Abstract
Previous studies of motor imagery have shown that the same neural correlates for actual movement are selectively activated during motor imagery of the same movement. However, little is known about motor imagery of isometric force. The aim of the present study was to investigate the neural correlates involved in motor imagery of isometric finger forces. Ten subjects were instructed to produce a finger flexion or extension force ranging from 10% to 60% of maximal isometric force and to mentally reproduce the force after an eight second delay period. Transcranial Magnetic Stimulation (TMS) was applied over the hand motor area during imagining the force. We measured the amplitude of Motor Evoked Potentals (MEPs) from the flexor digitorum superfialis (FDS) and the extensor digitorum communis (EDC) muscles and TMS-induced forces from the proximal phalanxes.
The results showed that, as compared to the rest condition, the MEP amplitude was greater in the FDS during imagining flexion forces, whereas it was greater in the EDC during imagining extension forces. MEP amplitudes were similar for motor imagery of graded flexion or extension forces. Also, TMS produced flexion forces during imagining flexion forces, whereas it produced extension forces during imagining extension forces. There was no change in the amplitude of TMS-induced forces across graded motor imagery task. These results support the notion that the same neural correlates for actual movement could be selectively activated during motor imagery of the same movement, but demonstrated that the magnitude of isometric force could not be mentally simulated.
Keywords: Motor Imagery, Isometric Finger Force, Motor Evoke Potential (MEP), flexor digitorum superfialis (FDS), extensor digitorum communis (EDC)
Motor imagery has been defined as the mental execution of action without any overt movements, i.e., no discernible EMG activity [5, 9]. TMS studies of motor imagery have been shown to subliminally enhance the excitability of corticospinal motor pathway at the cortical level [6, 8, 10] or at the spinal level [1, 13]. The subliminally enhanced excitability has been observed in terms of decreased motor threshold and/or facilitated MEPs [6, 10, 12], depressed MEPs [20], and faster RTs [11, 15] by the application of TMS during motor imagery tasks. Changes in MEP amplitude represent changes in corticospinal excitability in a manner of actual movement. The enhanced MEPs have been observed only in the muscles involved in the imagined movements. For example, the enhanced MEPs have been found in the wrist flexor during motor imagery of wrist flexion, and in the wrist extensor during motor imagery of wrist extension [8]. However, it is not clear whether neural correlates and motor pathways during motor imagery of muscle force are the same as those during actual muscle force production.
The purpose of the present study was to investigate the neural correlates involved in motor imagery of isometric finger forces with the questions. 1) Do neural correlates during motor imagery of isometric finger force are the same as those during actual force production? 2) Can isometric finger forces be graded during motor imagery? In order to answer these questions, we adopted a modified force matching task. During a force matching task, subjects were instructed to produce a force by a finger and to reproduce the force by the same or different finger after a few seconds delay [14, 17, 18]. In general, the magnitude of an isometric finger force can be reproduced by the same finger or different fingers of the hand as well as fingers of the other hand. This means that during the delay period, the information of force magnitude could be well maintained if there is no interference force [17]. In the present study, subjects were instructed to produce a force and to mentally reproduce the force (i.e., imagining the magnitude of the force) after an eight-second delay. The tested force levels were 10% – 60% MVC, to test whether if the magnitude of isometric finger forces can be graded during motor imagery.
Ten healthy subjects (28.0 ± 2.6 years old; 7 men and 3 women) participated in the experiment. Ten subjects were all right-handed according to their preferential use of the right hand during writing and eating. All subjects gave informed consent and all procedures were approved by our Institutional Review Board and conformed to the Declaration of Helsinki.
