Abstract
Vascularization is a primary challenge in tissue engineering. To achieve it in a tissue scaffold, an environment with the appropriate structural, mechanical, and biochemical cues must be provided enabling endothelial cells to direct blood vessel growth. While biochemical stimuli such as growth factors can be added through the scaffold material, the culture medium, or both, a well-designed tissue engineering scaffold is required to provide the necessary local structural and mechanical cues. As chitosan is a well-known carrier for biochemical stimuli, the focus of this study was on structure-property correlations, to evaluate the effects of composition and processing conditions on the three-dimensional architecture and properties of freeze-cast scaffolds; to establish whether freeze-cast scaffolds are promising candidates as constructs promoting vascularization; and to conduct initial tissue culture studies with endothelial cells on flat substrates of identical compositions as those of the scaffolds to test whether these are biocompatible and promote cell attachment and proliferation.
INTRODUCTION
Vascularization remains a primary challenge in tissue engineering.1 It is essential because cells more than 200–300 µm from a capillary cannot survive due to inadequate nutrient and oxygen diffusion.2–3 Early vascularization efforts focused on staged transfer, in which a tissue construct was inserted into a site of rich vascularization for vessel ingrowth, after which the construct was removed and implanted elsewhere.4 More recently, complex silicon-based structures have been seeded with endothelial cells to create a microvascular network for incorporation into tissue engineered structures.5,6 These strategies have met with some success, yet these techniques break down when reproducibility, large-scale production, and vascular integration in the tissue engineered construct are considered.
Instead, the ideal process to stimulate vascularization may involve creating a tissue engineering environment that provides the appropriate biochemical and mechanical cues for vascularization, allowing the endothelial cells to direct blood vessel growth. While biochemical stimuli such as growth factors can be added through the culture medium, a well-designed tissue engineering scaffold can provide local directional mechanical cues. Scaffold mechanics and porosity affect cell migration, phenotype, and nutrient diffusion, and critical porosities have been noted for endothelial cell tube formation.7–10 Defined feature size and shape can guide creation of tissue vasculature.6 For example, capillary networks can be formed in hydrogels with tube-shaped voids.11
A promising processing route to manufacture such complex, multifunctional scaffolds is freeze casting, the directional solidification of water-based solutions or slurries. It is a particularly promising technique for tissue engineering, because scaffolds with highly aligned porosity and well controlled pore size and geometry can be created.12,13 A wide range of mechanical properties can be achieved for different structures through an appropriate material choice. Additionally, biochemical cues can be incorporated without compromising their functionality due to low temperature processing.
STRUCTURE-PROPERTY CORRELATIONS IN CHITOSAN-BASED SCAFFOLDS
Choice of Scaffold Materials and Solution Preparation
Chitosan was chosen as a scaffold material because of its mild processing conditions—it can be dissolved at a pH lower than ~6 in weak acids such as acetic acid—and because it is an enzymatically degradable polysaccharide whose hydroxyl and amino groups offer sites for derivatization and grafting of desirable bioactive groups such as growth factors.14,15 Chitosan is partially deacetylated chitin, a structural molecule that, in the form of fibrils, is of great structural importance in the chitin-protein composite of arthropod exoskeletons. As a cationic molecule, chitosan allows for pH-dependent electrostatic interaction with negatively charged species such as glycosaminoglycans (GAG) and proteoglycans. Chitosan-GAG complexes are thought to provide a means to retain and concentrate desirable factors secreted by colonizing cells and even from surrounding tissue fluids, because GAGs are known to bind and modulate growth factors and cytokines.14,15 Chitosan’s chemistry is further attractive because it provides many options for ionic and covalent modifications and cross-linking, which allow the mechanical properties of the material to be adjusted and tailored for a particular application.14,15 Gelatin, a collagen derivative, was chosen to prepare blends with chitosan as it was shown to increase the stiffness, strength, and toughness of chitosan scaffolds in preliminary studies.
