W., Augustine G. J., Lafer E. M. (2000). A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 20, 8667-8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikko E., Pelham H. R. B. (2009). Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic 10, 1856-1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris F. A., Ungewickell E., Majerus P. W. (1995). Inositol hexakisphosphate binds to clathrin assembly protein 3(Ap-3/Ap180) and inhibits clathrin cage assembly in-vitro. J. Biol. Chem. 270, 214-217 [DOI] [PubMed] [Google Scholar]
- O'Donnell A. F., Apffel A., Gardner R. G., Cyert M. S. (2010). Alpha-arrestins Aly1 and Aly2 regulate intracellular trafficking in response to nutrient signaling. Mol. Biol. Cell 21, 3552-3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M. C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T., Bonifacino J. S. (1995). Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872-1875 [DOI] [PubMed] [Google Scholar]
- Olusanya O., Andrews P. D., Swedlow J. R., Smythe E. (2001). Phosphorylation of threonine 156 of the mu 2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 11, 896-900 [DOI] [PubMed] [Google Scholar]
- Overstreet E., Fitch E., Fischer J. A. (2004). Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development 131, 5355-5366 [DOI] [PubMed] [Google Scholar]
- Owen D. J., Evans P. R. (1998). A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Noble M. E. M., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. (1999). A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell 97, 805-815 [DOI] [PubMed] [Google Scholar]
- Owen D. J., Collins B. M., Evans P. R. (2004). Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20, 153-191 [DOI] [PubMed] [Google Scholar]
- Patwari P., Higgins L. J., Chutkow W. A., Yoshioka J., Lee R. T. (2006). The interaction of thioredoxin with Txnip-Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 281, 21884-21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari P., Chutkow W. A., Cummings K., Verstraeten V. L. R. M., Lammerding J., Schreiter E. R., Lee R. T. (2009). Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J. Biol. Chem. 284, 24996-25003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. (1981). Membrane recycling by coated vesicles. Annu. Rev. Biochem. 50, 85-101 [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. (1984). Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 3, 1951-1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao H. L., Machado I. M. P., Payne G. S. (2007). NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol. Biol. Cell 18, 57-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S., Di Fiore P. P. (2006). Endocytosis conducts the cell signaling orchestra. Cell 124, 897-900 [DOI] [PubMed] [Google Scholar]
- Polo S., Sigismund S., Faretta M., Guidi M., Capua M. R., Bossi G., Chen H., De Camilli P., Di Fiore P. P. (2002). A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416, 451-455 [DOI] [PubMed] [Google Scholar]
- Ramjaun A. R., McPherson P. S. (1998). Multiple amphiphysin II splice variants display differential clathrin binding: Identification of two distinct clathrin-binding sites. J. Neurochem. 70, 2369-2376 [DOI] [PubMed] [Google Scholar]
- Reider A., Barker S. L., Mishra S. K., Im Y. J., Maldonado-Báez L., Hurley J. H., Traub L. M., Wendland B. (2009). Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 28, 3103-3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta D., Conner S. D., Schmid S. L., von Figura K., Honing S. (2002). Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Biol. 156, 791-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger A. L., Kaiser C. A. (2008). Different ubiquitin signals act at the Golgi and plasma membrane to direct GAP1 trafficking. Mol. Biol. Cell 19, 2962-2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde G., Wenzel D., Haucke V. (2002). A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J. Cell Biol. 158, 209-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih S. C., Sloper-Mould K. E., Hicke L. (2000). Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 19, 187-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih S. C., Katzmann D. J., Schnell J. D., Sutanto M., Emr S. D., Hicke L. (2002). Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4, 389-393 [DOI] [PubMed] [Google Scholar]
- Shih W., Gallusser A., Kirchhausen T. (1995). A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J. Biol. Chem. 270, 31083-31090 [DOI] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, 2760-2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P. P. (2008). Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell 15, 209-219 [DOI] [PubMed] [Google Scholar]