We used a customized device to produce and measure finger flexion and extension forces. The customized device was comprised of in-out movable bars, up-down movable bars, a rotate plate, and hand and arm fixation bars. Subjects produced finger forces along with the horizontal axis, so the produced forces could be considered as the output of finger muscles’ activity with minimizing the effect of gravity on the hand and fingers. The configuration of the device also allowed subjects to move to accommodate different hand sizes. In order to measure finger forces, the proximal phalanx of each finger was tightened by a rubber-coated loop that was attached to the end plate. The four fingers simultaneously pulled rubber-coated loops to produce a flexion force or simultaneously pushed the end plates to produce an extension force.
Subjects were seated in an adjustable chair in front of a testing table. The right upper arm was placed on the customized device at approximately 45° of abduction in the frontal plane and 45° of flexion in the sagittal plane, the elbow at approximately 135° of flexion. The total force produced by the four fingers was displayed on the monitor screen placed on the testing table. Four bi-directional force sensors (208C02, PCB Piezotronics) were used to measure the isometric flexion and extension forces generated by individual fingers. Four signal conditioners (484B11, PCB Piezotronics) were connected to the force sensors for amplifying signals. The amplified signals were then digitized by a 16-bit analog-to-digital converter (PCI-6229, National Instruments) and stored in a personal computer. Two bipolar electrodes (The Bagnoli EMG system, Delsys Inc) were placed over the target muscle bellies to measure the surface electromyography (EMG) of the flexor digitorum superfialis (FDS) and of the extensor digitorum communis (EDC). The electrodes were connected to the main amplifier which was set to the 1000 gain. The amplified signals were then digitized by the same A/D converter mentioned above (PCI-6229, National Instruments) and stored in a personal computer. Both force and EMG signals were sampled at 1000 Hz.
During the experiment, a TMS was delivered over the motor cortex of the left hemisphere using a MagStim 200 stimulator (a figure-of-8-shaped stimulation coil with mean diameter of each wing 45 mm; MagStim Corp., UK). A tight elastic cap was placed on the subject’s head, and a grid of 2 × 2 cm was marked on the left side of the scalp, such that the central point was positioned 2 cm to the left of Cz. To find a hotspot of the FDS, the stimulus intensity was set at 50% of the stimulator output while subjects maintained a lower level of finger flexion force (usually less than 10% MVC). The hotspot was defined by where the largest increment in the total finger force was produced in three consecutive trials by moving the coil over the scalp in steps of 1 cm. The hotspot was then marked with a pen. The position of the coil was adjusted to obtain clear finger-force responses without any facial muscle twitches. To determine the motor threshold, the intensity of the stimulator was gradually decreased until the stimulation no longer induced any noticeable change in the finger forces or a visible muscle twitch, while keeping the stimulator at the hotspot. The same procedure was applied during finger extensions in order to find a hotspot of the EDC. During the experiment, the TMS coil was positioned at the middle of the FDS and EDC hotspots.
After finding the hotspots of the both muscles, the resting motor threshold (RMT) was measured at the FDS hotspot. The resting motor threshold was defined as the lowest stimulus capable of evoking at least four of eight consecutive MEPs with the amplitude ~50 μV while the FDS was at rest. During the experiment, the stimulus intensity was set at 150% of RMT of the FDS, and applied to the entire experiment. The actual stimulus intensity varied between 60% and 70% of the stimulator output across the subjects.
The experiment consisted of motor imagery of finger flexion and extension force tasks and the rest condition. The order of tasks was balanced across subjects. Each task included six target force levels ranging from 10% to 60% of the maximum voluntary contraction (MVC) force, and the target force levels were randomly presented in each task. Subjects performed five trials in a row in each force level. There was a rest condition between the flexion and extension tasks. Each subject performed the total of 65 trials during the experiment.