For scaffold preparation by freeze casting, aqueous solutions of chitosan (C) and gelatin (G) were prepared separately. Low molecular weight chitosan (75–85% deacetylated, Sigma Aldrich, St. Louis, MO) and Type B gelatin from bovine skin (Sigma Aldrich, St. Louis, MO) were used as received. Chitosan and gelatin solutions were prepared by dissolving 2.4% (w/v) chitosan and 5.5% (w/v) gelatin in 1% (v/v) glacial acetic acid (VWR International, West Chester, PA). Chitosan solutions were mixed on a roller at 10 rpm for 24 hours at room temperature. Solutions of gelatin were mixed by magnetic stirring at 60 rpm for 12 hours at 35°C. Blends of 63:37 (w/w) chitosan-geiatin (63C:37G) and 40:60 (w/w) chitosan-geiatin (40C:60G) were prepared by mixing the solutions in a high shear SpeedMixer (DAC 150 FVZ-K, FlackTek, Landrum, SC) at a speed of 1,600 rpm for 60 s.
Freeze Casting of Scaffolds
Freeze casting, the directional solidification of, in this case, a polymer solution, was chosen as the processing route for chitosan-based tissue scaffolds because it offers several advantages over other processing routes: (i) pore size, pore geometry, and overall porosity and architecture can be custom-made for a given application; (ii) scaffold architecture, properties, and degradation rates can be controlled not only through compositional modifications, but also by gelling and cross-linking before and after the freezing process; (iii) scaffolds can be cold processed so that the polymer can be functionalized without compromising their function during manufacture.
To test the influence of material composition and freezing rate as processing parameters, polymer solutions of the above three different compositions were freeze cast into 3-D tissue scaffolds using the freeze-casting system and method detailed in an earlier publication.13 Briefly, 12 mL of polymer solution were filled into a polytetrafluoroethylene (PTFE) mold, sealed with a copper bottom plate, and degassed at 1,600 rpm for 60 s in a DAC 150 FVZ-K SpeedMixer. For directional solidification, the filled mold was placed with its copper bottom on the temperature controlled copper coldfinger of the freeze caster, while the top of the mold remained open to ambient conditions. After precooling of the sample to 5°C, the coldfinger temperature was reduced at a constant cooling rate of either 1°C/min., 3°C/min., or 10°C/min. Once the sample was fully frozen, it was removed from the coldfinger, demolded with a wooden punch, and placed for at least 72 hours in a FreeZone 4.5 Liter Bench-top Freeze Dry System (Labconco, Kansas City, MO) to sublimate the ice-template.
Structure of Freeze-cast Chitosan-based Tissue Scaffolds
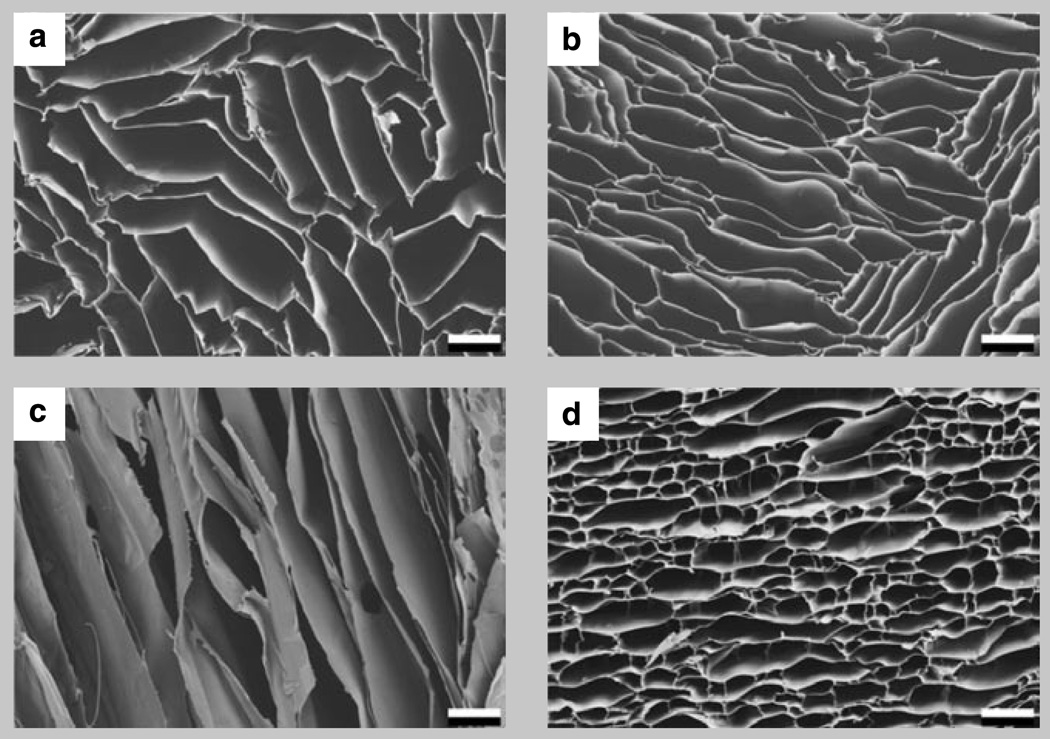
Figure 1 illustrates the distinct structural anisotropy that results from the highly aligned porosity in the scaffolds. Shown are one transverse and one longitudinal cross section of pure chitosan (Figure la,c) and transverse cross-sections of two chitosan-gelatin blends (Figure lb,d) frozen at l°C/min. Transverse to the freezing direction, the lamellar spacing is approximately 100 µm; parallel to it, pores are several millimeters in length and extend well beyond the depicted region. Comparing the transverse pore structure of pure chitosan with that of chitosan-gelatin blends (Figure 1) one finds striking differences, which, we will later find, are reflected also in the scaffolds’ mechanical properties.