- Smythe E., Ayscough K. R. (2003). The Ark1/Prk1 family of protein kinases-Regulators of endocytosis and the actin cytoskeleton. EMBO Rep. 4, 246-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenova S. D., Dunn R., Adler A. S., Hicke L. (2004). The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J. Biol. Chem. 279, 16017-16025 [DOI] [PubMed] [Google Scholar]
- Stamenova S. D., French M. E., He Y., Francis S. A., Kramer Z. B., Hicke L. (2007). Ubiquitin binds to and regulates a subset of SH3 domains. Mol. Cell 25, 273-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson H. E. M., Toret C. P., Cheng A. T., Pauly B. S., Drubin D. G. (2009). Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol. Biol. Cell 20, 4640-4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. Y., Xu J., Cai M. J. (2000). Pan1p, End3p, and Sla1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol. 20, 12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E., Harrison S. C., Kirchhausen T. (2000). Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc. Natl. Acad. Sci. USA 97, 1096-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell J., Shih S., Dunn R., Hicke L. (1998). A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell 1, 193-202 [DOI] [PubMed] [Google Scholar]
- Toret C. P., Lee L., Sekiya-Kawasaki M., Drubin D. G. (2008). Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic 9, 848-859 [DOI] [PubMed] [Google Scholar]
- Traub L. M. (2009). Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583-596 [DOI] [PubMed] [Google Scholar]
- Traub L. M., Lukacs G. L. (2007). Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J. Cell Sci. 120, 543-553 [DOI] [PubMed] [Google Scholar]
- Traub L. M., Downs M. A., Westrich J. L., Fremont D. H. (1999). Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc. Natl. Acad. Sci. USA 96, 8907-8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M., Borg J. P., Margolis B., Herz J. (1998). Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 273, 33556-33560 [DOI] [PubMed] [Google Scholar]
- Uhlik M. T., Temple B., Bencharit S., Kimple A. J., Siderovski D. P., Johnson G. L. (2005). Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J. Mol. Biol. 345, 1-20 [DOI] [PubMed] [Google Scholar]
- Walther K., Diril M. K., Jung N., Haucke V. (2004). Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc. Natl. Acad. Sci. USA 101, 964-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D. T., Andrews P. D., Gourlay C. W., Ayscough K. R. (2002). Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci. 115, 1703-1715 [DOI] [PubMed] [Google Scholar]
- Wendland B., Emr S. D. (1998). Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J. Cell Biol. 141, 71-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B., Steece K. E., Emr S. D. (1999). Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 18, 4383-4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Zaal K., Hailey D., Presley J., Lippincott-Schwartz J., Samelson L. E. (2002). Role of Grb2 in EGF-stimulated EGFR internalization. J. Cell Sci. 115, 1791-1802 [DOI] [PubMed] [Google Scholar]
- Ye W. L., Lafer E. M. (1995). Clathrin binding and assembly activities of expressed domains of the synapse-specific clathrin assembly protein Ap-3. J. Biol. Chem. 270, 10933-10939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung B. G., Phan H. L., Payne G. S. (1999). Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell 10, 3643-3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. W., Cai M. J. (2004). The yeast dynamin-related GTPase Vps1p functions in the organization of the actin cytoskeleton via interaction with Sia1p. J. Cell Sci. 117, 3839-3853 [DOI] [PubMed] [Google Scholar]
- Zaremba S., Keen J. H. (1985). Limited proteolytic digestion of coated vesicle assembly polypeptides abolishes reassembly activity. J. Cell. Biochem. 28, 47-58 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhang J., King M. L. (2003). Xenopus autosomal recessive hypercholesterolemia protein couples lipoprotein receptors with the AP-2 complex in oocytes and embryos and is required for vitellogenesis. J. Biol. Chem. 278, 44584-44592 [DOI] [PubMed] [Google Scholar]
- Zhuo Y., Ilangovan U., Schirf V., Demeler B., Sousa R., Hinck A. P., Lafer E. M. (2010). Dynamic interactions between clathrin and locally structured elements in a disordered protein mediate clathrin lattice assembly. J. Mol. Biol. 404, 274-290 [DOI] [PMC free article] [PubMed] [Google Scholar]