Each trial began with a beep sound. After waiting one second, subjects were instructed to produce a certain level of flexion or extension force with the fingers of the right hand for three seconds. In each trial, a pre-defined target force was displayed with a red colored horizontal line. The total force produced by the four fingers was displayed online with a white trace on the screen. Then subjects were instructed to close their eyes and keep the memory of the force. After the eight second delay period, subjects were instructed to mentally reproduce the force as they heard another beep sound. During imagining the force, subjects were instructed to relax their hand and arm muscle as much as possible. When the EMG trace was stable during imagining the force, TMS was unexpectedly delivered over the motor cortex of the left hemisphere. To confirm ‘no discernible EMG activity’ during the imagery task, the background EMG activity was monitored on-line. If noticeable spikes were seen in the EMG trace, then the trial was excluded. After the TMS delivery, subjects were instructed to open their eyes. Each trial lasted approximately 16 seconds, and the interval between trials was given for at least 30 seconds to prevent possible finger fatigue. A rest was given in the middle of the experiment.
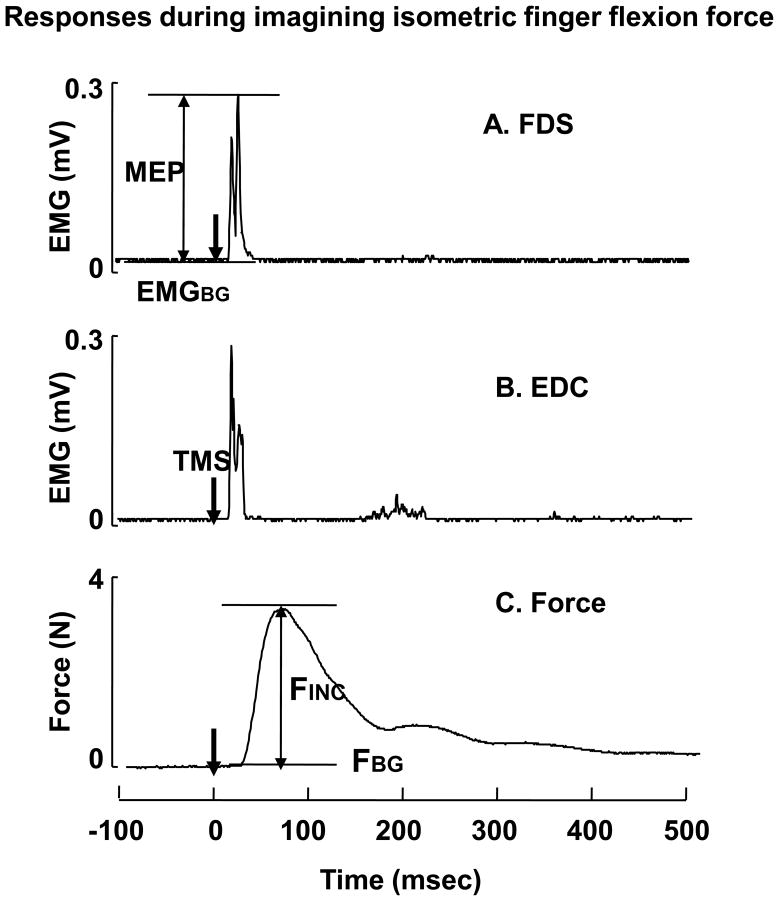
A typical trial in the present study is illustrated in Fig 1. In order to calculate the MEP amplitude, the raw EMG signals were rectified and filtered with the band-pass at 30~500 Hz using a fifth-order, zero lag, Butterworth filter. For each muscle, the background EMG was defined as the mean EMG calculated over a 100 ms window prior to the time of TMS application. The peak EMG value was calculated within a time window (i.e., from 10 ms to 40 ms following a TMS application). The MEP amplitude then calculated by the difference between the peak EMG and the background EMG. TMS also induced the force increment (Fig 1). The background force was defined as the mean force calculated over a 500 ms time window prior to the time of TMS application. Then TMS-induced force was calculated by the difference between the peak force and the background force.
Fig 1.
Typical responses to TMS. The rectified EMG traces from FDS (A) and EDC(B), and the finger force trace (C) are presented. TMS was triggered at 0 in time. MEP: motor evoked potential, FINC: TMS-induced force, FBG: background finger force, EMGBG: background EMG.