Figure 1.
Scanning electron micrographs of one transverse and one longitudinal cross-section of pure chitosan (a, c), and transverse cross sections of two chitosan-geiatin blends (b, 63C:36G) and (d, 40C:60G) frozen at a cooling rate of 1°C/min. Scale bar is 100 µm.
Pure chitosan has highly elongated pores with relatively straight cell walls. The addition of gelatin to chitosan forming the 63C:37G blend has only a small effect on the structure; the cell walls start to curve, but the highly elongated pore morphology is retained, if with a slightly lower aspect ratio. However, at the higher gelatin concentration of 40C:60G, the pores are bridged at regular intervals, resulting in an almost equiaxed, honeycomb-like pore structure. The reason for this change in cell geometry is a difference in freezing conditions. While chitosan is frozen from a viscous liquid, chitosan-gelatin blends are frozen from solutions that start gelling before the ice templating occurs in the freeze-casting process. This alters the conditions under which the water diffuses out from the polymer solution and, as a result, how and into which shape the ice crystals grow.
Scaffold Architecture and Mechanical Performance
The structural differences observed in scaffolds of different compositions were expected to be reflected in their mechanical properties. This is because three structural factors dominate the mechanical properties and performance of cellular solids like those prepared by freeze casting: the material properties from which the scaffold is made, the pore structure and morphology, and the relative density of the cellular solid, (ρ*/ρs), where ρ* is the density of the cellular solid and ρs is that of the solid from which it is made.16,17 It is important to note that these three most influential parameters are not independent since the composition of the solid from which the scaffold is made will not only affect the cell wall material properties, but as described above and illustrated in Figure 1, also the pore structure and morphology formed during freezing. Additionally, the solubility of a given polymer in a particular solvent can limit the achievable relative density of a scaffold.
Mechanical Performance of Dry and Wet 3-D Tissue Scaffolds
The mechanical properties of the freeze-cast scaffolds were investigated in compression on both dry and wet samples. For mechanical testing, the lyophilized samples were fixed with their flat bottoms onto ceramic disks which served as sample holders during manual cutting with a 220 µm diameter diamond-decorated steel wire and a wire speed of 0.7 m/s on a Well 4240 saw (Well Diamond Wire Saws, Inc., Norcross, GA). For mechanical testing, four adjacent 5 mm cubes were cut from the sample at a given height. Two samples of each layer were tested in the dry state and two in a rehydrated,
| (1a) |
| (1b) |
| (2) |
| (3) |
| (4) |
| (5) |
or wet, state. Compression tests were carried out on an Instron 4222 (Instron, Norwood, MA) with a 50 N load cell for dry samples and a 5 N load cell for wet samples and at a cross head speed of 0.05 mm/s, which corresponds to a strain rate of 10−2/s. To stabilize the cubes for wet testing, they were soaked in a 0.4% (w/v) reagent-grade sodium hydroxide in 95% ethanol solution for 15 min. to neutralize the samples, followed by rinsing twice with phosphate buffered saline (PBS) solution, before soaking in PBS for 24 hours prior to testing.
Testing on all samples was performed in the strong direction of the honeycomb-like material, thus parallel to the direction of solidification. A stress-strain response with three principal stress-strain regimes typical for low density cellular materials was observed: an initial linear elastic regime due to cell wall bending up to the elastic limit, at which cell walls start to buckle and continue to collapse at a nearly constant stress, the plateau stress, σ*pl, until the densification strain is reached at which opposite sides of the cells start to impinge on each other and the stress starts to rise rapidly.16,17 Young’s modulus and plateau strength were determined for each sample.
Structure-Property Correlations
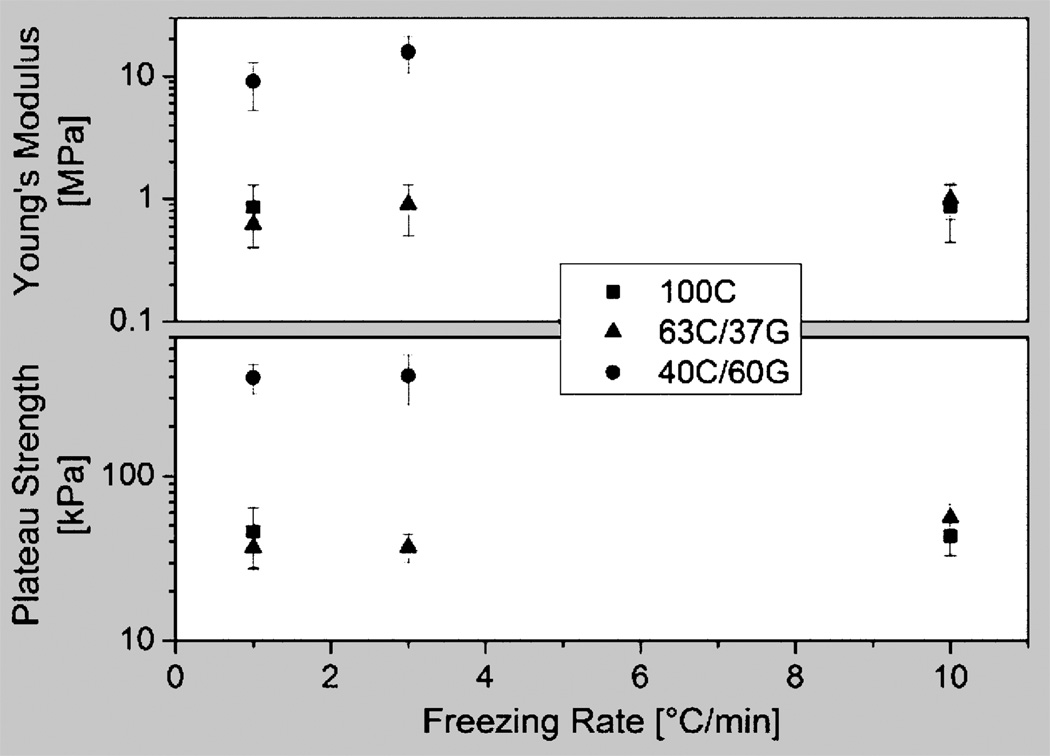
To compare the mechanical properties of the samples of different compositions and processing conditions, Young’s modulus and plateau strength of the dry material were plotted against the sample cooling rate in Figure 2. It yielded the interesting result that the freezing rate had only a small effect on these properties, as did the addition of 37 wt.% gelatin. However, both Young’s modulus and plateau strength increased by about one order of magnitude from 0.86±0.43 MPa to 12.45±4.55 MPa and 44.6±14.4 kPa to 402.3±107.2 kPa, respectively, when 60 wt.% of gelatin was added to the chitosan. This marked increase can partly be attributed to the compositional change, which, assuming values for pure chitosan and gelatin of 534 MPa18 and 3,000 MPa,19 and according to the rule of mixture, increases the Young’s modulus by a factor of less than four. This means that the larger contribution to the property increase must be due to the 40C:60G blend’s pore morphology. This is plausible since the observed lamella bridging significantly increases the second moment of area of each lamella, stiffening it against both bending and buckling and, as a result, significantly increases both Young’s modulus and plateau strength.
Figure 2.
Young’s modulus (top) and plateau strength (bottom) plotted against the sample cooling rate for dry materials. The freezing rate had only a small effect on these properties, as did the addition of 37 wt.% gelatin. However, both Young’s modulus and plateau strength increased by more than one order of magnitude when 60 wt.% of gelatin was added to the chitosan.