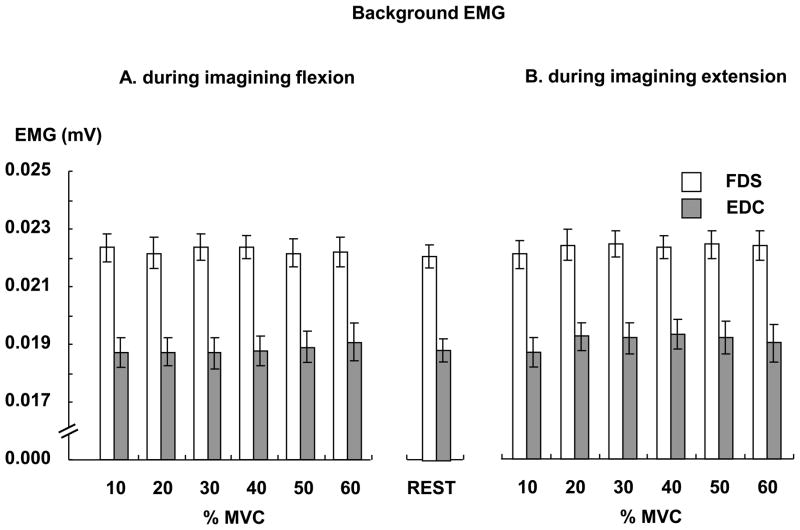
During the experiment, subjects were asked to produce an actual isometric force in the range of 10% – 60% MVC and to mentally reproduce the force without any muscle activations after the eight second delay period. We applied TMS during imagining the finger force and measured background EMG, MEP and TMS-induced force. We also calculated these measurements while subjects were at rest. During the rest condition, the mean background EMG was 0.022 (mV) in the FDS and 0.019 (mV) in the EDC, respectively (Fig 2). Separate T tests showed that the background EMG at each force level was similar to the one at rest in each muscle.
Fig 2.
Background EMGs during imagining finger flexion force (A) and during imagining finger extension force (B). In each muscle, the background EMG of each force level was similar to the one of the rest condition.
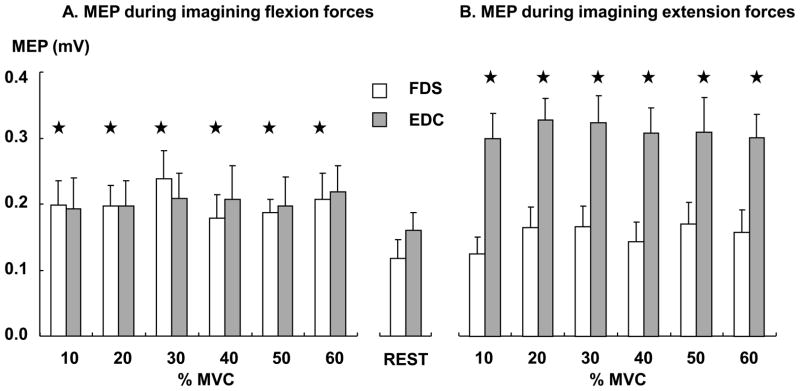
We compared MEP at each force level and the one at rest (Fig 3). The result showed that the MEP increased in the FDS during motor imagery of flexion forces, while it increased in the EDC during motor imagery of extension forces, as compared to the rest condition. Separate T-tests showed that as compared to the rest condition, the MEP was greater during imagining finger flexion forces at all force levels in the FDS (T-tests, all p values are less than 0.009), while the MEP was greater during imagining finger extension forces at all force levels in the EDC (T-tests, all p values are less than 0.002).
Fig 3.
MEPs during imagining finger flexion forces (A) and during imagining finger extension forces (B). ★ represents that the MEP is significantly greater than the MEP at rest.