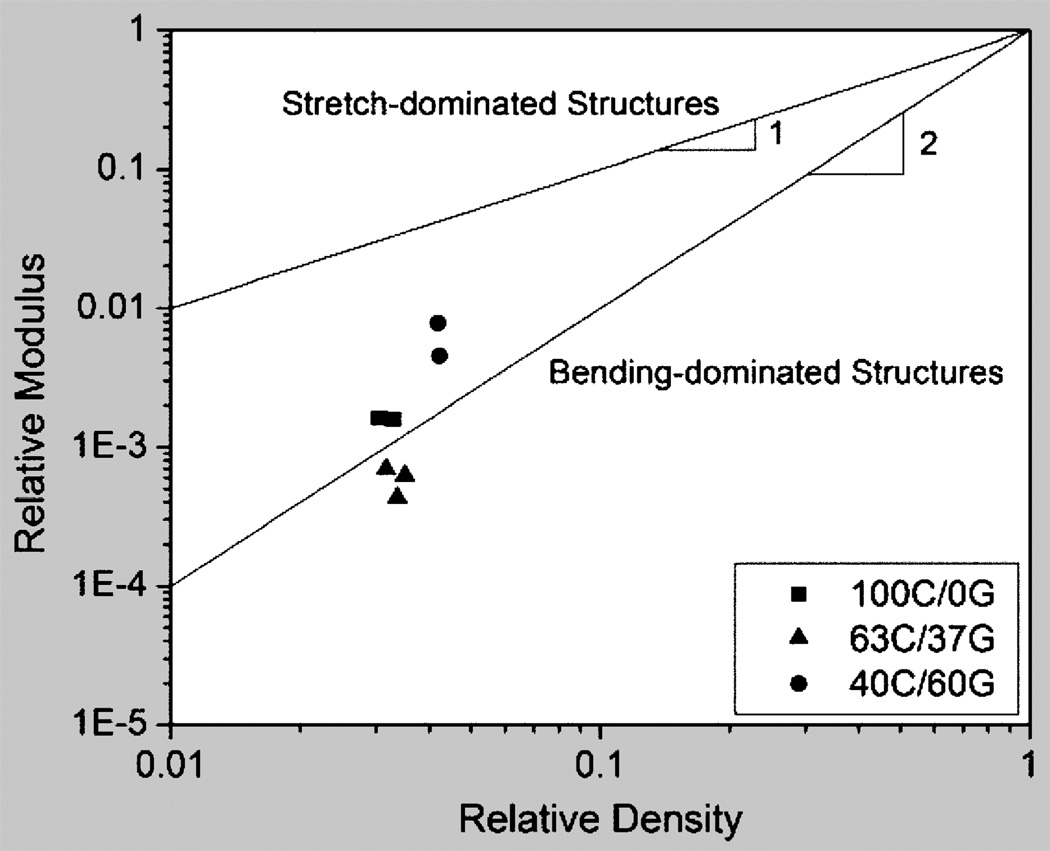
According to the Gibson-Ashby model,17 the relative Young’s modulus, E*/Es, where E* is the Young’s modulus of the foam and Es is that of the solid from which it is made, of a cellular solid is expected to scale linearly with the relative density (Equation la), if its deformation is stretch dominated, as in a honeycomb-like structure, and quadratically (Equation lb), if it is bending dominated, as in an equiaxed foam.
Figure 3 is a plot of relative Young’s modulus, E*/Es, versus relative density, ρ*/ρs, in the dry state for the different scaffold compositions, assuming pure chitosan and pure gelatin to have densities and Young’s moduli of 1.22 Mg/m3 (own measurement) and 1.34 Mg/m3 and 534 MPa and 3,000 MPa, respectively.18–20 A line of slope 1 indicates honeycomb-like stretch dominated behavior; a line of slope 2 indicates foam-like, bending-dominated behavior.16 The plot illustrates that the materials with the more lamellar structure, i.e. pure chitosan and the 63C:37G blend, have properties more akin to bending-dominated foams while the 40C:60G blend performs, as their structure would suggest, in their deformation more like stretch-dominated honeycombs. This makes the 40C:60G blend more efficient; for a given overall porosity, they are stiffer and stronger.
Figure 3.
Relative Young’s modulus, E*Es, plotted versus relative density, ρ*/ρs, in the dry state, assuming pure chitosan and pure gelatin to have densities and Young’s moduli of 1.22 Mg/m3 and 1.34 Mg/m3 and 534 MPa and 3,000 MPa, respectively.18–20 A line of slope 1 indicates honeycomb-like stretch dominated behavior; a line of slope 2 indicates foam-like, bending-dominated behavior.
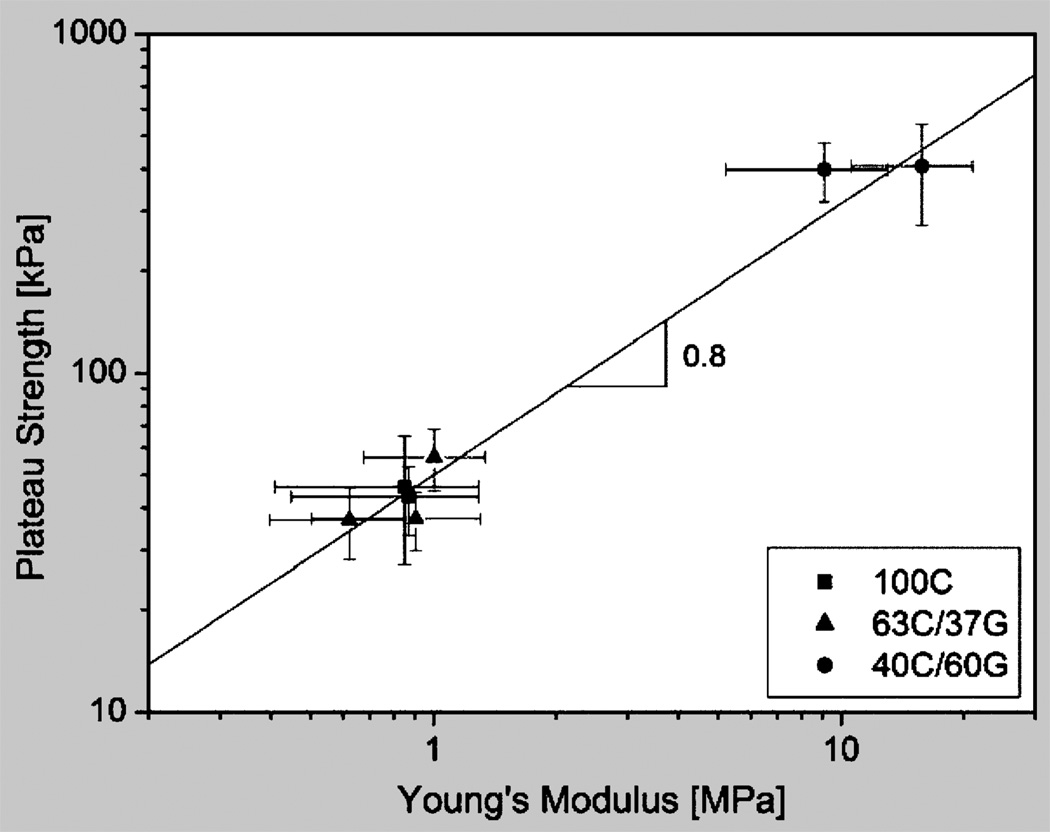
Assuming for further analysis that the Young’s modulus, E*, of the freeze-cast scaffolds scales with relative density as it does in an ideal honeycomb and that the scaffolds collapse by elastic buckling when the plateau strength, σ*pl, is reached, relations that are expressed as Equations 2 and 3, respectively,16,17 where C is a structure-related constant of proportionality—we would expect plateau strength and Young’s modulus of the scaffolds to scale as Equation 4. To test for this correlation, the plateau strength was plotted versus Young’s modulus for the dry freeze-cast materials with relative densities (ρ*/ρs) = 0.035±0.005. Figure 4 shows them to be correlated as shown in Equation 5 with a constant of proportionality C = 1.4, suggesting that the earlier assumptions were realistic. The good agreement between experiment and model is remarkable in light of the fact that we are comparing materials of similar relative density, but different composition and structure, and of variable and imperfect pore alignment.
Figure 4.
A graph showing plateau strength plotted against Young’s modulus of the freeze-cast materials in their dry state. Plateau strength and Young’s modulus of the scaffolds are related as σpl* = 0.05 (E*)08.