Also, the result showed that MEPs were greater in the EDC than in the FDS, and during the agonist than during the antagonist. However, the MEP amplitude was similar across all force levels in each muscle during each imagery task. A three-way ANOVA, with factors of MUSCLE (two muscles: FDS and EDC), ROLE (two roles: agonist and antagonist) and FL (six force levels: 10% – 60% MVCs), showed main effects of MUSCLE (F(1,9) =7.82, p=0.021) and ROLE (F(1,9) =15.5, p=0.003), but no effect of FL and no interaction.
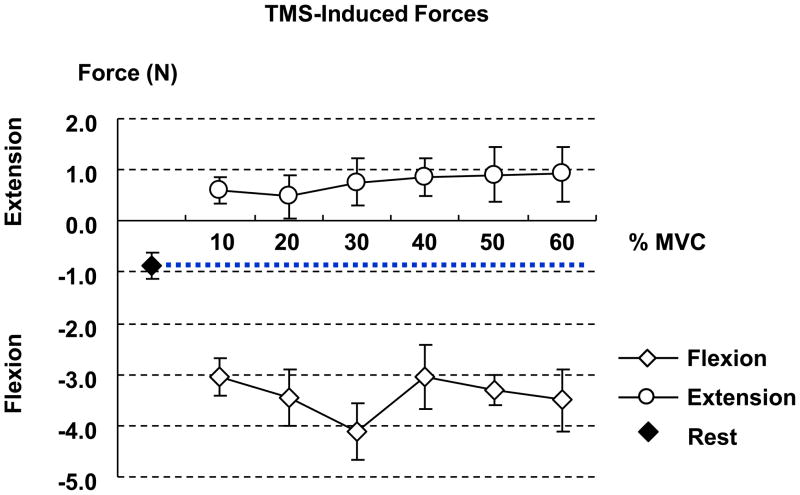
TMS-induced force was increased in the direction of flexion during imagining flexion forces, while it was increased in the direction of extension during imagining extension force, as compared to the rest condition (Fig 4). T-tests showed that p values were less than 0.006 for motor imagery of flexion forces and were less than 0.016 for motor imagery of extension forces. In both flexion and extension motor imagery tasks, TMS-induced force was similar across all force levels.
Fig 4.
TMS-induced force during motor imagery of finger flexion and extension forces. The positive and negative values represent the finger extension and flexion forces, respectively. TMS-induced force at each force level is significantly different from that the one at rest.
The present study showed that, as compared to the rest condition, the MEP was increased in the FDS during motor imagery of isometric flexion force, while it was increased in the EDC during motor imagery of isometric extension force. The MEP amplitude was similar across the force levels in each muscle (Fig 3), i.e., no graded MEP responses during motor imagery of submaximal isometric forces. Similarly, TMS induced flexion forces during motor imagery of flexion forces, while it induced extension forces during motor imagery of extension forces. Also, the TMS-induced force was similar across the force levels (Fig 4), i.e., no graded force responses.
Most TMS studies have clearly demonstrated that motor imagery modulates the excitability of the corticospinal motor system in a movement specific manner [6, 8, 10, 12]. For example, MEP in the wrist flexor was increased during imagining wrist flexion movement, while it was increased in the wrist extensor during imagining wrist extension movement [8, 10]. Consistent with these findings, the present results showed that, as compared to the rest condition, the increased MEP was found in the FDS during imagining finger flexion forces, while the increased MEP was found in the EDC during imagining finger extension forces (Fig 3). A similar result was also found in TMS-induced forces (Fig 4). The result showed that TMS induced flexion forces during imagining flexion forces, while it induced extension forces during imagining extension forces. It has been shown that the direction of TMS-induced force was highly correlated with the direction of isometric force exerted [3]. Taken together, these results support the notion that the same neural correlates for actual movement could be specifically and selectively activated during motor imagery of the same movement.