Similar, if less marked, trends and correlations were observed when testing the scaffolds in their hydrated or wet state. When wet, the mechanical properties are about three magnitudes lower than dry; both Young’s modulus and plateau strength increased from 3.43±1.32 kPa to 9.57±4.95kPa and 0.72±0.18 kPa to 1.02±0.45 kPa, respectively, when 60 wt.% of gelatin were added to the chitosan. Swelling, which is larger in the chitosan-gelatin blends than in the pure chitosan, thickens the cell walls so significantly that lamellar bridging plays a much reduced role in the mechanical performance of the structure.
ENDOTHELIAL CELL ATTACHMENT
Endothelial cell attachment to thin films was investigated in vitro on 22 mm square glass coverslips that had been coated with solutions of pure chitosan, 63C:73G and 40C:60G. Samples were soaked in a 0.4% (w/v) reagent-grade sodium hydroxide in 95% ethanol for 15 min. to neutralize the samples and prevent dissolution. The coated coverslips were placed into 6 well tissue culture plates, washed in PBS for two hours, and soaked overnight in Dulbec-co’s modification of Eagle’s Medium (DMEM); 100,000 primary porcine aortic endothelial cells were seeded on each sample in 2 mL of DMEM supplemented with 5% fetal bovine serum and 1% penicillin/streptomycin. Cells were imaged daily by phase contrast microscopy (Nikon Eclipse TS100, Nikon Instruments, Melville, NY). Endothelial cells attached to and remained viable on the substrates (Figure 5) and appeared to proliferate, since cells were initially seeded at subconfluent levels, but reached confluence after four days.
Figure 5.
Porcine aortic endothelial cells remained attached and viable on a variety of chitosan samples, including (a) pure chitosan, and (b) 40C:60G blend. Scale bar is 100 µm.
Our results confirmed the variable endothelial cell attachment on chitosan substrates reported in the literature. Some have found that endothelial cells adhere well to chitosan alone,21 while others have shown that chitosan decreased attached cell area perhaps through changes to integrins and the actin cytoskeleton.22 Some of these inconsistencies may be related to different endothelial cell sources, however we also observed cell attachment variability in our experiments, and on some substrates the cells appeared to peel off after several days of culture. We hypothesize that this may be related to variability in protein absorption to chitosan, since proteins present in the culture medium such as fibronectin, collagen, and laminin will coat the chitosan surface and have been shown to enhance endothelial cell attachment to chitosan.23 It may be necessary to either pre-coat chitosan surfaces with adhesive proteins, or incorporate adhesive proteins into the chitosan scaffold to ensure reproducible cell attachment.
CONCLUSIONS
Freeze casting of chitosan and chitosan-gelatin blends offers a promising route to create a tissue engineering environment that combines in one scaffold the appropriate biochemical, structural, and mechanical cues to promote vascularization by allowing endothelial cells to direct blood vessel growth. Chitosan is known as a biopolymer ideally suited for functionalization; the chitosan-based scaffolds created were shown to have a highly aligned porosity which is desired for cell guidance. Custom scaffold design can easily be achieved through an appropriate selection of processing parameters and compositional changes. By cross-linking with genipin, for example, the mechanical properties of the wet scaffolds can easily be brought into the range of natural vasculature. To increase reliability in cell attachment and proliferation, adhesive proteins can be either incorporated into or coated onto the scaffolds.
How would you….
…describe the overall significance of this paper?
Efficient vascularization, the “Achilles heel” of tissue engineering, requires a scaffold that combines the appropriate structural, mechanical, and biochemical cues to allow endothelial cells direct blood vessel growth. Novel freeze-cast chitosan and chitosan-gelatin scaffolds are shown to possess the required property combination. Reported are structural and mechanical properties of dry and wet scaffolds and how they correlate as well as endothelial cell culture responses to these materials.
…describe this work to a materials science and engineering professional with no experience in your technical specialty?
Vascularization is promoted when scaffolds provide the appropriate structural, mechanical, and biochemical cues to enable endothelial cells to direct blood vessel growth. We show that freeze-cast biopolymer scaffolds combine all design requirements. Additionally, we provide newly established, systematic structure-property linkages based on composition and processing conditions, which ease the custom-design of scaffolds for a given application.
…describe this work to a layperson?
Essential to tissue engineering, which aims to repair or replace body tissues, is the development of porous structures—scaffolds—that guide tissue formation. Using directional freezing and subsequent freeze-drying, we can create from biopolymer (chitosan and gelatin) solutions scaffolds with highly aligned porosity which are templated by the ice crystals during freezing. The appeal of this technique is that by varying composition and freezing conditions both structure and mechanical properties of these scaffolds can be tuned to match those of the tissues whose formation they are supposed to promote, in our case blood vessels.
ACKNOWLEDGEMENTS
The authors thank A. Lowman, Drexel University, Philadelphia, PA for the kind permission to use his Instron for mechanical testing, and the Central Research Facility at Drexel University for expert support. They are grateful to the members of both the Biomimetic Design Group and the Vascular Kinetics Lab for experimental guidance and support, particularly P.M. Hunger for scanning electron microscopy and graph preparation, J.R. Smith for help with the manuscript, and A. Basnet and S.F. Kemenyfor cell culture assistance. They acknowledge financial support by the NIH/NIDCR under grant No. 5R01 DE015633 and from NSF through a GAANN (AED) and an REU (EJM) fellowship, and from the Steinbright Career Development Center, Drexel University through a 2010 Fall/Winter Research Co-op Award (NWM). The authors further wish to express their particular gratitude to Anne L. Stevens for her generous support of UGKW and the research carried out by her group.
Contributor Information
Nichols W. Meghri, Department of Materials Science and Engineering, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104
Amalie E. Donius, Department of Materials Science and Engineering, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104
Benjamin W. Riblett, Department of Materials Science and Engineering, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104
Elizabeth J. Martin, Department of Materials Science and Engineering, The Ohio State University, Columbus, OH
Alisa Morss Clyne, Department of Mechanical Engineering of Drezel University.
Ulrike G.K. Wegst, Department of Materials Science and Engineering, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104
References
- 1.Lavik E, Langer R. Applied Microbiology and Biotechnology. 2004;65(1):1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 2.Greene H. Exp. Med. 1941;73:461–474. doi: 10.1084/jem.73.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muschler GF, Nakamoto C, Griffith LG. J. Bone & Joint Surgery. 2004;86-A(7):1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Polykandriotis E, et al. J. Cellular and Molecular Medicine. 2007;11(1):6–20. doi: 10.1111/j.1582-4934.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin M, et al. Biomedical Microdevices. 2004;6(4):269–278. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- 6.Borenstein JT, et al. Biomedical Microdevices. 2002;4(3):167–175. [Google Scholar]
- 7.Kong HJ, et al. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Ingber DE. Nature Cell Biology. 1999;1(5):E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 9.Karageorgiou V, Kaplan D. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Greisler HP, et al. Surgery. 1992;112(2):244–255. [PubMed] [Google Scholar]
- 11.Chrobak KM, Potter DR, Tien J. Microvascular Research. 2006;71(3):185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Deville S, et al. Science. 2006;27(5760):515–518. doi: 10.1126/science.1120937. [DOI] [PubMed] [Google Scholar]
- 13.Wegst UGK, et al. Phil. Trans, R. Soc. A. 2010;368:2099–2121. doi: 10.1098/rsta.2010.0014. [DOI] [PubMed] [Google Scholar]
- 14.Muzzarelli RAA. Carbohydrate Polymers. 2009;76:167–182. [Google Scholar]
- 15.Madihally SV, Matthew HWT. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 16.Ashby MF. Phil. Trans. R. Soc. A. 2006;364:15–30. doi: 10.1098/rsta.2005.1678. [DOI] [PubMed] [Google Scholar]
- 17.Gibson LJ, Ashby MF. Cellular Solids: Structure and Properties. Cambridge, U.K.: Cambridge University Press; 1997. [Google Scholar]
- 18.Suyatma NE, et al. J. Polym. Environ. 2004;12:1–6. [Google Scholar]
- 19.Cao N, Fu Y, He J. Food Hydrocolloids. 2007;21:1153–1162. [Google Scholar]
- 20.Fels G. Journal of Applied Polymer Science. 1964;8:1813–1824. [Google Scholar]
- 21.Chupa JM, et al. Biomaterials. 2000;21:2315–2322. doi: 10.1016/s0142-9612(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, et al. Biomaterials. 2005;26:7616–7627. doi: 10.1016/j.biomaterials.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Cuy JL, et al. J. Biomed. Mater. Res. 2003;67A:538–547. doi: 10.1002/jbm.a.10095. [DOI] [PubMed] [Google Scholar]