Human and animal studies have shown that the activity of the CNS is different during flexion and extension movements of the upper limb due to the structural and functional differences in the neuromuscular control system for controlling flexion and extension movements of the upper limb [2, 16, 19]. For example, a TMS study of humans showed that corticospinal projections to the elbow flexor muscle are stronger than those to the extensor muscle [16]. Some electrophysiological studies of primates showed that more monosynaptic connections were found in the biceps brachii than in the triceps brachii [19]. Similarly, our study showed that the absolute magnitude of TMS-induced force was greater during imagining flexion forces than during imagining extension forces (Fig 4). In addition to these, neurons in the cortices related to the wrist extension showed greater discharges compared with the neurons related to the wrist flexion for each unit of force increment [2]. Also, an EEG study demonstrated that EEG-derived movement-related cortical potential was stronger during extension movements than during flexion movements of the forearm [22]. Consistent with the results of these actual movement studies, our study of motor imagery showed that MEPs were greater in the EDC than in the FDS during motor imagery of isometric finger forces (Fig 3), although EMG activities were clearly less in the EDC than in the FDS (Fig 2).
Meanwhile, the finding of no differences in MEPs and TMS-induced force responses in motor imagery of submaximal finger flexion or extension force is not trivial. As discussed above, enhanced responses during motor imagery indicate that the corticospinal excitability is enhanced, and this enhancement occurs in subthreshold level [12, 15]. No change in the response magnitude during graded motor imagery tasks, therefore, suggests that subthreshold enhancement in corticospinal excitability during motor imagery could not be further graded.
TMS studies have shown that MEPs and TMS-induced forces increase with increasing force level [4]. So, one can expect graded responses to TMS during motor imagery of different levels of force. Our results did not support this expectation. Our results of no graded response do not mean that the CNS cannot represent or compute different levels of force. Previous studies have demonstrated that a force can be stored in the brain for a few seconds [14, 17, 18], and that humans even can half or double the force [21]. These studies adopted a force matching task such that subjects were instructed to produce a reference force (i.e., to-be-remembered force) and to reproduce the force (i.e., reproduction period) after a delay period. The reference force was well reproduced by the same finger or different fingers of the hand as well as fingers of the other hand after a few seconds delay [14, 17, 18]. The present study modified the reproduction period in the task such that subjects were instructed to mentally reproduce the reference force after a delay period. So we assumed that subjects maintained the representation of force during the motor imagery. No graded response during the motor imagery may be related to the lack of afferent signals during motor imagery. According to a hypothesis raised by Feldman and Latash (1982), the CNS needs an efferent copy (i.e., motor plan), afferent signals, and the comparison of the two, in order to produce a target force [7]. In the present study, however, there is no afferent signal available for comparisons during the motor imagery period. So, the implicit motor plan (i.e., the representation of the reference force) could not be processed further to the explicit motor processing, resulting in no graded response to TMS.
In conclusion, motor imagery of isometric finger forces increases the excitability of the corticospinal system as involved in actual isometric finger force productions, while the magnitude of isometric force could not be simulated during motor imagery of finger forces. Such conclusion needs to be used with cautions, however. As an anonymous reviewer pointed out, the result of no difference in imagery ability with respect to force level is not expected from internal models. The difference is most likely due to the fact that motor imagery is theoretically an explicit processing of a typical implicit motor plan.
Acknowledgments
We thank anonymous reviewers for helpful comments. Dr. Sheng Li is in part supported by an NIH/NINDS grant (R01NS060774).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonnet M, Decety J, Jeannerod M, Requin J. Mental simulation of an action modulates the excitability of spinal reflex pathways in man. Brain Res Cogn Brain Res. 1997;5:221–228. doi: 10.1016/s0926-6410(96)00072-9. [DOI] [PubMed] [Google Scholar]
- 2.Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol. 1980;44:773–791. doi: 10.1152/jn.1980.44.4.773. [DOI] [PubMed] [Google Scholar]
- 3.Cros D, Soto O, Chiappa K. Transcranial magnetic stimulation during voluntary action: directional facilitation of outputs and relationships to force generation. Brain Res. 2007;1185:103–116. doi: 10.1016/j.brainres.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Danion F, Latash M, Li S. Finger interactions studied with transcranial magnetic stimulation during multi-finger force production tasks. Clin Neurophysiol. 2003;114:1445–1455. doi: 10.1016/s1388-2457(03)00105-6. [DOI] [PubMed] [Google Scholar]
- 5.Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996;77:45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 6.Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- 7.Feldman AG, Latash ML. Interaction of afferent and efferent signals underlying joint position sense: empirical and theoretical approaches. J Mot Behav. 1982;14:174–193. doi: 10.1080/00222895.1982.10735272. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto R, Rothwell J. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 1999;125:75–81. doi: 10.1007/s002210050660. [DOI] [PubMed] [Google Scholar]
- 9.Jeannerod M, Decety J. Mental motor imagery: a window into the representational stages of action. Curr Opin Neurobiol. 1995;5:727–732. doi: 10.1016/0959-4388(95)80099-9. [DOI] [PubMed] [Google Scholar]
- 10.Kasai T, Kawai S, Kawanishi M, Yahagi S. Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res. 1997;744:147–150. doi: 10.1016/s0006-8993(96)01101-8. [DOI] [PubMed] [Google Scholar]
- 11.Kumru H, Soto O, Casanova J, Valls-Sole J. Motor cortex excitability changes during imagery of simple reaction time. Exp Brain Res. 2008;189:373–378. doi: 10.1007/s00221-008-1433-6. [DOI] [PubMed] [Google Scholar]
- 12.Li S. Movement-specific enhancement of corticospinal excitability at subthreshold levels during motor imagery. Exp Brain Res. 2007;179:517–524. doi: 10.1007/s00221-006-0809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Kamper D, Stevens J, Rymer W. The effect of motor imagery on spinal segmental excitability. J Neurosci. 2004;24:9674–9680. doi: 10.1523/JNEUROSCI.2781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Leonard CT. The effect of enslaving on perception of finger forces. Exp Brain Res. 2006;172:301–309. doi: 10.1007/s00221-005-0332-3. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Stevens J, Rymer W. Interactions between imagined movement and the initiation of voluntary movement: a TMS study. Clin Neurophysiol. 2009;120:1154–1160. doi: 10.1016/j.clinph.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park W, Leonard C. The effect of intervening forces on finger force perception. Neurosci Lett. 2008;438:286–289. doi: 10.1016/j.neulet.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Park W, Leonard C, Li S. Finger force perception during ipsilateral and contralateral force matching tasks. Exp Brain Res. 2008;189:301–310. doi: 10.1007/s00221-008-1424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PHILLIPS C, PORTER R. THE PYRAMIDAL PROJECTION TO MOTONEURONES OF SOME MUSCLE GROUPS OF THE BABOON’S FORELIMB. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- 20.Sohn Y, Dang N, Hallett M. Suppression of corticospinal excitability during negative motor imagery. J Neurophysiol. 2003;90:2303–2309. doi: 10.1152/jn.00206.2003. [DOI] [PubMed] [Google Scholar]
- 21.Van Doren CL. Halving and doubling isometric force: evidence for a decelerating psychophysical function consistent with an equilibrium-point model of motor control. Percept Psychophys. 1996;58:636–647. doi: 10.3758/bf03213096. [DOI] [PubMed] [Google Scholar]
- 22.Yue G, Liu J, Siemionow V, Ranganathan V, Ng T, Sahgal V. Brain activation during human finger extension and flexion movements. Brain Res. 2000;856:291–300. doi: 10.1016/s0006-8993(99)02385-9. [DOI] [PubMed] [Google Scholar]